Abstract
Background
Surgeon-dependent factors such as experience and volume are associated with patient outcomes. However, it is unknown whether a surgeon’s research productivity could be related to outcomes. The main aim of this study is to investigate the association between the surgeon’s academic productivity and clinical outcomes following neurosurgical clipping of ruptured aneurysms.
Methods
We performed a post-hoc analysis of 3567 patients who underwent clipping of ruptured intracranial aneurysms in the randomized trials of tirilazad mesylate from 1990 to 1997. These trials included 162 centers and 156 surgeons from 21 countries. Primary and secondary outcomes were: Glasgow outcome scale score and mortality, respectively. Total publications, H-index, and graduate degrees were used as academic indicators for each surgeon. The association between outcomes and academic factors were assessed using a hierarchical logistic regression analysis, adjusting for patient covariates.
Results
Academic profiles were available for 147 surgeons, treating a total of 3307 patients. Most surgeons were from the USA (62, 42%), Canada (18, 12%), and Germany (15, 10%). On univariate analysis, the H-index correlated with better functional outcomes and lower mortality rates. In the multivariate model, patients under the care of surgeons with higher H-indices demonstrated improved neurological outcomes (p = 0.01) compared to surgeons with lower H-indices, without any significant difference in mortality. None of the other academic indicators were significantly associated with outcomes.
Conclusion
Although prognostication following surgery for ruptured intracranial aneurysms primarily depends on clinical and radiological factors, the academic impact of the operating neurosurgeon may explain some heterogeneity in surgical outcomes.
Introduction
Prognostication of surgical outcomes is largely dependent upon a number of factors involving the patient, the disease, the surgeon, and the treatment center. It is increasingly recognized that surgeon-dependent factors including, but not limited to surgical experience, time spent in practice, and age can influence patient outcomes following surgery [1, 2]. For instance, a number of previous studies have demonstrated better patient outcomes at centers where surgeons perform a high volume of certain procedures [3, 4]. This effect was seen across almost all surgical specialties (general[5], cardiothoracic[6], vascular[7], plastics[8], pediatric[1], neurosurgery[9], orthopedics[10], and urology[11]). However, despite the value placed by academic institutions on academia in surgical practice, the impact of a surgeon or hospital’s academic productivity on patient outcomes following surgery is controversial and remains largely unexplored [12]. Most studies on this topic have focused on a hospital’s teaching status, and have demonstrated that patient outcomes following surgery at teaching hospitals are superior to those at non-teaching centers [13–19]. However, none of these studies focus directly on a surgeon’s academic profile and whether his/her academic output and impact correlate with his/her clinical outcomes.
Importantly, aneurysmal subarachnoid hemorrhage (SAH) represents a disease with widely heterogeneous outcomes, with almost 40% of patients suffering permanent neurological and cognitive deficits afterwards [20]. These patients are usually admitted under the care of neurosurgeons since they often require urgent surgical intervention with either open aneurysm clipping or endovascular coiling with extensive inpatient follow-up afterwards for complications such as delayed cerebral ischemia [21]. The prognosis of patients with SAH is not only determined by their initial neurological status. Clinical factors following aneurysmal SAH linked to functional outcome and mortality include: age, aneurysm location, history of hypertension, and presence of intracerebral or intraventricular hemorrhage [21, 22]. Studies have demonstrated notable differences in clinical outcomes following aneurysm surgery at different operative centers [23]. This heterogeneity could be related to the clinical characteristics of the patients, case volume of the institution,[24] and/or surgeon-dependent factors such as surgical experience or academic involvement [25].
This study aims to explore the understudied role of a surgeon’s academic productivity and citation impact (measured by total number of publications, the H-index, and possession of a graduate degree) on their patients’ functional outcomes and mortality following surgical clipping of ruptured intracranial aneurysms.
Methods and materials
Study population
In order to perform this analysis, we used individual patient data from the randomized controlled trials of tirilazad mesylate following SAH who underwent aneurysm clipping. We examined data on 3567 patients enrolled in 4 studies of tirilazad mesylate in SAH. The studies were conducted from 1990–1997 across 162 neurosurgical institutions in 21 countries in North and Central America, Europe, Africa, and Australia [26–30]. The studies included data submitted by 156 neurosurgeons/investigators for patients 18-years and older, with ruptured saccular aneurysms confirmed on angiography and evidence of SAH seen on computed tomography (CT) or via lumbar puncture. Patients with non-saccular aneurysms, and/or significant cardiac disease were excluded. All patients were admitted to hospital within 48 hours of their SAH and were randomized to receive either placebo, or 2, 6 or 15 mg/kg/day of tirilazad mesylate. All patients received nimodipine. Most ruptured aneurysms were surgically clipped by the neurosurgeons/investigators (97.6%). The remainder were repaired with coiling by interventional neuroradiologists.
Patient-level covariates
Clinical variables recorded included age, sex, history of hypertension, initial neurological status based on the World Federation of Neurological Societies grading scale (WFNS),[31] dichotomized into good (WFNS I-III) and poor (WFNS IV-V) grades, as well as systolic blood pressure on admission. Radiographic variables on the admission cranial CT included Fisher grade dichotomized into I-II and III-IV, hydrocephalus, and/or intraventricular hemorrhage. Aneurysm location (anterior versus posterior circulation) and size (categorized as small, medium, and large with respective sizes of <12 mm, 12–24 mm, and ≥25 mm), as well as radiographic signs of cerebral infarction on admission were also included.
