Abstract
The polyphagous agromyzid fly, Liriomyza trifolii, is a significant and important insect pest of ornamental and vegetable crops worldwide. The adaptation of insects to different environments is facilitated by heat shock proteins (HSPs), which play an important role in acclimation to thermal stress. In this study, we cloned and characterized five HSP-encoding genes of L. trifolii (Lthsp20, Lthsp40, Lthsp60, Lthsp70, and Lthsp90) and monitored their expression under different thermal stresses using real-time quantitative PCR. Pupae of L. trifolii were exposed to 19 different temperatures ranging from -20 to 45°C. The results revealed that Lthsp20, Lthsp40, Lthsp70 and Lthsp90 were significantly upregulated in response to both heat and cold stress, while Lthsp60 was induced only by heat temperatures. The temperatures of the onset (Ton) and maximal (Tmax) expression of the five Lthsps were also determined and compared with published Ton and Tmax values of homologous genes in L. sativae and L. huidobrensis. Although L. trifolii occurs primarily in southern China, it has cold tolerance comparable with the other two Liriomyza species. Based on the heat shock proteins expression patterns, L. trifolii has the capacity to tolerate extreme temperatures and the potential to disseminate to northern regions of China.
Introduction
Liriomyza trifolii is an economically important invasive insect pest in China [1]. It was initially discovered in Guangdong in 2005 [2] and has since proliferated throughout the southern region of China [3]. Both larvae and adults of L. trifolii can cause damage to crop plants. The larvae mine tunnels in the leaf tissues, and female adults puncture the leaf tissues for oviposition. Both activities can reduce photosynthesis and increase leaf drop, resulting in lower crop quality and yield [4–5]. In recent years, L. trifolii has spread rapidly throughout the country, causing significant damage to various vegetable and horticultural crops [6–8].
Insects are poikilothermic organisms, and their physiological activities can be greatly affected by temperatures [9]. The tolerance of insects to temperature stress is a definitive factor in their survival [10–11]. Multiple studies have shown that insect tolerance to thermal stress is multifactorial and has genetic, physiological, and biochemical components [12–17]. Insects exposed to temperature stress may exhibit alterations in behavior, such as seeking shelter. Additionally, changes in morphology, life history and physiological characteristics, which include changes in membrane fluidity, the accumulation of carbohydrate alcohols, and the generation of heat shock proteins (HSPs) and antioxidant enzymes are also shown to effect in tolerate extreme temperatures [18–19]. Alternations of hsps expression in insects as affected by temperature stress has been widely studied and is one of the best predictors of insect tolerance to temperature stress [20].
Insect HSPs are divided into several families based on molecular weight and homology, including HSP90, HSP70, HSP60, HSP40 and small heat shock proteins (sHSPs) [21–23]. In addition to increasing heat tolerance and protecting organisms from thermal injury, HSPs function as molecular chaperones to promote proper protein folding and prevent the aggregation of denatured proteins [20, 24–26].
Previous studies have examined the response of hsp90, hsp70, hsp60, hsp40 and hsp20 to temperature stress in L. sativae and L. huidobrensis [27], and hsp90 and hsp70 in L. trifolii [28–29]. However, the expression profiles of hsp60, hsp40 and hsp20 in L. trifolii during temperature stress has not yet been investigated. In this study, we characterized the five hsps in L. trifolii, hsp90, hsp70, hsp60, hsp40 and hsp20 to better understand hsp expression in response to both high and low temperature stress. In addition, we also compared the expression of hsps in L. trifolii with the homologous genes in L. sativae and L. huidobrensis, which provides insights into the competition between Liriomyza spp. and the distribution and dissemination of leaf mining insects in response to temperature.
Materials and methods
Study insect
L. trifolii were originally collected on celery in Yangzhou (32.39°N, 119.42°E) in 2010 and reared on beans in the laboratory at 26°C with a 16:8 h (L: D) photoperiod as described by Chen & Kang [30]. Beans (Vigna unguiculata) were seeded at the rate of 5–6 plants per pot (12 cm in diameter) and moved into cages (40×40×65cm) for insect feeding when plants had five to six true leaves. About 150 adults were reared per cage and the larvae inside the tunneling leaves were collected in plastic bags until pupation. The pupae were collected in glass tubes and no field populations were added during experimental period. No specific permissions were required for these activities and the field studies did not involve endangered or protected species.
Temperature treatments
Two-day-old pupae (n = 30) were collected and placed in small glass tubes. The glass tubes along with the pupae were placed into a temperature controller (DC-3010, Ningbo, China) and exposed for 1 h at low temperatures of -20, -17.5, -15–12.5, -10, and -7.5°C; moderate temperatures of -5, -2.5, 0.0, 2.5, 27.5, and 30°C; and high temperatures of 32.5, 35, 37.5, 40, 42.5, and 45°C. The control group consisted of the pupae maintained at 25°C. After exposure to thermal treatments, pupae were allowed to recover at 25°C for 1 h, frozen in liquid nitrogen, and stored at -70°C. Each treatment was repeated four times.
RNA isolation and cloning experiments
Total RNA was extracted from L. trifolii using the SV Total RNA isolation system (Promega, USA). The integrity and purity of RNA was determined by agarose gel electrophoresis and spectrophotometry (Eppendorf Bio Photometer plus, Germany). Total RNA (1 μg) was transcribed into cDNA using oligo (dT) primers. Degenerate primers (Table 1) were used to amplify partial segments of the five hsps, and then 5′ and 3′ RACE were utilized to obtain the full-length cDNAs as recommended by the manufacturer (SMART RACE cDNA Amplification Kit, Clontech, USA).
