Abstract
Resveratrol (RSV), a polyphenolic compound and naturally occurring phytoalexin, has been reported to exert cardio-protective effects in several animal studies. However, the outcome of initial clinical trials with RSV was less effective compared to pre-clinical studies. Therefore, RSV treatment protocols need to be optimized. In this study we evaluated prophylactic versus therapeutic effect of resveratrol (RSV) in mitigating doxorubicin (Dox)-induced cardiac toxicity in rats. To investigate prophylactic effects, RSV was supplemented for 2 weeks along with Dox administration. After 2 weeks, Dox treatment was stopped and RSV was continued for another 4 weeks. To study therapeutic effects, RSV treatment was initiated after 2 weeks of Dox administration and continued for 4 weeks. Both prophylactic and therapeutic use of RSV mitigated Dox induced deterioration of cardiac function as assessed by echocardiography. Also RSV treatment (prophylactic and therapeutic) prevented Dox induced myocardial damage as measured by cardiac enzymes (LDH and CK-MB) in serum. Which was associated with decrease in Dox induced myocardial apoptosis and fibrosis. Interestingly our study also reveals that prophylactic use of RSV was more effective than its therapeutic use in mitigating Dox induced apoptosis and fibrosis in the myocardium. Therefore, prophylactic use of resveratrol may be projected as a possible future adjuvant therapy to minimize cardiotoxic side effects of doxorubicin in cancer patients.
Introduction
Doxorubicin (Dox) is an effective broad spectrum anti-neoplastic agent. The therapeutic index of this highly potent anti-cancer drug is reduced due to cardio-toxic side effects [1,2]. Dox induced deterioration of cardiac function mainly occurs due to myocardial apoptosis [3,4]. Doxorubicin induces cardiac cell apoptosis via intrinsic and extrinsic pathways [5,6]. Both these pathways are linked and that molecules in these two pathways influence each other. Caspase 3 is one such molecule which is involved in both intrinsic and extrinsic pathways [7,8]. Caspase 3 protein is a member of cysteine aspartic acid protease family. The nuclear factors of activated T-cells (NFATs) family members are transcriptional factors that play an important role in cardiac and skeletal functions [9]. The deletion of NFAT1 gene during embryogenesis causes lethal cardiac defects [10,11]. On the contrary, activation of NFAT 2,3 in a Ca/calcenurin-dependent manner mediate adverse remodeling and apoptosis [12–14].
Resveratrol (RSV: trans-3,5,4′-trihydroxystilbene), a polyphenolic compound and naturally occurring phytoalexin was shown to exert strong cardioprotective actions. RSV prevented H2O2 induced oxidative stress in adult cultured cardiomyocytes [15]. Also RSV administration exerted cardioprotective effects by reducing oxidative stress in a rat model of diabetic cardiomyopathy [16]. Similarly, RSV inhibited hypoxia-induced apoptosis in myocardial cells by preventing an increase in Bax and caspase 3 activation [17–20]. Resveratrol also prevents Dox-induced cardiotoxicity by mitigating cardiomyocyte apoptosis, oxidative stress and cardiac fibrosis [21–23]. The cardio protective effects of RSV are reported to be mediated by different cell signaling pathways, including AMP-activated Protein Kinase and Akt survival pathways [24,25]. The beneficial effects of RSV administration in experimental models of cardiac complications have been reported in several animal models [26–28]. However, the outcome of initial clinical trials in cardiac patients suggested marginal benefits after RSV supplementation [29,30]. Therefore, treatment protocols and timing of RSV supplementation during cardiac injury need to be optimized. In this study we aimed to compare potential preventive and therapeutic role of RSV in an experimental model of Dox induced cardiotoxicity. In the current study we also sought to investigate the mechanisms of RSV mediated improvement in cardiac function.
Materials and methods
Animals
Adult male Wister rats (140-150g), (n = 32) were used for the current study, all the animals were kept in the animal care facility of the Cairo University and were provided ordinary rat chow and water ad libitum with a 12 hrs light-dark cycle. The experimental protocol and procedures were approved by the Institutional Animal Care and Use Committee of the Cairo University. Animals were allowed to acclimatize for 10 days prior to the start of study.