Surgeon-level covariates
Surgeon-related academic covariates, which were decided upon a priori and merged into the tirilazad database, were 1) total publications, 2) H-index, and 3) possession of a graduate degree (MSc and/or PhD). Data for the total number of publications and the H-index were accessed from the Scopus database on April 2016 and averaged from the surgeon's publications between 1990–1997, the same as the study enrollment dates. The last and first name of each surgeon were used to search the Scopus database. Scopus provided unique author identification numbers (Author ID) for each author that listed all their publications based on academic affiliations and country. The accuracy of the Scopus ID has been estimated to be very high [32]. To include the unlisted papers of specific surgeons, we viewed unmatched author names and determined whether additional papers found should be correctly categorized under specific authors and their unique author ID. The H-index of the author, with self-citations excluded, was then calculated. The H-index is an author metric that measures both an author’s number of publications as well as the number of times an author’s work has been cited in other papers. A higher H-index has been interpreted as a general indicator of higher academic impact. Lastly, each surgeon’s graduate degree was determined based on what was listed in their publications during the study period.
Outcomes
Our primary outcome was clinical and functional status based on the 5-point Glasgow outcome scale (GOS) assessed at 3-months following surgery for ruptured aneurysms, dichotomized into 'favorable' (GOS 4–5; moderate or low disability) and 'unfavorable' (GOS 1–3; severe disability, persistent vegetative state or death) groups. Secondary outcome was mortality, dichotomized as alive or deceased, and assessed also at 3-months follow-up.
Statistical analysis
We used a hierarchical or multi-level mixed-effects analysis, in which data were analyzed as a hierarchically structured set with first-level covariates (pertaining to patients) nested within second-level variables (pertaining to surgeons). The advantage of this hierarchical structure is that it allows variance across the higher level (surgeons) to explain heterogeneity in first-level response outcomes (clinical outcomes and mortality). Patients were excluded from analysis if they were treated by two neurosurgeons, unknown surgeons, or by surgeons who did not have a unique Scopus ID.
Means, standard deviations (SD), frequencies, or percentages were used for descriptive statistics. In order to evaluate heterogeneity in patient outcomes, both mortality and disability, an empty model was constructed and visualized as the intercept (and its SD) for patient outcomes for each individual surgeon. A univariate random coefficient mixed-effects model was utilized first to determine associations between our collected post-hoc variables and outcomes in the dataset. The surgeon-level variables were entered into the final multivariate models if they achieved a p<0.25 on univariate analysis. A multivariate mixed-effects logistic regression was employed to assess the association between the abovementioned variables and both primary and secondary outcomes 3-months post surgery. The multivariate analysis was adjusted for patient-related variables that were previously associated with patient outcomes in earlier studies [33–36]. We compared the associations and direction of the effects using parameter estimates (β), and standard error (SE). Negative β-values indicate an inverse relationship with outcome and a protective effect against unfavorable outcomes or mortality. P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using R, an open-source statistical computing and graphics platform developed by the R Foundation for Statistical Computing (www.r-project.org).
Additional analysis
We also performed additional analysis to confirm the effects of H-index on patient outcomes by adjusting for the number of years a neurosurgeon has been in academic practice (Adjusted H-index = H-index / number of years after neurosurgery residency graduation). Time of graduation for the neurosurgeons was collected from a number of different sources that included: the official website of their affiliated departments, and biographies listed on the websites or newsletters of official neurosurgical organizations (e.g. American Association of Neurological Surgeons, Society of Neurological Surgeons, and Congress of Neurological Surgeons, and European Association of Neurosurgical Societies). We excluded surgeons without listed residency graduation dates from this analysis.
Ethics statement
All procedures in the randomized controlled trials of tirilazad mesylate were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Our post-hoc analysis meets the exclusion criteria of the Canadian Tri-Council Policy Statement for research that necessitates a review by an institutional research ethics board, since our study relies exclusively on secondary use of anonymous patient information.
Results
Academic profiles were available for 147 surgeons, who treated 3307 patients. Data associated with 9 surgeons were omitted from the analysis due to unavailability of their academic metrics or because data of individual patients were submitted by two surgeons.
Patient level covariates
Patient demographics, clinical and radiologic characteristics are presented in Table 1. Most patients had good neurological status with poor radiological grade of SAH upon presentation (WFNS I-III, Fisher III-IV, respectively). Acute complications of SAH, such as cerebral infarction and hydrocephalus, were noted in nearly half of all patients. Almost all patients were surgically clipped within 48 hours of admission. Most aneurysms were in the anterior circulation (85%), and were less than 12 mm in maximum diameter (73%).
Table 1. Patients' clinical, radiographic, and outcome characteristics.