Table 1. Primers used in the cDNA cloning and real-time quantitative PCR.
| Gene* | Primer sequences(5’→3’) | Fragment length (bp) | ||
|---|---|---|---|---|
| Primers for cDNA cloning | ||||
| hsp20 | F | ATGTDCAACARTTYGCYCC | 187 | |
| R | ACGCCGTCDGADGAMARTTG | |||
| 5’ | CCTCCACTACCACATAGTTGTCCACCA | 451 | ||
| 3’ | ACGCTACCGTCTACCTAAGGGTGTCA | 405 | ||
| hsp40 | F | GCGGTGGYGCYTTYCGTT | 338 | |
| R | CACTGCCATCCCGTTTGA | |||
| 5’ | GGAAATAGTTTTGACCACCTGGGCTG | 609 | ||
| 3’ | CCTTCCCCAAAGAGGGTGACCAATC | 510 | ||
| hsp60 | F | CAAGTRGCCACMATCTCA | 931 | |
| R | TTGCCGTAYTCRCCCTTM | |||
| 5’ | TTCCACCTTAGCTCCCTTGGAGGAA | 948 | ||
| 3’ | GCCGCATAGAGAAGGTATTGTGCCC | 621 | ||
| hsp70 | F | TCAAACGCAAATACAAGAAGGAT | 731 | |
| R | TACATTCAGTATGCCGTTAGCGT | |||
| 5’ | GACAGATTCAGGCTCTTGCCACAG | 927 | ||
| 3’ | ACTTGGACGCTAACGGCATACTGA | 683 | ||
| hsp90 | F | CCAAGTCTGGTACTAAGGCA | 776 | |
| R | CAAATCTTCACAGTTGTCCATGA | |||
| 5’ | TATGTATGGGAATCATCAGCTGG | 618 | ||
| 3’ | GTGCGCCGTGTGTTCATCATGGA | 1441 | ||
| β-actin | F | TACCAACTGGGATGATATGGAA | 322 | |
| R | TCGACCAGCCAAGTCCAAACGC | |||
| Primers for qRT-PCR | ||||
| hsp20 | F | GAAATCAATGTGAAAGTGGTGGA | 175 | |
| R | GAACCTTCAACAAGCCATCAGAT | |||
| hsp40 | F | AAAGTCTCACTCAAGCAAGCATT | 127 | |
| R | GTCCAGTTATGCGTTTGACAGTT | |||
| hsp60 | F | AAATAGTGCGTCGTTCATTGCGT | 99 | |
| R | CGGATTGTGTTTCAACTTTAGCC | |||
| hsp70 | F | GTCGCATACCAAGCAAACAAAC | 198 | |
| R | CGTTAGCGTCCAAGTCAAATGT | |||
| hsp90 | F | CAAAACTAAACCCATCTGGACACG | 158 | |
| R | GCACAAACAAAAGAGCACGGA | |||
| β-actin | F | TTGTATTGGACTCTGGTGACGG | 73 | |
| R | GATAGCGTGAGGCAAAGCATAA | |||
* F, forward; R, reverse; 5’, 5’ RACE primer; 3’, 3’ RACE primer.
Quantitative real-time reverse transcriptase PCR (qRT-PCR)
RNA (0.5 μg) was reverse-transcribed into first-strand cDNA using the Bio-Rad iScript™ cDNA Synthesis Kit (Bio-Rad, CA, USA). Reactions were conducted in a 20 μl reaction volume consisting of 10 μl Bio-Rad iTaq™ Universal SYBR® Green Supermix (2×), 1 μl of each gene-specific primer (10 μM) (Table 1), 2 μl of each cDNA template, and 6 μl of ddH2O. Real-time PCR were performed using an Applied Biosystems 7500 real-time PCR system (Thermo Fisher Scientific, USA) under the following conditions: 3 min at 95°C, 40 cycles of denaturation at 95°C for 30 s, and annealing at the Tm of primer pairs (Table 1) for 30 s. Each treatment contained four replications, and each reaction was run in triplicate. β-actin was cloned from L. trifolii (GenBank accession no: KY231150) and used as a reference gene.
Sequence alignment and data analysis
Full-length cDNAs sequences of the five Lthsps were used as queries to search for other insect hsps using the BLAST programs available at the NCBI website (http://www.ncbi.nlm.gov/BLAST/). Sequence alignments were conducted using Clustal X software [31]. Open reading frames (ORFs) were identified using ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/). Sequence analysis tools of the ExPASy Molecular Biology Server (Swiss Institute of Bioinformatics, Switzerland) were used to analyze the deduced hsps sequences.
The 2−ΔΔCt method was used to evaluate fold changes in mRNA expression levels [32]. Geometric means of the reference genes were utilized to normalize expression under different experimental conditions. One-way ANOVA was used to detect significant differences in mRNA levels among treatments, followed by Tukey’s multiple comparison (P<0.05) in SPSS v. 16.0 (SPSS, Chicago, IL, USA). For ANOVA tests, original data were log-transformed for homogeneity of variances. The temperature where expression was significantly higher than that at 25°C was designated as the onset temperature (Ton), whereas the temperature where expression was significantly higher than that of other temperatures was denoted as Tmax.