Animal grouping and treatment protocol
Animals were randomly allocated into four groups; Control group (n = 8): received vehicle solution, saline (0.2 ml ip); Dox group (n = 8): injected with Dox (ADRICIN, EIMC United Pharmaceuticals, Cairo) in six equal doses (cumulative dose 2.5 mg/kg body wt, ip) over a period of 2 weeks; RSV-Dox group (n = 8): in order to determine the prophylactic effect of resveratrol in preventing the Dox induced cytotoxicity, this group received both Dox (2.5 mg/kg body wt, ip) and RSV (Sigma Aldrich, USA, at a dose of 20mg/kg body wt, orally dissolved in saline) for 2 weeks, after 2 weeks Dox was stopped, and RSV was continued for next 4 weeks; Dox–RSV group (n = 8), in order to investigate the therapeutic effect of RSV, this group received Dox (2.5 mg/kg body wt, ip) for 2 weeks, then Dox treatment was stopped and RSV treatment (20mg/kg body wt, orally dissolved in saline) was initiated and continued for 4 weeks.
Animals were monitored for body weight at the beginning, 2weeks and 6weeks from the start of experiments. At the end of the study, rats were given halothane inhalation anesthesia, after confirmation of deep anesthesia, hearts were removed surgically and divided for histopathological and biochemical analysis.
Heart function
Echocardiography was performed in different groups at the beginning, after 2 weeks of Dox administration and at the end of the experimental period (6 weeks). Two –dimensional and M-mode recording of short axis view was performed using a 8-10MHz liner transducer (maximum depth of 3 cm) attached to an ultra-sonographic machine (Samsung Madison, SONOACE-R3-Korea). The following measurements were recorded: left ventricular internal dimension at end-diastole (LVIDd), left ventricular internal dimension at end-systole (LVIDs), fractional shortening (FS%) and ejection fraction (%EF).
Serum cardiac enzymes
Blood samples were collected at the beginning and at the end of the study (6 weeks) to measure cardiac enzymes creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH) in serum. Briefly, blood samples were collected in dry test tubes and allowed to coagulate at room temperature for 30 min. Serum was separated by centrifugation at 3000 rpm for 10 min. The cardiac enzymes CK-MB and LDH were measured by commercial kits (Stanbio Laboratory, Boerne, TX, USA).
NFAT3 and NFAT5 levels
Total RNA was extracted from homogenized rat tissues using the RNAeasy mini kit (Qiagen, Germany). cDNA synthesis was performed using a superscript kit from Invitrogen and RT-PCR was performed using a Step One Plus™ sequence detection system from Applied Biosystems (Germany). The sequences of PCR primer pairs used for each gene are presented in Table 1. Relative expression of NFAT3 and NFAT5 were normalized to the housekeeping gene ß-actin.
Table 1. List of primers used for RT-PCR analysis.
| Gene | Primers sequence (5′-3′) | Gene bank accession number | Product Length | |
|---|---|---|---|---|
| NFAT 3 | Forward | CCACCAACTGCTCTGACTGC | NM_001108447.1 | 365 |
| Reverse | CCTAGCTATGCAACCAGGTCAC | |||
| NFAT5 | Forward | AGTGGATGCCAGAGTGTTGTC | NM_001107425.1 | 237 |
| Reverse | CGAACAGAAGCCACCACACA | |||
| ß-actin | Forward | TATCCTGGCCTCACTGTCCA | NM_031144.3 | 120 |
| Reverse | AACGCAGCTCAGTAACAGTC | |||
Histopathological analysis
Hematoxylin and eosin (H&E) staining was performed to assess myocardial damage (Kiernan 2001). Briefly cardiac tissue samples from all the groups were fixed in 10% formalin for 48 hours and paraffin blocks were prepared. Each sample was cut into 5μm thick sections and taken onto poly-lysine coated slides. Each slide was then deparaffinized in xylene, rehydrated in different descending concentrations of ethanol and stained in hematoxylin. Next, the slides were counter stained in eosin, dehydrated in increasing concentrations of ethanol as well as xylene and mounted. The images were captured under the microscope. Using Leica Qwin 500 LTD computer assisted image analysis software (Cambridge, UK) assessment of the area of degenerated (deeply acidophilic) cardiac myocytes was measured in H&E stained sections. The measurements were done in 10 high power fields (HPF).