| Variable | Number | Percentage | |
|---|---|---|---|
| Clinical Characteristics | Age (years) | 51.7 (mean; SD 13.2) | |
| Sex | |||
| Female | 2720 | 82.2% | |
| History of hypertension | 1058 | 32.0% | |
| History of diabetes | 126 | 3.8% | |
| Initial neurologic grade | |||
| Good (WFNS I-III) | 2556 | 77.3% | |
| Poor (WFNS IV-V) | 751 | 22.7% | |
| Study treatment: | |||
| Tirilazad | 2034 | 61.5% | |
| Placebo | 1273 | 38.5% | |
| Systolic Blood Pressure | 141 (mean; SD 24.5) | ||
| Time from admission to clipping (hours) | 32.7 (mean; SD 11.1) | ||
| Use of rescue therapy | 776 | 23.4% | |
| Radiographic Characteristics | Fisher Grade | ||
| I-II | 1075 | 32.5% | |
| III- IV | 2232 | 67.5% | |
| Intraventricular hemorrhage | 1445 | 43.7% | |
| Hydrocephalus | 1361 | 41.2% | |
| Cerebral infarction | 909 | 27.5% | |
| Anterior circulation aneurysms | 2799 | 84.6% | |
| Aneurysm Size | |||
| < 12 mm | 2418 | 73.1% | |
| 12–24 mm | 749 | 22.6% | |
| ≥ 25 mm | 140 | 4.2% | |
| Outcomes | Unfavorable (GOS 1–3) | 978 | 29.6% |
| Mortality | 524 | 15.8% |
Abbreviations used: WFNS, World Federation of Neurological Societies; SD, standard deviation; GOS, Glasgow Outcome Score
Surgeon-level covariates
Most surgeons were from USA (62, 42%), Canada (18, 12%), Germany (15, 10%), Italy (14, 10%), and Australia (8, 5%). This group between 1990 and 1997 produced a total of 6770 publications. The average number of publications per surgeon was 46 (SD: 40, range 0–217) and the average H-index was 14 (SD: 40, range 0–47). Only 37 surgeons (25%) had a graduate degree (Table 2).
Table 2. Surgeons' academic indicators and univariate analysis of their association with outcomes.
| Variable | Mean (SD) or n. (%) | Estimate, β (SE) for dichotomized GOS | Estimate, β (SE) for mortality |
|---|---|---|---|
| Average total publications (1990–1997) | 46.0 (39.9) | 0.018 (0.046) | -0.001 (0.002) |
| Average H- index (1990–1997) | 14.4 (9.7) | -0.007 (0.006)* | -0.010 (0.007)* |
| Presence of graduate degree (MSc or PhD) | 37 (25.1%) | -0.003 (0.099) | -0.057 (0.123) |
Negative values indicate an inverse relationship with outcomes.
* denotes a p value equal or less than 0.25
Abbreviations used: SD, standard deviation; SE, standard error; GOS, Glasgow Outcome Score
Heterogeneity in outcomes
Fig 1 shows a substantial heterogeneity among surgeons using an empty model intercept for primary and secondary outcomes, some of which is explained by first-level (patient level) covariates, as previously studied in the tirilazad trials and subsequent post hoc analyses [22, 37, 38].
Fig 1. Heterogeneity in empty model intercept.
Heterogeneity by surgeons in Glasgow Outcome Scale (GOS) (top plot), and mortality (bottom plot).
Univariate analysis
Univariate analysis was performed to assess the contribution of the three a priori surgeon academic indicators (total publications, H-index, graduate degree) to our primary and secondary outcomes. On univariate mixed-effects analysis, the H-index and the possession of a graduate degree were related to better functional outcomes and lower mortality. The H-index was the only academic indicator that met threshold for inclusion in the multivariate model, as shown in Table 2.
Multivariate analysis
Ten patient-level covariates and 1 calculated academic indicator with p<0.25 on univariate analysis were included as fixed effects in separate multilevel logistic regression models with clinical and functional status (primary outcome) and mortality (secondary outcome) as the dependent variables. For primary outcome, 9 clinical and radiological factors were significantly associated with clinical and functional status measured by the GOS (Table 3). The H-index had a positive and statistically significant association with improved functional outcomes. With respect to secondary outcome (Table 4), similar results were seen in patient-level covariates in their association with mortality. The H-index also had a protective effect on mortality, however, this was not statistically significant.
Table 3. Fixed-effects analysis of the association of patient- and surgeons' H-index on dichotomized Glasgow outcome score.
| Variable | Estimate, β (SE) | p-value |
|---|---|---|
| Age | 0.039 (0.004) | <0.001* |
| History of hypertension | 0.266 (0.108) | 0.013* |
| Poor WFNS grade (vs. good WFNS) | 1.797 (0.115) | <0.001* |
| Systolic blood pressure | 0.006 (0.002) | <0.001* |
| Hydrocephalus | 0.066 (0.110) | 0.548 |
| Fisher grade (I-II vs. III- IV) | 0.496 (0.127) | <0.001* |
| Intraventricular hemorrhage | 0.542 (0.110) | <0.001* |
| Cerebral infarction | 2.080 (0.112) | <0.001* |
| Aneurysm location (Ant. vs. post): | 0.479 (0.143) | <0.001* |
| Aneurysm size (vs. < 12 mm): | ||
| ≥25 mm | 0.834 (0.255) | 0.001* |
| 13–24 mm | 0.335 (0.117) | 0.004* |
| Surgeon's H- index | - 0.015 (0.005) | 0.010* |
Negative values indicate an inverse relationship with outcomes.
* denotes significance at p<0.05. Dichotomized variables are compared with ‘no’ unless otherwise specified.
Abbreviations used: WFNS, World Federation of Neurological Societies; Ant, anterior circulation; Post, posterior circulation.