Results
Cloning and characterization of five hsps from L. trifolii
The five hsps cloned from L. trifolii were designated as Lthsp20, Lthsp40, Lthsp60, Lthsp70, and Lthsp90, respectively, and were deposited in GenBank with accession nos. KY231145, KY231146, KY231147, KY231148 and KY231149, respectively. The full-length cDNAs of Lthsp20, Lthsp40, Lthsp60, Lthsp70, and Lthsp90 were 911, 1490, 2065, 2293, and 2639bp, respectively. The sequence information of the five Lthsps and its predicted amino acids were detailed in Table 2.
Table 2. The sequence information of the five Lthsp genes and its predicted amino acids.
| Gene | cDNA length (bp) | 5’UTR length (bp) |
3’UTR length (bp) |
ORF length (bp) | molecular weight (kDa) |
isoelectric point | Accession number |
|---|---|---|---|---|---|---|---|
| hsp20 | 911 | 132 | 218 | 561 | 21.23 | 6.38 | KY231145 |
| hsp40 | 1490 | 289 | 181 | 1020 | 37.92 | 8.94 | KY231146 |
| hsp60 | 2065 | 169 | 177 | 1719 | 60.96 | 5.73 | KY231147 |
| hsp70 | 2293 | 184 | 186 | 1923 | 70.43 | 5.43 | KY231148 |
| hsp90 | 2639 | 132 | 362 | 2145 | 81.63 | 4.96 | KY231149 |
The alignment of LtHSP20 with sHSPs from L. sativae and L. huidobrensis revealed a conserved region in the middle, which constitutes an α-crystalline domain (Fig 1A). The N- and C-terminal ends of the predicted proteins were highly variable in the three Liriomyza spp. HSP20s from L. trifolii and L. sativae showed 95.70% amino acid identity, whereas HSP20 orthologs in L. trifolii and L. huidobrensis only showed 72.73% identity. LtHSP40 showed 96.76 and 88.53% amino acid identity with orthologous proteins in L. sativae and L. huidobrensis, respectively. The N-terminal 65 amino acids (positions 4–68), which constitute the most conserved region of HSP40, comprise the DnaJ domain (Fig 1B). LtHSP60 showed a high degree of identity to related proteins in L. sativae and L. huidobrensis (95.63 and 95.98% identity, respectively). LtHSP60 contained a conserved GGM motif at the C-terminal end (Fig 1C). Multiple ATP/Mg2+ binding sites were distributed throughout the predicted protein product in L. trifolii, which were consistent with the structure of HSP60s in the two other Liriomyza spp. Amino acid alignments revealed that LtHSP70 was closely related to analogous proteins in L. sativae and L. huidobrensis, which showed 99.06 and 95.96% amino acid identity, respectively. Similarly, HSP90 in L. trifolii showed a high degree of identity relative to that in L. huidobrensis and L. sativae (97.34 and 99.30%, respectively). Conserved EEVD motifs were identified in the C-terminal ends of LtHSP90 and LtHSP70 (Fig 1D and 1E).
Fig 1. Salient features of five genes encoding HSPs in Liriomyza spp.
The amino acid sequences of the deduced protein products of hsp20 (A), hsp40 (B), hsp60 (C), hsp70 (D) and hsp90 (E) were aligned and conserved motifs or domains are indicated. Dots (.) indicate alignment. Abbreviations: Lt20, L. trifolii hsp20; Lh20, L. huidobrensis hsp20 (DQ452370.1); Ls20, L. sativae hsp20 (DQ452371.1); Lt40, L. trifolii hsp40; Lh40, L. huidobrensis hsp40 (DQ452364.1); Ls40, L. sativae hsp40 (DQ452365.1); Lt60, L. trifolii hsp60; Lh60, L. huidobrensis hsp60 (AY845949.2); Ls60, L. sativae hsp60 (AY851366.2); Lt70, L. trifolii hsp70; Lh70, L. huidobrensis hsp70 (AY842476.2); Ls70, L. sativae hsp70 (AY842477.2); Lt90, L. trifolii hsp90; Lh90, L. huidobrensis hsp90 (AY851367.2); and Ls90, L. sativae hsp90 (AY851368.2).
Expression of Lthsps at different temperatures
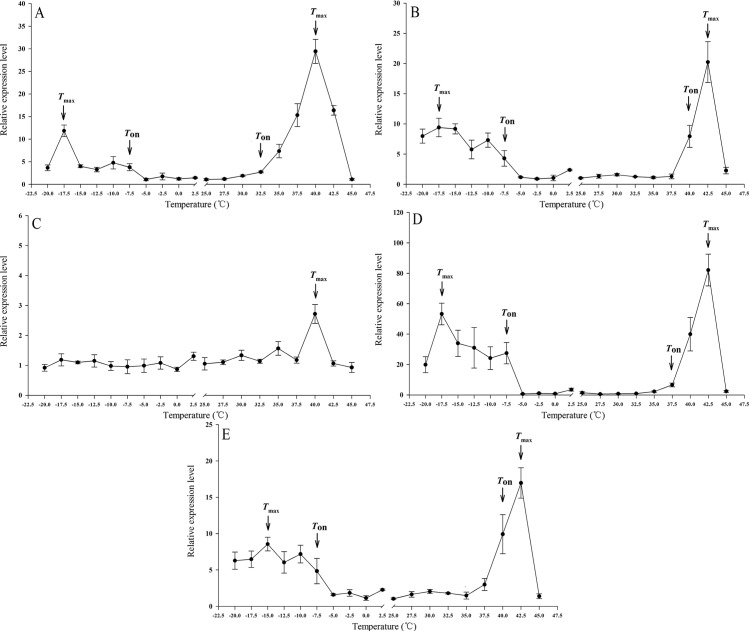
The expression of Lthsps in response to temperature stress (-20 to 45°C) was examined using qRT-PCR. The results showed that the Lthsp20, 40, 70, and 90 were all significantly upregulated in response to both cold and heat stress (cold stress: F6, 21 ≥ 9.818, P < 0.001; heat stress: F6, 21 ≥ 6.631, P < 0.001). The Lthsp60 also showed significant differences (F6, 21 = 6.994, P < 0.001) under heat stress, with mRNA levels increased by 2.72-fold after 1 h at 40°C, while it did not show different responses to cold stress (F6, 21 = 0.412, P = 0.863) (Fig 2C). The expression of the five Lthsps was inhibited when temperatures were lower than -17.5°C or higher than 42.5°C (Fig 2). The Lthsps showed different expression patterns in response to temperature. Lthsp20, 40, 70, and 90 showed a dramatic increase in expression in response to heat stress with mRNA expression increased by 29.43-, 20.24-, 82.15-, and 16.97-fold, respectively, after 1 h at 40°C or 42.5°C (Fig 2A, 2B, 2D and 2E). However, the five Lthsps were not induced by relatively mild temperatures (-5°C, -2.5°C, 0°C, 2.5°C, 27.5°C, and 30°C) (F6, 21 ≤ 2.491, P ≥ 0.056) (Fig 2).