To assess myocardial tissue fibrosis, heart sections were stained with Masson’s Trichrome (Bancroft and Gamble 2008) and % area of collagen fibres was calculated using Leica Qwin 500 LTD computer assisted image analysis software (Cambridge, UK).
Immunohistochemistry
Serial sections were cut and taken onto poly-lysine coated slides. Tissue sections were boiled in 10 mM citrate buffer pH 6.0 for 10–20 minutes and then left to cool at room temperature for 20 min. After washing twice in phosphate buffer saline (PBS), sections were incubated with 0.1 ml of rabbit anti-caspase 3 (3015–100, Biovision, USA) antibody. After 1hr of incubation with primary antibody slides were washed with PBS and biotin labelled secondary antibody was applied for 10 minutes at room temperature. After washing slides were incubated with DAB chromogen mixture for 10–15 minutes at room temperature. The slides were counterstained with Mayer-Haematoxylin for 1–3 minutes. Tonsils specimens were used as positive controls. Negative controls were myocardial sections without primary antibody. The images were captured under the microscope. Using Leica Qwin 500 LTD computer assisted image analysis software (Cambridge, UK) caspase 3 positive area (area%) was measured in the sections. The measurements were done in 10 high power fields (HPF).
Bax and Bcl-xl levels
Bax and Bcl-xl protein levels were measured by Western blotting. Briefly, myocardial tissue protein extracts were prepared from control and treated samples in different groups, and suspended in PBS containing protease inhibitor cocktail. 30μg of protein was loaded onto 10% FastCast Acrylamide gel (Bio-Rad Laboratories Ltd). SDS-PAGE electrophoresis, immunoblotting, and protein detection were done for Bax, Bcl-xl and ß-actin using anti Bax (sc-493, Santa Cruz), anti Bcl-xl (sc-8392, Santa Cruz) and anti ß-actin (sc-47778, Santa Cruz) antibodies. Band intensity was analyzed by ChemiDoc™ imaging system with Image Lab™ software version 5.1 (Bio-Rad Laboratories Inc., USA). The results were normalized with ß-actin.
Statistical analysis
Data is presented as mean±SD. One-way analysis-of-variance (ANOVA) followed by Bonferroni post-hoc test was performed using SPSS software. p values <0.05 were considered statistically significant.
Results
Body weight
A significant increase (P < 0.05) in the body weight in control group, RSV-Dox and Dox-RSV groups was observed after 2 and 6 weeks of treatment. However, we found a decrease in the body weight in Dox administered animals after 6 weeks of treatment (Table 2).
Table 2. Body weight (in grams) in different groups measured at baseline, after 2 and 6 weeks.
| Body weight (gms) | ||||
|---|---|---|---|---|
| Control | Dox | RSV-Dox | Dox-RSV | |
| Baseline | 144.28±4.94 | 146.66±4.71 | 144 ±4.89 | 144± 4.47 |
| 2 weeks | 166.6±4.67* | 153.33±7.90 | 166.6±4.71* | 167.6 ±4.71* |
| 6 weeks | 168.57±13.55* | 124.2±4.94# | 170±5.34* | 178.5± 6.38* |
Data are mean ± SD,
*P < 0.05, significantly different from respective baseline group,
#P <0.05, compared to baseline in Dox group.
Echocardiography
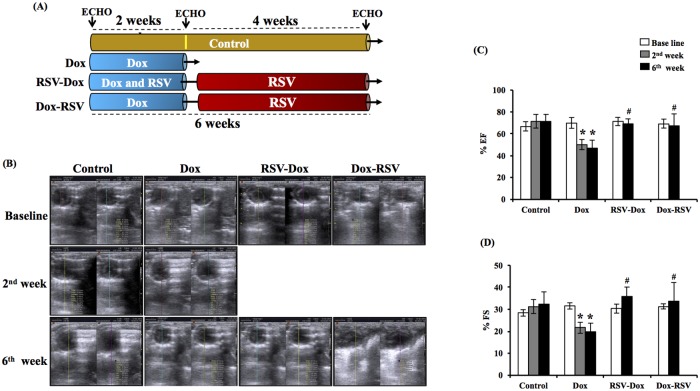
Dox induced deterioration of cardiac function was evident after 2 weeks of drug administration with a significant decrease in %EF and %FS (Fig 1). Cardiac function further deteriorated at 6 weeks of Dox administration. Supplementation of RSV along with Dox prevented Dox induced deterioration of heart function. Also RSV treatment for 4 weeks following Dox therapy prevented Dox induced deterioration of cardiac function (Fig 1A–1D).