Table 4. Fixed-effects analysis of the association of patient- and surgeons' H-index on mortality.
| Variable | Estimate, β (SE) | p-value |
|---|---|---|
| Age | 0.025 (0.004) | <0.001* |
| History of hypertension | 0.216 (0.123) | 0.078* |
| Poor WFNS grade (vs. good WFNS) | 1.409 (0.120) | <0.001* |
| Systolic blood pressure | 0.004 (0.002) | 0.041* |
| Hydrocephalus | 0.019 (0.126) | 0.877 |
| Fisher grade (I-II vs. III- IV) | 0.594 (0.156) | <0.001* |
| Intraventricular hemorrhage | 0.481 (0.127) | 0.001* |
| Cerebral infarction | 1.457 (0.120) | <0.001* |
| Aneurysm location (Ant. vs. post): | 0.393 (0.125) | 0.013* |
| Aneurysm size (vs. < 12 mm): | ||
| ≥25 mm | 0.952 (0.250) | <0.001* |
| 13–24 mm | 0.374 (0.128) | 0.003* |
| Surgeon's H- index | - 0.015 (0.005) | 0.078 |
Negative values indicate an inverse relationship with outcomes.
* denotes significance at p<0.05. Dichotomized variables are contrasted with ‘no’ unless otherwise specified.
Abbreviations used: WFNS, World Federation of Neurological Societies; Ant, anterior circulation; Post, posterior circulation.
Additional analysis
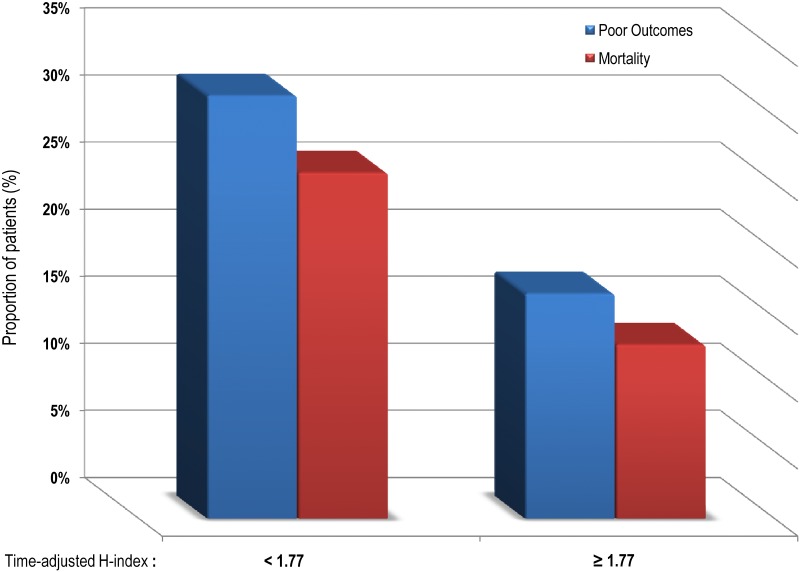
The time since graduation from neurosurgical residency was available for 121 neurosurgeons (2704 patients). Most surgeons graduated before 1985 (75%) and their mean graduating year was 1978 (SD: 7 years). In the additional multilevel logistic regression model, the time-adjusted H-index for years after neurosurgical residency had a positive, statistically significant association with both improved functional outcomes (β -0.058, SE 0.21, P = 0.007) and survival (β -0.125, SE 0.036, P = 0.001). The effect of time-adjusted H-index on outcomes can be visualized in Fig 2.
Fig 2. Bar chart of patient outcomes in percentages and divided into two groups as per the mean of time-adjusted H-index = 1.77.
Higher rates of poor outcomes and mortality by surgeons with a time-adjusted H-index below the mean.
Discussion
Clinically significant differences in surgical outcomes across a variety of surgical specialties have been demonstrated between different centers and even between individual surgeons [1, 4, 5, 7, 39]. These variations were primarily attributed to differences in case volume as well as the duration and specialization of training, including the completion of fellowships [1]. However, despite the growing role of academia in many surgical specialties, the importance of a surgeon's academic impact on their clinical outcomes remains largely unexplored. In the current analysis, we studied a cohort of surgeons from 1990 to 1997, an era strongly favoring open surgical clipping of ruptured intracranial aneurysms as opposed to current endovascular interventions. This allowed us to investigate the role of academic productivity and impact exclusively on surgical outcomes over a substantial time and for a large number of practicing surgeons. Importantly, we identified heterogeneity in patient outcomes following surgery that were associated with a surgeon’s academic impact. These findings, which suggest an association between academia and improved patient care, may provide a rationale for encouraging the optimization of funding allocation and research opportunities in surgery moving forwards.
The H-index as a tool to measure academic impact
The H-index is currently one of the most well-known and accepted metrics for evaluating the scientific impact of individuals, centers, and journals. As aforementioned, the H-index accounts for both the number of publications produced and the number of citations per publication in different papers [40]. The H-index is currently still being used as a tool to rank universities based on their total academic productivity and to compare individual researchers in and amongst various departments and journals for the purposes of promotion [41]. Like all other metrics of scientific impact, the H-index has its shortcomings. For example, the H-index does not take into consideration the author's rank on the publication nor any self-citations. But in spite of these limitations, the H-index has been accepted as a highly effective metric of academic impact that overcomes these flaws, particularly within the research-heavy field of neurosurgery. In a study by Lee et al [40], the H-index of particular academic neurosurgeons were randomly selected, from a large sample of academic programs in the US, to examine its robustness in assessing academic impact. The position of authorship and self-citations did not significantly affect the H-index of the neurosurgeons included in the study. However, the academic rank of the neurosurgeon, a major confounder missing in our study, correlated with higher H-indices.