Fig 2. The mRNA expression profiles of genes encoding five HSPs in L. trifolii.
Panels: (A) hsp20; (B) hsp40; (C) hsp60; (D) hsp70; and (E) hsp90. The first temperature where expression was significantly higher than that the control (25°C) was described as the onset temperature (Ton) or the hsp, and the temperature at which the expression level was significantly higher than expression at other temperatures was denoted as Tmax. Ton and Tmax are marked by arrows (→), and the notable temperature shifts of Ton and Tmax are indicated on the curves. The relative level of hsp expression represented the fold increase as compared with the expression in controls. The data were denoted as mean ± SE.
The relative mRNA levels of Lthsps were compared and the onset (Ton) and maximal (Tmax) temperature values were identified. Under cold temperature stress, the Ton values of these five Lthsps were all in -7.5°C and the Tmax values were -17.5°C, except for Lthsp90, which peaked at -15°C. In response to heat stress, the Ton values were 32.5°C for Lthsp20, 40°C for Lthsp40 and Lthsp90, and 37.5°C for Lthsp70. The Tmax was 40°C for Lthsp60 and Lthsp20 and 42.5°C for the other three Lthsps (Fig 2).
Interspecific differences in hsps
A total of ten TATA-box-like regulatory elements were identified in the 5’ untranslated regions (5’UTRs) of the five Lthsps. In comparison, five and eleven TATA-box-like elements were identified in the 5’UTRs of hsps in L. sativae and L. huidobrensis, respectively (Fig 3). Liriomyza huidobrensis contained a single TATA-box-like element in the 5’UTR of hsp20, which was not present in the other two Liriomyza spp. (Fig 3A). The 5’UTR of hsp40 contained three, four, and one TATA-box in L. trifolii, L. huidobrensis, and L. sativae, respectively (Fig 3B). In hsp60, L. trifolii and L. sativae possessed a single TATA-box-like element but L. huidobrensis had four (Fig 3C). Five TATA-box-like elements were found in Lthsp70, whereas L. huidobrensis and L. sativae contained one and two, respectively (Fig 3D). All three leafminers contained a single TATA-box-like element in the 5’ UTR of hsp90 (Fig 3E).
Fig 3. Alignment of 5’UTRs of Liriomyza hsp genes.
The TATA-box-like elements are indicated by shading and the dots indicate alignment. Abbreviations are identical to those in Fig 1. Panels: (A) hsp20; (B) hsp40; (C) hsp60; (D) hsp70; and (E) hsp90.
Under cold stress, the Ton and Tmax values of the five hsps in L. trifolii and L. huidobrensis were 2.5 to 7.5°C lower than that in L. sativae (Table 3). The Ton and Tmax values were generally similar among the three leafminer species when exposed to heat stress, with the exception of Ton for hsp90, which varied from 30 to 40°C in the three leafminer species (Table 3).
Table 3. Interspecific differences in Ton and Tmax between three species of Liriomyza leafminers.
| Gene | L. trifolii | L. huidobrensis* | L. sativae* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| heat | cold | heat | cold | heat | cold | |||||||
| Ton | Tmax | Ton | Tmax | Ton | Tmax | Ton | Tmax | Ton | Tmax | Ton | Tmax | |
| hsp20 | 32.5°C | 40°C | -7.5°C | -17.5°C | 30°C | 40°C | -7.5°C | -17.5°C | 32.5°C | 42.5°C | 0°C | -12.5°C |
| hsp40 | 40°C | 42.5°C | -7.5°C | -17.5°C | 37.5°C | 40°C | -5°C | -12.5°C | 40°C | 42.5°C | -2.5°C | -10°C |
| hsp60 | - | 40°C | - | - | - | 37.5°C | - | - | - | 42.5°C | - | - |
| hsp70 | 37.5°C | 42.5°C | -7.5°C | -17.5°C | 32.5°C | 40°C | -7.5°C | -17.5°C | 35°C | 42.5°C | -2.5°C | -10°C |
| hsp90 | 40°C | 42.5°C | -7.5°C | -15°C | 30°C | 40°C | -5°C | -15°C | 40°C | 42.5°C | -2.5°C | -12.5°C |
*Data for L. huidobrensis and L. sativae were obtained from Huang and Kang [27].