Fig 1. Effect of Dox, RSV-Dox and Dox-RSV on %EF, %FS was measured by echocardiography. RSV treatment prevented Dox induced deterioration of cardiac function.
(A) Graphical representation of time points for treatment protocol. (B). Representative M-mode images from different groups. (C). % Ejection fraction (%EF). (D). % Fractional shortening (%FS). Dox treatment for 2 weeks significantly decreased both %EF and %FS, RSV treatment along with Dox or after 2 weeks of Dox supplementation mitigated Dox induced deterioration of cardiac function. Data are mean ± SD, *P < 0.05, significantly different from respective control group, #P <0.05, compared to respective Dox group.
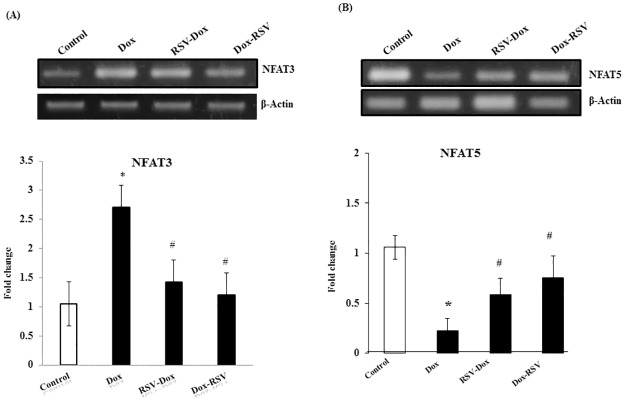
NFAT3 and NFAT5
NFAT3 and NFAT5 levels were measured by RT-PCR. We found a significant increase in NFAT3 in Dox group that was mitigated by co-treatment with RSV. Resveratrol treatment after 2 weeks of Dox administration also prevented NFAT3 increase. However, NFAT5 levels were significantly decreased in Dox group, co-treatment with RSV or RSV treatment after 2 weeks of Dox administration increased NFAT5 levels (Fig 2A and 2B).
Fig 2. Effect of Dox, RSV-Dox and Dox-RSV on NFAT 3 and NFAT5 expression.
(A&B) NFAT3 and NFAT5 expression was measured by RT-PCR. Dox treatment for 2 weeks significantly increased NFAT3 (A) and decreased NFAT5 (B), RSV supplementation along with Dox or after 2 weeks of Dox treatment prevented NFAT3 increase and NFAT5 decrease. ß-actin was used as internal control. Data are mean ± SD. *P < 0.05, significantly different from respective control group, #P < 0.05, significantly different from respective Dox group.
Histological examination
H&E staining
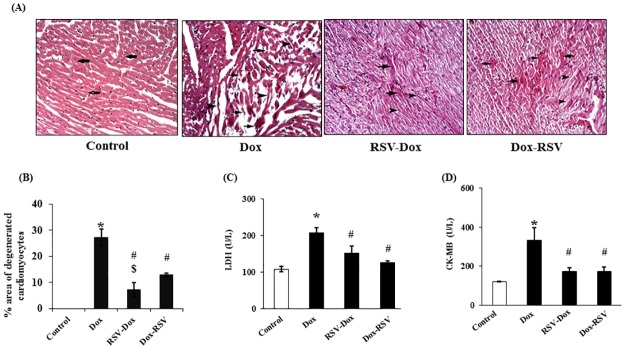
Control sections revealed normal architecture of cardiac muscle fibers arranged in different directions. Myocardial sections in Dox group showed wide areas of widely spaced deep acidophilic fibers including multiple disrupted and thin attenuated fibers exhibiting dark peripheral nuclei (Fig 3A and 3B). In RSV-Dox group few congested blood vessels were detected among muscle fibers, besides few deeply acidophilic and few thin attenuated fibers were detected. In Dox-RSV group also some congested blood vessels were evident among muscle fibers. In addition, some deeply acidophilic fibers, some thin attenuated and some fibers exhibiting dark peripheral nuclei were seen compared to Dox group (Fig 3A and 3B). Interestingly when we compared the % area of degenerated cardiomyocytes in both RSV-Dox and Dox-RSV groups, resveratrol performed better when it was supplemented prophylactically compared to its therapeutic use.