Academic impact and hospitals quality
The true role of high academic productivity and impact on clinical outcomes is controversial [42]. Two studies recently examined the association between the quality of a hospital’s research and their quality of patient care. Pons et al. found a statistically significant, negative correlation between mortality rates and academic impact for congestive heart failure and acute myocardial infarction among Spanish public hospitals [43]. Similarly, Tchetchik et al. collected the number of total publications and H-index amongst three different clinical specializations (Cardiology, Oncology, and Orthopedics) in 50 US-based university hospitals and found that the quality of research produced had a positive correlation with the perceived quality of care provided by various hospitals as per the U.S. News &World Report’s ranking for the top hospitals [42]. However, these studies did not focus on other surgical specialties or examine each physician's individual academic profile and its impact on their own patient outcomes, as our study aimed to do.
Role of academia in SAH
With respect to intracranial aneurysms and SAH, there are numerous studies investigating the contributions of hospital- and surgeon-related factors to patient outcomes. Overall, these studies have suggested that teaching hospitals are associated with improved outcomes following SAH compared to their non-teaching counterparts [16, 18, 44–46]. One explanation was that patients may have better results because academic staff have more subspecialized knowledge and may be more up to date on the most recent practice and research updates. However, it should be noted that these studies were conducted using retrospective data and that the analysis was not adjusted for the patient’s presenting clinical and radiographic phenotype (i.e. the WFNS, Hunt and Hess or Fisher scales). Furthermore, bias in patient prognostic factors may have been present since obviously the patients are not randomized to type of hospital (teaching vs non-teaching), and propensity score adjusted studies could not be done since the data mostly do not include prognostic factors for outcome after SAH. Our results provide important, novel evidence that factors favoring the academic setting may be associated with improved clinical outcomes.
Strengths and limitations
The current analysis used a mixed-effects model, which overcomes the limitation of conventional regressions that assume that all patients are independent observations. A hierarchical mixed-effect model allows for hypothetical modeling of variables that might explain the variance between observations [47]. Moreover, a mixed-effects method does not presume a normal distribution for the outcome variables or their absolute independence. Using a hierarchical model, we are able to test our a priori hypothesis that surgeon-specific features, namely the academic impact are associated with first-level response variables, such as outcome. It cannot, however, be concluded that surgeons with a higher H-index provide better care. These findings do merit further investigation both by the neurosurgical community and other specialties for other disease processes, and potentially also include academic parameters in combination with health-quality rankings to see whether this association truly exists and importantly, in which direction. These findings may have important implications for research funding and surgical residents’ enrollment in research fellowships and post-graduate programs (MSc, PhD) during residency. Moreover, finding a positive relationship between academic impact and clinical outcomes will overcome the common myth that significant time investment in successful research projects may take time away from clinical practice and affect the care provided by surgeons for their patients [48].
Our study has several limitations: The annual case-volume of both the surgeons and centers involved were not available and consequently were not taken into consideration for analysis. Therefore, case volume is a significant confounder in that it remains uncertain for this cohort of surgeons whether case-volume would have a similar or greater effect on outcome compared to academic productivity independently. Moreover, the data we obtained were insufficient to effectively scrutinize a surgeon’s complication rates (e.g. rates of intraoperative aneurysm rupture, post-operative wound infection, etc.) and this hinders our ability to make any conclusive statements about surgical performance as another confounder leading to differences in outcome. Finally, we utilized only one citation database, which may fail to account for the comprehensive research records of all the surgeons analyzed.
Implications for future research
Our study further highlights the importance of surgeon-related factors in the study of surgical outcomes. A number of prior studies have previously established the role of surgeon-dependent factors [1, 4, 6, 9–11, 49–51]. A recent systematic review of the general surgical oncology literature found 29 published studies from several specialties (e.g. gynecology, urology, general surgery, thoracic surgery, and dermatology), the majority of which showed that a surgeon’s training, certification, and experience were all associated with patient outcomes [39]. The main limitation of these studies was inconsistencies in defining sub-specialization and experience, and not adjusting for confounders such as surgeon age, experience, and case-volume, thereby limiting our ability to draw any causal conclusions. Future studies investigating surgeon—dependent factors should standardize these definitions, and most importantly, investigate their causal effect on patient’s outcomes to fully separate surgeon-related factors from hospital- and center-related factors. This requires a multi-collaborative effort to design comprehensive and well-defined protocols that take these factors into consideration.
Conclusion
The current study suggests that differences in patient outcomes following surgical clipping of ruptured intracranial aneurysms could be in part related to the academic impact of surgeons. The associations described in this analysis reflect the large-scale trends obtained from a large cross-international database. Additional studies with better-defined outcomes and health quality measures, spanning different specialties and disease processes will likely further expand our understanding of how a surgeon’s academic role could be related to their clinical outcomes and what factors mediate this association. The results of these studies may provide rationale for the optimization of funding allocation and research opportunities within surgery.
Abbreviations
- β
Parameter Estimates
- CT
Computed Tomography
- GOS
Glasgow Outcome Scale
- SAH
Subarachnoid Hemorrhage
- SD
Standard Deviation
- SE
Standard Error
- WFNS
World Federation of Neurological Societies
Data Availability
Data are available from the SAHIT (Subarachnoid Hemorrhage International Trialists) Repository for researchers who meet the criteria for access to confidential data. Contact link: http://www.stmichaelshospital.com/neuroscience/SAHIT.php.
Funding Statement
The authors received no specific funding for this work.