Discussion
Heat shock proteins function in various biological and physiological processes and may be produced in response to temperature, starvation, or disease [33–37]. In this study, we showed that the coding regions of five L. trifolii hsps are highly conserved relative to that in L. huidobrensis and L. sativae. The C-terminal ends of LtHSP90 and LtHSP70 both contain EEVD motifs, which is consistent with their role as molecular chaperones for interaction with other proteins [38]. LtHSP60 also contained C-terminal (GGM)n repeats, which are typical of mitochondrial forms of HSP60 [39]. However, the other Liriomyza HSPs are likely located in the cytosol. LtHSP40 contained a DnaJ domain near the N-terminus, which is consistent with its function in ATPase activity and role as a co-chaperone with HSP70 in multiple processes (e.g. protein folding, trafficking, assembly, and dissociation) [40–41]. The central portion of LtHSP20 contained an α-crystalline domain like other sHSPs, may have essential functions in various processes including diapause and insect immunity [42–43].
Although the coding regions of Liriomyza species hsps are highly conserved, the nucleotide sequences of the 5’UTRs in the Lthsp were different from the other two Liriomyza spp. Regulatory elements in the hsp promoter regions play important roles in hsp expression and may be a contributing factor in establishing specific patterns of hsp expression [44–45]. Ten TATA-box-like elements were identified in the 5’UTRs of the five Lthsps, and the number of 5’UTRs and their locations varied among the three Liriomyza spp. (Fig 3). Data on different Ton and Tmax values of hsps expression in the three Liriomyza may explain the differences in the ability of three Liriomyza species to tolerate cold and heat stress. Although L. huidobrensis prefers cold climates [46], L. trifolii may be the most cold-tolerant when compared to the other two Liriomyza species based on hsps expression. The differences of heat tolerance among the three species were relatively small. L. trifolii and L. sativae may have comparative thermotolerance than L. huidobrensis according to hsps expression pattern.
Furthermore, the super cooling points (SCPs) of L. trifolii and L. huidobrensis were less than -20°C, which was much lower than that of L. sativae (-11.7°C) [30, 47–48]. SCP is an important predictor for cold tolerance [49–51] and field populations of Liriomyza appear to enhance their cold tolerance by depressing the SCP of the puparial stage [1, 52]. The SCP value for L. trifolii was low enough to enable the leafminer to safely overwinter in most regions of China. However, L. trifolii is primarily distributed in southern China, thus further research is needed to discover underlying reasons for its southern distribution patterns. Other studies have shown that the developmental threshold temperature and effective accumulated temperature of the three leafminers were different [53–55]. The developmental threshold temperature of each life stage of L. trifolii was lower than that of L. sativae. Therefore, the first generation of L. trifolii should occur earlier than L. sativae. In addition, the effective accumulated temperature of L. trifolii was higher than that of L. sativae, resulting in the longer dissemination period of L. trifolii relative to L. sativae. In contrast, L. huidobrensis occurs at relatively high latitudes and altitudes. The developmental threshold temperature of L. huidobrensis was the lowest among the three leafminers, and the effective accumulated temperature was in-between L. sativae and L. trifolii. The earlier occurrence of the first generation and longer dissemination in L. trifolii suggest that this pest could probably expose to cold climates in spring/post-winter and autumn/pre-winter, and its relatively lower cold tolerance may play an important role in the survival and development of the population. [1, 56–57].
Although it has the potential to be widely dispersed throughout the country, L. trifolii was currently limited to southern China [3, 58]. There may be several reasons for the geographical limitation of L. trifolii. Liriomyza sativae and L. huidobrensis were first identified in China in 1994 [59–60] while L. trifolii was first reported eleven years later in 2005. Liriomyza trifolii may not have had enough time to disperse into other regions of China. In addition, L. sativae has a higher reproductive capacity than L. trifolii [61] and may not be as prolific as L. sativae. Other possible reasons include the occurrence of natural enemies, pesticide resistance, and the availability of host plants [61–69].
With the rapid development of facility agriculture in China, L. sativae has been displaced by L. trifolii in the southern region of the country. In Hainan province, L. trifolii has become the dominant species, and it constitutes about 95% of the leafminer population in the city of Sanya [70]. Field investigations of Liriomyza spp. have revealed that the damage caused by L. trifolii has become a more serious pest in the northern part of Jiangsu from 2008 to 2015 [71–72]. In the context of predictions of global warming, and the development of facility agriculture and frequent trade exchange, it is high possible that the range of L. trifolii will expand in China. Therefore, research is critical regarding the factors affecting the distribution of L. trifolii in China.
Acknowledgments
We sincerely thank Dr. Tom for editing the manuscript prior to submission. We express our deep gratitude to the Testing Center of Yangzhou University. This research was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the National Science and Technology Support Program (2012BAD19B06), the Jiangsu Science & Technology Support Program (BE2014410), the Science and Technology Program of Yangzhou (YZ2014171), and the Basic Research Program of Agricultural application of Suzhou (SNG201602).
Data Availability
All relevant data are within the paper.