Fig 3. Resveratrol treatment rescued Dox induced myocardial damage.
(A). H & E staining: Photomicrographs of rat myocardial sections (x 200). Control group showed muscle fibers arranged in different directions (arrows), Dox group showing a wide area of widely spaced deeply acidophilic fibers including multiple disrupted (arrows), multiple thin attenuated (arrowheads) and multiple fibers exhibiting dark peripheral nuclei. RSV-Dox showed few congested blood vessels among muscle fibers, few deeply acidophilic (arrows) and few thin attenuated fibers (arrowheads) were present. In Dox-RSV group also sections showed some congested blood vessels among apparently normal muscle fibers, in addition to some deeply acidophilic fibers (arrows), some thin attenuated (arrowheads) and some fibers exhibiting dark peripheral nuclei were detected. (B). Quantitative analysis of (percent area) degenerated myocytes. (C&D). LDH and CK-MB levels in serum measured by commercial kits purchased from Stanbio Laboratory USA. Data are mean ± SD. *P < 0.05, significantly different from respective control group, #P < 0.05, significantly different from respective Dox group, $P < 0.05, significantly different from respective Dox-RSV group.
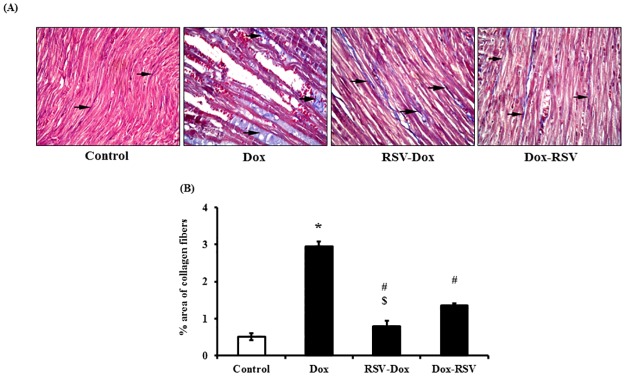
Masson's trichrome staining
In control group fine collagen fibers were found among muscle fibers. In Dox group, dense collagen fibers were observed among thin fibers. In both RSV-Dox and Dox-RSV groups, we observed decreased level of fibrosis, as there was lesser amount of collagen deposition among muscle fibers in RSV treated groups. However, prophylactic use of RSV was more effective than its therapeutic use in mitigating Dox induced fibrosis, as % area of collagen deposition was significantly greater in Dox-RSV group compared to RSV-Dox group (Fig 4A and 4B).
Fig 4. Resveratrol treatment mitigates Dox induced cardiac fibrosis.
(A) Masson’s trichrome staining: Photomicrographs of rat myocardial sections (X 200): Control group showed fine collagen fibers (arrows) among muscle fibers. Dox group showed dense collagen fibers (arrows) among thin muscle fibers. RSV-Dox group showed fine collagen fibers (arrows) among muscle fibers. In Dox-RSV group lesser amount of dense collagen fibers (arrows) among muscle fibers were detected. (B) Quantitative analysis (percent area) of collagen fibers. Data are mean ± SD. *P < 0.05, significantly different from respective control group, #P < 0.05, significantly different from respective Dox group, $P < 0.05, significantly different from respective Dox-RSV group.
Cardiac enzymes
Both CK-MB and LDH are markers of cardiac damage after myocardial infarction. In the current study we found a significant elevation in CK-MB and LDH in the blood from Dox treated rats. Treatment with RSV along with Dox therapy or after 2 weeks of Dox administration decreased the levels of both CK-MB and LDH (Fig 3C and 3D).