References
- 1.McAteer JP, LaRiviere CA, Drugas GT, Abdullah F, Oldham KT, Goldin AB. Influence of surgeon experience, hospital volume, and specialty designation on outcomes in pediatric surgery: a systematic review. JAMA Pediatr. 2013;167(5):468–75. Epub 2013/03/27. doi: 10.1001/jamapediatrics.2013.25 . [DOI] [PubMed] [Google Scholar]
- 2.Duclos A, Peix JL, Colin C, Kraimps JL, Menegaux F, Pattou F, et al. Influence of experience on performance of individual surgeons in thyroid surgery: prospective cross sectional multicentre study. BMJ. 2012;344:d8041 Epub 2012/01/13. doi: 10.1136/bmj.d8041 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137(6):511–20. Epub 2002/09/17. . [DOI] [PubMed] [Google Scholar]
- 4.Boudourakis LD, Wang TS, Roman SA, Desai R, Sosa JA. Evolution of the surgeon-volume, patient-outcome relationship. Ann Surg. 2009;250(1):159–65. Epub 2009/06/30. doi: 10.1097/SLA.0b013e3181a77cb3 . [DOI] [PubMed] [Google Scholar]
- 5.Peltoniemi P, Peltola M, Hakulinen T, Hakkinen U, Pylkkanen L, Holli K. The effect of hospital volume on the outcome of breast cancer surgery. Ann Surg Oncol. 2011;18(6):1684–90. Epub 2011/01/06. doi: 10.1245/s10434-010-1514-1 . [DOI] [PubMed] [Google Scholar]
- 6.Ch'ng SL, Cochrane AD, Wolfe R, Reid C, Smith CI, Smith JA. Procedure-specific Cardiac Surgeon Volume associated with Patient outcome following Valve Surgery, but not Isolated CABG Surgery. Heart Lung Circ. 2015;24(6):583–9. Epub 2015/01/27. doi: 10.1016/j.hlc.2014.11.014 . [DOI] [PubMed] [Google Scholar]
- 7.Cowan JA Jr., Dimick JB, Thompson BG, Stanley JC, Upchurch GR Jr. Surgeon volume as an indicator of outcomes after carotid endarterectomy: an effect independent of specialty practice and hospital volume. J Am Coll Surg. 2002;195(6):814–21. Epub 2002/12/24. . [DOI] [PubMed] [Google Scholar]
- 8.Pacella SJ, Butz DA, Comstock MC, Harkins DR, Kuzon WM Jr., Taheri PA. Hospital volume outcome and discharge disposition of burn patients. Plast Reconstr Surg. 2006;117(4):1296–305; discussion 306–7. Epub 2006/04/04. doi: 10.1097/01.prs.0000204962.85336.51 . [DOI] [PubMed] [Google Scholar]
- 9.Basques BA, Louie PK, Shifflett GD, Fice MP, Mayo BC, Massel DH, et al. The Effect of Surgeon Volume on Complications, Length of Stay, and Costs Following Anterior Cervical Fusion. Spine (Phila Pa 1976). 2016. Epub 2016/07/01. doi: 10.1097/BRS.0000000000001756 . [DOI] [PubMed] [Google Scholar]
- 10.Lau RL, Perruccio AV, Gandhi R, Mahomed NN. The role of surgeon volume on patient outcome in total knee arthroplasty: a systematic review of the literature. BMC Musculoskelet Disord. 2012;13:250 Epub 2012/12/18. doi: 10.1186/1471-2474-13-250 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilt TJ, Shamliyan TA, Taylor BC, MacDonald R, Kane RL. Association between hospital and surgeon radical prostatectomy volume and patient outcomes: a systematic review. J Urol. 2008;180(3):820–8; discussion 8–9. Epub 2008/07/19. doi: 10.1016/j.juro.2008.05.010 . [DOI] [PubMed] [Google Scholar]
- 12.Bennett WO, Bird JH, Burrows SA, Counter PR, Reddy VM. Does academic output correlate with better mortality rates in NHS trusts in England? Public Health. 2012;126 Suppl 1:S40–3. Epub 2012/07/17. doi: 10.1016/j.puhe.2012.05.021 . [DOI] [PubMed] [Google Scholar]
- 13.Paulsen T, Kjaerheim K, Kaern J, Tretli S, Trope C. Improved short-term survival for advanced ovarian, tubal, and peritoneal cancer patients operated at teaching hospitals. Int J Gynecol Cancer. 2006;16 Suppl 1:11–7. Epub 2006/03/07. doi: 10.1111/j.1525-1438.2006.00319.x . [DOI] [PubMed] [Google Scholar]
- 14.Meguid RA, Brooke BS, Chang DC, Sherwood JT, Brock MV, Yang SC. Are Surgical Outcomes for Lung Cancer Resections Improved at Teaching Hospitals? The Annals of Thoracic Surgery. 2008;85(3):1015–25. http://dx.doi.org/10.1016/j.athoracsur.2007.09.046. [DOI] [PubMed] [Google Scholar]
- 15.Hyder O, Sachs T, Ejaz A, Spolverato G, Pawlik TM. Impact of hospital teaching status on length of stay and mortality among patients undergoing complex hepatopancreaticobiliary surgery in the USA. J Gastrointest Surg. 2013;17(12):2114–22. Epub 2013/09/28. doi: 10.1007/s11605-013-2349-4 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald RJ, Cloft HJ, Kallmes DF. Impact of admission month and hospital teaching status on outcomes in subarachnoid hemorrhage: evidence against the July effect. J Neurosurg. 2012;116(1):157–63. Epub 2011/09/29. doi: 10.3171/2011.8.JNS11324 . [DOI] [PubMed] [Google Scholar]