Funding Statement
This research was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the National Science and Technology Support Program [2012BAD19B06 (YZD)] (http://jsycw.ec.js.edu.cn/), the Jiangsu Science & Technology Support Program [BE2014410 (YZD)] (http://www.jstd.gov.cn/), the Science and Technology Program of Yangzhou [YZ2014171 (YZD)] (http://kjj.yangzhou.gov.cn/), and the Basic Research Program of Agricultural application of Suzhou [SNG201602 (JYC)] (http://szkj.gov.cn/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kang L, Chen B, Wei JN, Liu TX. Roles of thermal adaptation and chemical ecology in Liriomyza distribution and control. Annu Rev Entomol. 2009; 54: 127–145. doi: 10.1146/annurev.ento.54.110807.090507 [DOI] [PubMed] [Google Scholar]
- 2.Wang ZG, Guan W, Chen DH. Preliminary report of the Liriomyza trifolii in Zhongshan area. Plant Q. 2007; 21: 19–20. [Google Scholar]
- 3.Gao YL, Reitz S, Xing ZL, Ferguson S, Lei ZR. A decade of a leafminer invasion in China: Lessons learned. Pest Manag Sci. 2017; doi: 10.1002/ps.4591 [DOI] [PubMed] [Google Scholar]
- 4.Johnson MW, Welter SC, Toscano NC. Reduction of tomato leaflet photosynthesis rates by mining activity of Liriomyza sativae (Diptera: Agromyzidae). J Econ Entomol. 1983; 76: 1061–1063. [Google Scholar]
- 5.Parrella MP, Jones VP, Youngman RR, Lebeck LM. Effect of leaf mining and leaf stippling of Liriomyza spp. on photosynthetic rates of chrysanthemum. Ann Entomol Soc Am. 1984; 78: 90–93. [Google Scholar]
- 6.Spencer KA. Agromyzidae (Diptera) of economic importance, 9: Series Entomologica, ed. by Schimitschek E, Gottingen. Bath: The Hague Publishers; 1973. pp. 19–28. [Google Scholar]
- 7.Reitz SR, Kund GS, Carson WG, Phillip PA, Trumble JT. Economics of reducing insecticide use on celery through low-input pest management strategies. Agr Ecosyst Environ. 1999; 73: 185–197. [Google Scholar]
- 8.Yang F, Du YZ, Cao JM, Huang FN. Analysis of three leafminers' complete mitochondrial genomes. Gene. 2013; 529: 1–6. doi: 10.1016/j.gene.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 9.Taylor F. Ecology and Evolution of Physiological Time in insects. Am Nat. 1981; 117: 1–23. [Google Scholar]
- 10.Chen B, Kang L. Implication of pupal cold tolerance for the northern over-wintering range limit of the leafminer Liriomyza sativae (Diptera: Agromyzidae) in China. Appl Entomol Zool. 2005; 40: 437–446. [Google Scholar]
- 11.Sinclair BJ, Coello Alvarado LE, Ferguson LV. An invitation to measure insect cold tolerance: methods, approaches, and workflow. J Therm Biol. 2015; 53: 180–197. doi: 10.1016/j.jtherbio.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 12.Bale JS. Insects and low temperatures: from molecular biology to distributions and abundance. Phil. Trans. R. Soc. Lond. B. 2002; 357: 849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chidawanyika F, Terblanche JS. Rapid thermal responses and thermal tolerance in adult codling moth Cydia pomonella (Lepidoptera: Tortricidae). J Insect Physiol. 2011; 57: 108–117. doi: 10.1016/j.jinsphys.2010.09.013 [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann AA, Parsons PA. Evolutionary genetics and environmental stress New York: Oxford University Press; 1991. [Google Scholar]
- 15.Hoffmann AA, Sørensen JG, Loeschcke V. Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J Therm Biol. 2003; 28: 175–216. [Google Scholar]
- 16.Kelly SA, Panhuis TM, Stoehr AM. Phenotypic plasticity: molecular mechanisms and adaptive significance. Compr Physiol. 2012; 2: 1417–1439. doi: 10.1002/cphy.c110008 [DOI] [PubMed] [Google Scholar]
- 17.Lü ZC, Wang YM Zhu SG, Yu H, Guo JY, Wan FH. Trade-Offs between survival, longevity, and reproduction, and variation of survival tolerance in Mediterranean Bemisia tabaci after temperature Stress. J Insect Sci. 2014; 14: 124 doi: 10.1093/jis/14.1.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duman JG. Antifreeze and ice nucleator proteins in terrestrial arthropods. Annu Rev Physiol. 2001; 63: 327–357. doi: 10.1146/annurev.physiol.63.1.327 [DOI] [PubMed] [Google Scholar]
- 19.Lu MX, Hua J, Cui YD, Du YZ. Five small heat shock protein genes from Chilo suppressalis: characteristics of gene, genomic organization, structural analysis, and transcription profiles. Cell Stress Chaperon. 2014; 19(1): 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response. Annu Rev Physiol.1999; 61: 243–282. doi: 10.1146/annurev.physiol.61.1.243 [DOI] [PubMed] [Google Scholar]
- 21.Sørensen JG, Kristensen TN, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol Lett. 2003; 6: 1025–1037. [Google Scholar]
- 22.Huang LH, Wang CZ, Le K. Cloning and expression of five heat shock protein genes in relation to cold hardening and development in the leafminer, Liriomyza sativa. J Insect Physiol. 2009; 55: 279–285. doi: 10.1016/j.jinsphys.2008.12.004 [DOI] [PubMed] [Google Scholar]
- 23.Tang XT, Sun M, Lu MX, Du YZ. Expression patterns of five heat shock proteins in Sesamia inferens, (Lepidoptera: Noctuidae) during heat stress. J Asia Pac Entomol. 2015; 18: 529–533. [Google Scholar]