Myocardial apoptosis
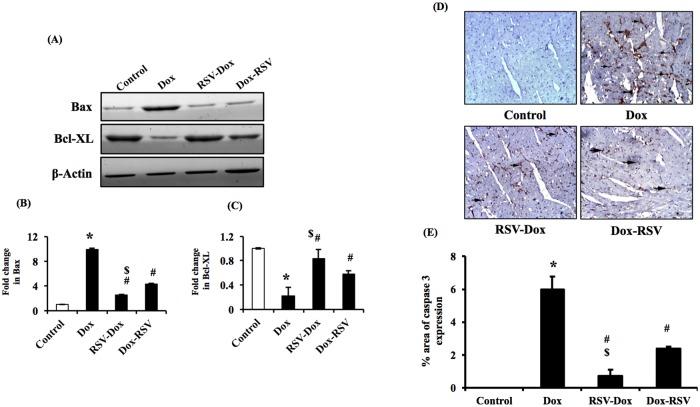
Myocardial apoptosis was detected by measuring the levels of pro-apoptotic protein Bax and anti-apoptotic protein Bcl-xl. Our data demonstrate that protein levels of Bax increased and Bcl-xl decreased in Dox group compared to control. However, co-treatment with RSV along with Dox or RSV treatment following Dox administration prevented Dox induced increase in Bax and decrease in Bcl-xl levels (Fig 5A–5C). We also performed immunohistochemistry to measure caspase 3 expression in myocardial tissue. Caspase 3 is a well-known marker for cellular apoptosis, in control group, we could not detect any caspase 3 positive areas (Fig 5D and 5E). Whereas, in Dox group, there was a significant increase in caspase 3 expression. Therefore, Dox treatment lead to an increase in myocardial apoptosis. Co-treatment with RSV along with Dox or RSV treatment following Dox administration was associated with decrease in myocardial apoptosis, as there was a downregulation of Dox induced increase in caspase 3 expression in both RSV-Dox and Dox-RSV groups (Fig 5D and 5E). However, prophylactic use of RSV was more effective than its therapeutic use in mitigating Dox induced apoptosis.
Fig 5. Resveratrol treatment prevents Dox induced myocardial apoptosis.
(A, B & C) Bax and Bcl-xl protein levels by Western blot. The protein levels of Bax increased and Bcl-xl decreased in Dox group compared to control animals. Treatment with RSV along with Dox or RSV treatment following Dox administration prevented Dox induced increase in Bax, and decrease in Bcl-xl levels. Histograms depict densitometric analysis (B and C). The results were normalized to ß-actin. (D & E) Caspase 3 expression by immunohistochemistry: Photomicrographs of rat myocardial sections (X 200). Control group showed –ve immunostaining among muscle fibers. Dox group showed caspase3 +ve areas (arrows) in myocardial sections. RSV-Dox group showed decreased expression of caspase 3 in myocardial sections. In Dox-RSV group also there was a decrease in caspase 3 +ve area. Data are mean ± SD. *P < 0.05, significantly different from respective control group, #P < 0.05, significantly different from respective Dox group, $P < 0.05, significantly different from respective DOX-RSV group.
Discussion
Several studies in animal models have reported cardio-protective effects of resveratrol [21, 26–28]. Based on the encouraging outcome of these preclinical studies, various clinical trials were conducted recently [29–31]. The outcome of these trials showed mixed response, some of the studies reported positive effects after RSV supplementation, while others found no significant changes in RSV treated subjects. In patients with diabetic cardiomyopathy, RSV supplementation (250 mg/daily) for 3 months demonstrated improvement in systolic volume, mean arterial blood pressure and blood glucose levels [32]. In another trial, in patients at high risk for cardiovascular disease, daily intake of 8 mg resveratrol for 6 months caused 20% decrease in oxidized LDL cholesterol, but only a modest decrease in LDL cholesterol [33]. Another clinical study tested the efficacy of RSV in MI patients, it was reported that treatment with 10 mg/day of RSV had significantly improved diastolic function with a modest increase in systolic function [34]. On the other hand, in patients with metabolic syndrome, RSV supplementation did not have any effect on systolic and diastolic blood pressure [35,36]. Furthermore, in a recent randomized trial in type II diabetes patients, ingestion of 500mg/day of RSV for 5 weeks failed to show any beneficial effects [37]. Majority of clinical trials were centered on the therapeutic effects of RSV supplementation, whereas studies based on preventive approaches are still lacking. Therefore, in current study, we compared potential preventive and therapeutic role of RSV in an experimental model of Dox induced cardiotoxicity. We found that prophylactic supplementation of RSV is more effective than its therapeutic use in mitigating Dox induced cardiotoxicity in the myocardium.