- 17.Meguid RA, Brooke BS, Perler BA, Freischlag JA. Impact of hospital teaching status on survival from ruptured abdominal aortic aneurysm repair. J Vasc Surg. 2009;50(2):243–50. Epub 2009/05/19. doi: 10.1016/j.jvs.2009.01.046 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai PM, Lin N, Du R. Effect of teaching hospital status on outcome of aneurysm treatment. World Neurosurg. 2014;82(3–4):380–5.e6. Epub 2013/07/31. doi: 10.1016/j.wneu.2013.07.015 . [DOI] [PubMed] [Google Scholar]
- 19.Yaghoubian A, de Virgilio C, Lee SL. Appendicitis outcomes are better at resident teaching institutions: a multi-institutional analysis. Am J Surg. 2010;200(6):810–3; discussion 3. Epub 2010/12/15. doi: 10.1016/j.amjsurg.2010.07.028 . [DOI] [PubMed] [Google Scholar]
- 20.Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10(1):44–58. Epub 2013/12/11. doi: 10.1038/nrneurol.2013.246 . [DOI] [PubMed] [Google Scholar]
- 21.Connolly ES Jr., Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43(6):1711–37. doi: 10.1161/STR.0b013e3182587839 . [DOI] [PubMed] [Google Scholar]
- 22.Guha D, Ibrahim GM, Kertzer JD, Macdonald RL. National socioeconomic indicators are associated with outcomes after aneurysmal subarachnoid hemorrhage: a hierarchical mixed-effects analysis. J Neurosurg. 2014;121(5):1039–47. Epub 2014/08/16. doi: 10.3171/2014.7.JNS132141 . [DOI] [PubMed] [Google Scholar]
- 23.Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8(7):635–42. Epub 2009/06/09. doi: 10.1016/S1474-4422(09)70126-7 . [DOI] [PubMed] [Google Scholar]
- 24.Bardach NS, Zhao S, Gress DR, Lawton MT, Johnston SC. Association between subarachnoid hemorrhage outcomes and number of cases treated at California hospitals. Stroke. 2002;33(7):1851–6. Epub 2002/07/10. . [DOI] [PubMed] [Google Scholar]
- 25.Jaja BN, Saposnik G, Nisenbaum R, Schweizer TA, Reddy D, Thorpe KE, et al. Effect of Socioeconomic Status on Inpatient Mortality and Use of Postacute Care After Subarachnoid Hemorrhage. Stroke. 2013. Epub 2013/08/01. doi: 10.1161/STROKEAHA.113.001368 . [DOI] [PubMed] [Google Scholar]
- 26.Haley EC Jr., Kassell NF, Alves WM, Weir BK, Hansen CA. Phase II trial of tirilazad in aneurysmal subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. J Neurosurg. 1995;82(5):786–90. Epub 1995/05/01. doi: 10.3171/jns.1995.82.5.0786 . [DOI] [PubMed] [Google Scholar]
- 27.Kassell NF, Haley EC Jr., Apperson-Hansen C, Alves WM. Randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in Europe, Australia, and New Zealand. J Neurosurg. 1996;84(2):221–8. Epub 1996/02/01. doi: 10.3171/jns.1996.84.2.0221 . [DOI] [PubMed] [Google Scholar]
- 28.Haley EC Jr., Kassell NF, Apperson-Hansen C, Maile MH, Alves WM. A randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in North America. J Neurosurg. 1997;86(3):467–74. Epub 1997/03/01. doi: 10.3171/jns.1997.86.3.0467 . [DOI] [PubMed] [Google Scholar]
- 29.Lanzino G, Kassell NF, Dorsch NW, Pasqualin A, Brandt L, Schmiedek P, et al. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part I. A cooperative study in Europe, Australia, New Zealand, and South Africa. J Neurosurg. 1999;90(6):1011–7. Epub 1999/06/01. doi: 10.3171/jns.1999.90.6.1011 . [DOI] [PubMed] [Google Scholar]
- 30.Lanzino G, Kassell NF. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part II. A cooperative study in North America. J Neurosurg. 1999;90(6):1018–24. Epub 1999/06/01. doi: 10.3171/jns.1999.90.6.1018 . [DOI] [PubMed] [Google Scholar]
- 31.Drake CG, Hunt WE, Sano K, Kassell N, Teasdale G, Pertuiset B, et al. Report of World Federation of Neurological Surgeons committee on a universal subarachnoid hemorrhage grading scale. Journal of Neurosurgery. 1988;68(6):985–6. [DOI] [PubMed] [Google Scholar]
- 32.Kawashima H, Tomizawa H. Accuracy evaluation of Scopus Author ID based on the largest funding database in Japan. Scientometrics. 2015;103(3):1061–71. doi: 10.1007/s11192-015-1580-z [Google Scholar]
- 33.Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38(8):2315–21. Epub 2007/06/16. doi: 10.1161/STROKEAHA.107.484360 . [DOI] [PubMed] [Google Scholar]
- 34.Helbok R, Kurtz P, Vibbert M, Schmidt MJ, Fernandez L, Lantigua H, et al. Early neurological deterioration after subarachnoid haemorrhage: risk factors and impact on outcome. J Neurol Neurosurg Psychiatry. 2013;84(3):266–70. Epub 2012/09/27. doi: 10.1136/jnnp-2012-302804 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berman MF, Solomon RA, Mayer SA, Johnston SC, Yung PP. Impact of hospital-related factors on outcome after treatment of cerebral aneurysms. Stroke. 2003;34(9):2200–7. Epub 2003/08/09. doi: 10.1161/01.STR.0000086528.32334.06 . [DOI] [PubMed] [Google Scholar]