- 24.Gehring WJ, Wehner R. Heat shock protein synthesis and thermo tolerance in Cataglyphis, an ant from the Sahara desert. Proc Natl Acad Sci U S A. 1995; 92: 2994–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998; 143: 1883–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glover JR, Lindquist S. Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998; 94: 73–82. [DOI] [PubMed] [Google Scholar]
- 27.Huang LH, Kang L. Cloning and interspecific altered expression of heat shock protein genes in two leafminer species in response to thermal stress. Insect Mol Biol. 2007; 16(4): 491–500. doi: 10.1111/j.1365-2583.2007.00744.x [DOI] [PubMed] [Google Scholar]
- 28.Ji QZ, Wang HH, Lei ZR, Zhang KW, Wang J, Zhang Y. Cloning and expression of heat shock protein 90 gene in relation to heat stress in the leafminer, Liriomyza trifolii. Plant Prot. 2013; 39: 110–116. [Google Scholar]
- 29.Zheng D, Cui XH, Li HL, Cai C, Gao YS, Shang HW. Cloning of heat shock protein gene, hsp70, in Liriomyza trifolii and its expression under temperature stress. Acta Phytophy Sin. 2010; 37: 159–164. [Google Scholar]
- 30.Chen B, Kang L. Cold hardiness and supercooling capacity in the pea leafminer Liriomyza huidobrensis. Cryo Letters. 2002; 23: 173–182. [PubMed] [Google Scholar]
- 31.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL-Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997; 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmitgen TD. Analysis of relative gene expression data using real-time quantification PCR and the 2(-Delta Delta CT) Method. Methods. 2001; 25: 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 33.Lu MX, Li HB, Zheng YT, Shi L, Du YZ. Identification, genomic organization and expression profiles of four heat shock protein genes in the western flower thrips, Frankliniella occidentalis. J Therm Biol. 2016; 57: 110–118. doi: 10.1016/j.jtherbio.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 34.Sun M, Lu MX, Tang XT, Du YZ. Characterization and Expression of Genes Encoding Three Small Heat Shock Proteins in Sesamia inferens (Lepidoptera: Noctuidae). Int J Mol Sci. 2014; 15: 23196–23211. doi: 10.3390/ijms151223196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu MX, Liu ZX, Cui YD, Du YZ. Expression Patterns of Three Heat Shock Proteins in Chilo suppressalis (Lepidoptera: Pyralidae). Ann Entomol Soc Am. 2014; 107: 667–673. [Google Scholar]
- 36.Huang LH, Wang HS, Kang L. Different evolutionary lineages of large and small heat shock proteins in eukaryotes. Cell Res. 2008; 18: 1074–1076. doi: 10.1038/cr.2008.283 [DOI] [PubMed] [Google Scholar]
- 37.Wang XH, Kang L. Molecular mechanisms of phase change in locusts. Annu Rev Entomol. 2014; 59: 225–244. doi: 10.1146/annurev-ento-011613-162019 [DOI] [PubMed] [Google Scholar]
- 38.Tomanek L, Somero GN. Interspecific- and acclimation induced variation in levels of heat-shock proteins 70 (hsp70) and 90 (hsp90) and heat-shock transcription factor-1 (HSF1) in congeneric marine snails (genus Tegula): implications for regulation of hsp gene expression. J Exp Biol. 2002; 205: 677–685. [DOI] [PubMed] [Google Scholar]
- 39.Tsugekils R, Mori H, Nishimura M. Purification, cDNA cloning and Northern-blot analysis of mitochondrial chaperonin 60 from pumpkin cotyledons. Eur J Biochem. 1992; 209: 453–458. [DOI] [PubMed] [Google Scholar]
- 40.Qiu XB, Shao YM, Miao S, Wang L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol Life Sci. 2006; 63: 2560–2570. doi: 10.1007/s00018-006-6192-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheetham M, Caplan A. Structure, function and evolution of dnaj: conservation and adaptation of chaperone function. Cell Stress Chaperon. 1998; 3: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayward SAL, Pavlidesb SC, Tammariellob SP, Rineharta JP, Denlinger DL. Temporal expression patterns of diapause associated genes in flesh fly pupae from the onset of diapause through post-diapause quiescence. J Insect Physiol. 2005; 51: 631–640. doi: 10.1016/j.jinsphys.2004.11.009 [DOI] [PubMed] [Google Scholar]
- 43.Rinehart JP, Li A, Yocum GD, Robich RM, Hayward SA, Denlinger DL. Up-regulation of heat shock proteins is essential for cold survival during insect diapauses. Proc Natl Acad Sci U S A. 2007; 3: 11130–11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Struhl K. Naturally occurring poly (dA-dT) sequences are upstream promoter elements for constitutive transcription in yeast. Proc Natl Acad Sci U S A. 1985; 82: 8419–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen B, Jia TL, Ma RH, Zhang B, Kang L. Evolution of hsp70 gene expression: a role for changes in at-richness within promoters. PLoS ONE. 2011; 6: e20308 doi: 10.1371/journal.pone.0020308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang LH, Chen B, Kang L. Impact of mild temperature hardening on thermotolerance, fecundity, and Hsp gene expression in Liriomyza huidobrensis. J Insect Physiol. 2007; 53: 1199–205. doi: 10.1016/j.jinsphys.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 47.Zhao YX, Kang L. Cold tolerance of the leafminer Liriomyza sativae (Diptera: Agromyzidae). J Appl Entomol. 2000; 124: 185–189. [Google Scholar]
- 48.Xiao T, Chen XY, Zhuang YQ, Pan YL, Yang JH. Study on supercooling and freezing point of Liriomyza trifolii (Burgess). J Southwest China Agri Sci. 2012; 25: 1289–1293. [Google Scholar]
- 49.Worland MR, Convey P. Rapid cold hardening in Antarctic microarthropods. Funct Ecol. 2001; 15:515–524. [Google Scholar]
- 50.Sinclair BJ, Jaco KC, Scott MB, Terblanche JS, Chown SL. Diurnal variation in supercooling points of three species of Collembola from Cape Hallett, Antarctica. J Insect Physiol. 2003; 49: 1049–1061. [DOI] [PubMed] [Google Scholar]
- 51.Klok CJ, Chown SL, Gaston KJ. The geographic ranges structure of the holly leaf-miner. III. Cold hardiness physiology. Funct Ecol. 2003; 17:858–868. [Google Scholar]
- 52.Chen B, Kang L. Variation in cold hardiness of Liriomyza huidobrensis (Diptera: Agromyzidae) along the latitudinal gradients. Environ Entomol. 2004; 33:155–164. [Google Scholar]
- 53.Luo JC, Liu YY, Wei YH. Threshold temperature and effective temperature sum of Liriomyza huidobrensis. Entomol Knowl. 2002; 39: 136–137. [Google Scholar]
- 54.Xiao T, Chen XY, Yang HT, Guo J, Yang JH, Pan YL. Study on the developmental zero and effective accumulative temperature of Liriomyza trifolii (Burgess). J Environ Entomol. 2011; 33: 8–12. [Google Scholar]
- 55.Chen SJ, Sun JD, Wu CQ. Study on the threshold temperature and effective accumulative temperature of Liriomyza sativae. Jiangsu Agri Sci. 2000; 2: 44–45. [Google Scholar]
- 56.Chen B, Kang L. Insect population differentiation in response to environmental thermal stress. Prog Nat Sci. 2005; 15:289–296. [Google Scholar]
- 57.Chen B, Kang L. Can greenhouses eliminate the development of cold resistance of the leafminers? Oecologia. 2005; 144:187–195. doi: 10.1007/s00442-005-0051-2 [DOI] [PubMed] [Google Scholar]
- 58.Lei ZR, Yao JM, Zhu CJ, Wang HH. Prediction of suitable areas for Liriomyza trifolii (Burgess) in China. Plant Prot. 2007; 33: 100–103. [Google Scholar]
- 59.Wen JZ, Wang Y, Lei ZR. New record of Liriomyza sativae Blanchard (Diptera: Agromyzidae) from China. Entomotaxonomia. 1996; 18: 311–312. [Google Scholar]
- 60.Wen JZ, Lei ZR, Wang Y. Survey of Liriomyza huidobrensis in Yunnan Province and Guizhou Province, China. Plant Prot. 1998; 24:18–20. [Google Scholar]
- 61.Wang HH, Reitz SR, Xiang JC, Smagghe G, Lei ZR. Does temperature-mediated reproductive success drive the direction of species displacement in two invasive species of leafminer fly? PLoS ONE. 2014; 9: e98761 doi: 10.1371/journal.pone.0098761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abe Y, Kawahara T. Coexistence of the vegetable leafminer, Liriomyza sativae (Diptera: Agromyzidae), with L. trifolii and L. bryoniae on commercially grown tomato plants. Appl. Entomol. Zool. 2001; 36: 277–281. [Google Scholar]
- 63.Abe Y, Tokumaru S. Displacement in two invasive species of leafminer fly in different localities. Biol Invasions. 2008; 10: 951–953. [Google Scholar]
- 64.Reitz SR, Trumble JT. Interspecific and intraspecific differences in two Liriomyza leafminer species in California. Entomol Exp Appl. 2002; 102: 101–113. [Google Scholar]
- 65.Reitz SR, Trumble JT. Competitive displacement among insects and arachnids. Annu Rev Entomol. 2003; 47: 435–465. [DOI] [PubMed] [Google Scholar]
- 66.Gao YL, Reitz SR, Wei QB, Yu WY, Lei ZR. Insecticide-mediated apparent displacement between two invasive species of leafminer fly. PLoS ONE. 2012; 7: e36622 doi: 10.1371/journal.pone.0036622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsuda K, Saito T. Insecticide susceptibility and carboxylesterase activity in leafminers (Diptera: Agromyzidae) and their associated hymenopteran parasitoids. Crop Prot. 2014; 55: 50–54. [Google Scholar]
- 68.Gao YL, Reitz SR, Wei QB, Yu WY, Zhang Z, Lei ZR. Local crop planting systems enhance insecticide-mediated displacement of two invasive leafminer fly. PLoS ONE. 2014; 9: e92625 doi: 10.1371/journal.pone.0092625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao YL, Reitz SR. Emerging themes in our understanding of species displacements. Annu Rev Entomol. 2017; 62:165–183. doi: 10.1146/annurev-ento-031616-035425 [DOI] [PubMed] [Google Scholar]
- 70.Wang KG, Hao YI, Lei ZR, Xiang JC, Lian ZM. Surveys and analysis of competition and displacement between two invasive species of leafminer fly in Hainan province. Sci Agri Sin. 2013; 46: 4842–4848. [Google Scholar]
- 71.Yang F, Cao JM, Du YZ. Survey and molecular identification of Liriomyza trifolii in Jiangsu, China. Plant Prot. 2010; 36: 108–111. [Google Scholar]
- 72.Chang YW, Shen Y, Dong CS, Gong WR, Tian ZH, Du YZ. Occurrence and population dynamics of Liriomyza trifolii and Liriomyza sativae in Jiangsu. Chinese Bull Entomol. 2016; 54: 884–891. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.