- 36.Ibrahim GM, Fallah A, Macdonald RL. Clinical, laboratory, and radiographic predictors of the occurrence of seizures following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2013;119(2):347–52. Epub 2013/04/16. doi: 10.3171/2013.3.JNS122097 . [DOI] [PubMed] [Google Scholar]
- 37.Lipsman N, Tolentino J, Macdonald RL. Effect of country or continent of treatment on outcome after aneurysmal subarachnoid hemorrhage. Clinical article. J Neurosurg. 2009;111(1):67–74. Epub 2009/03/31. doi: 10.3171/2008.10.JNS08184 . [DOI] [PubMed] [Google Scholar]
- 38.Fergusen S, Macdonald RL. Predictors of cerebral infarction in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2007;60(4):658–67; discussion 67. Epub 2007/04/07. doi: 10.1227/01.NEU.0000255396.23280.31 . [DOI] [PubMed] [Google Scholar]
- 39.Bilimoria KY, Phillips JD, Rock CE, Hayman A, Prystowsky JB, Bentrem DJ. Effect of surgeon training, specialization, and experience on outcomes for cancer surgery: a systematic review of the literature. Ann Surg Oncol. 2009;16(7):1799–808. Epub 2009/05/16. doi: 10.1245/s10434-009-0467-8 . [DOI] [PubMed] [Google Scholar]
- 40.Lee J, Kraus KL, Couldwell WT. Use of the h index in neurosurgery. Clinical article. J Neurosurg. 2009;111(2):387–92. Epub 2009/04/28. doi: 10.3171/2008.10.JNS08978 . [DOI] [PubMed] [Google Scholar]
- 41.Patel VM, Ashrafian H, Ahmed K, Arora S, Jiwan S, Nicholson JK, et al. How has healthcare research performance been assessed?: a systematic review. J R Soc Med. 2011;104(6):251–61. Epub 2011/06/11. doi: 10.1258/jrsm.2011.110005 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tchetchik A, Grinstein A, Manes E, Shapira D, Durst R. From Research to Practice: Which Research Strategy Contributes More to Clinical Excellence? Comparing High-Volume versus High-Quality Biomedical Research. PLoS One. 2015;10(6):e0129259 Epub 2015/06/25. doi: 10.1371/journal.pone.0129259 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pons J, Sais C, Illa C, Mendez R, Sunen E, Casas M, et al. Is there an association between the quality of hospitals' research and their quality of care? J Health Serv Res Policy. 2010;15(4):204–9. Epub 2010/05/15. doi: 10.1258/jhsrp.2010.009125 . [DOI] [PubMed] [Google Scholar]
- 44.Vermeulen MJ, Schull MJ. Missed diagnosis of subarachnoid hemorrhage in the emergency department. Stroke. 2007;38(4):1216–21. Epub 2007/02/27. doi: 10.1161/01.STR.0000259661.05525.9a . [DOI] [PubMed] [Google Scholar]
- 45.Attenello FJ, Reid P, Wen T, Cen S, Kim-Tenser M, Sanossian N, et al. Evaluation of time to aneurysm treatment following subarachnoid hemorrhage: comparison of patients treated with clipping versus coiling. J Neurointerv Surg. 2016;8(4):373–7. Epub 2015/03/15. doi: 10.1136/neurintsurg-2014-011642 . [DOI] [PubMed] [Google Scholar]
- 46.Kim HS, Park CW, Yoo CJ, Kim EY, Kim YB, Kim WK. Impact of admission month on outcomes in spontaneous subarachnoid hemorrhage: evidence against the march effect. J Cerebrovasc Endovasc Neurosurg. 2013;15(2):67–75. Epub 2013/07/12. doi: 10.7461/jcen.2013.15.2.67 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabe-Hesketh S, S A, Pickles A. Estimation of generalized linear mixed models. Stata J. 2002;2:1–21. [Google Scholar]
- 48.Seaburg LA, Wang AT, West CP, Reed DA, Halvorsen AJ, Engstler G, et al. Associations between resident physicians' publications and clinical performance during residency training. BMC Med Educ. 2016;16:22 doi: 10.1186/s12909-016-0543-2 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gooden KM, Howard DL, Carpenter WR, Carson AP, Taylor YJ, Peacock S, et al. The effect of hospital and surgeon volume on racial differences in recurrence-free survival after radical prostatectomy. Med Care. 2008;46(11):1170–6. Epub 2008/10/28. doi: 10.1097/MLR.0b013e31817d696d ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Almatar A, Wallis CJ, Herschorn S, Saskin R, Kulkarni GS, Kodama RT, et al. Effect of radical prostatectomy surgeon volume on complication rates from a large population-based cohort. Can Urol Assoc J. 2016;10(1–2):45–9. Epub 2016/03/16. ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barocas DA, Mitchell R, Chang SS, Cookson MS. Impact of surgeon and hospital volume on outcomes of radical prostatectomy. Urol Oncol. 2010;28(3):243–50. Epub 2009/04/28. doi: 10.1016/j.urolonc.2009.03.001 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the SAHIT (Subarachnoid Hemorrhage International Trialists) Repository for researchers who meet the criteria for access to confidential data. Contact link: http://www.stmichaelshospital.com/neuroscience/SAHIT.php.