Doxorubicin is a highly potent anticancer drug, however cardiotoxicity due to Dox limits its use for cancer patients [1,2,38]. Doxorubicin induced cardiac complications have been characterized by thinning and dilatation of the ventricular wall and a reduced ejection fraction. In the present study, we observed a significant reduction in %EF and %FS values in Dox treated animals. Which was associated with an increase in myocardial damage as there was an elevation in CK-MB and LDH levels following Dox administration. In several animal models a similar response has been reported [4,21,22]. Dox induced cardiotoxicity and oxidative damage is reported to be manifested by a significant increase in serum CK-MB levels [21,39]. Doxorubicin induced deterioration of cardiac function was also detected in cancer patients on anti-cancer therapy [40,41]. In our study, co-treatment of RSV with Dox or RSV treatment after 2 weeks of Dox administration were both effective in improving cardiac function. Also there was a significant decrease in myocardial damage after RSV treatment. RSV has been reported to increase myocardial anti-oxidant reserve and free radical scavenging capacity [23,30]. Multiple mechanisms have been reported to be involved in RSV mediated antioxidant reserve. RSV can inhibit nicotinamide adenine dinucleotide phosphate (NADPH) and prevent lipid peroxidation [42,43].
Myocardial apoptosis has been suggested to play a significant role in Dox induced cardiomyopathy and deterioration of heart function. It has been reported that Dox induced oxidative stress lead to activation of intrinsic as well as extrinsic pro-apoptotic pathways in myocardial cells [3–6]. In the current study we observed a significant increase in Bax and a decrease in Bcl-xl as well as an upregulation of caspase 3 expression in Dox group. The Bax protein plays a critical role in intracellular apoptosis. On the other hand, Bcl-xl suppresses apoptosis. Therefore, the ratio of Bax/Bcl-xl determines the susceptibility of a cell to undergo apoptosis. Caspase 3 is also reported to play a central role in the execution of cellular apoptosis [7,8]. Doxorubicin induced oxidative damage lead to alteration in the balance between pro-apoptotic and anti-apoptotic proteins that disturbs mitochondrial membrane permeability and leakage of cytochrome c from mitochondria to the cytosol. In the cytosol cytochrome c binds to apoptotic-protease-activating factor-1 (Apaf-1) that further activates caspase 3 [44,45]. We found that co-treatment with RSV and Dox, as well as RSV treatment after Dox administration decreased the caspase 3 levels in myocardial sections. Interestingly, our studies revealed that prophylactic supplementation of RSV along with Dox was more effective than therapeutic use of RSV in mitigating Dox induced myocardial apoptosis.
It has been previously reported that NFAT family of transcription factors have been involved in Dox induced apoptosis [8]. shRNA mediated inhibition of NFAT3 in glioma cells prevented caspase 3 activation and apoptosis [46]. Doxorubicin treatment activates Ca/calcineurine –NFAT pathway leading to upregulation of FAS/FASL dependent apoptosis [46]. Under basal conditions NFAT3 is present in the cytoplasm in its phosphorylated state. Upon de-phosphorylation at multiple serine residues NFAT3 trans-locates to the nucleus [12,13]. Dox treatment has been reported to mediate translocation of NFAT3 to the nucleus [46,47]. Another NFAT family member NFAT5 is identified as a transcription factor involved in cellular responses to hypertonic stress [48]. NFAT5 regulates expression of several target genes responsible for metabolism of organic osmolytes. Unlike other members of NFAT family, NFAT5 is Ca/calceneurin independent and is regulated by Dox in a different manner [49]. NFAT5 has been reported to play a role in cardiomyocyte survival [49,50]. Previously, Dox induced apoptosis have been suggested to result from proteasome-mediated degradation of NFAT5 [44–51]. In the current study, we found a significant increase in NFAT3 and a decrease in NFAT5 levels in myocardial tissue from Dox treated animals. Resveratrol administration along with Dox or after 2 weeks of Dox treatment decreased NFAT3 and increased NFAT5 expression.
Our study also demonstrate that Dox administration increases myocardial fibrosis as manifested by increased deposition of collagen in the myocardial tissue. Previously it has been reported that Dox induced increase in cardiac fibrosis is associated with upregulation of TGF-ß1 levels [21]. TGF-ß1 is responsible for fibroblasts to myofibroblasts conversion and increase in cardiac fibrosis. Co-treatment with RSV and Dox or RSV treatment after Dox administration decreased Dox induced cardiac fibrosis. In the current study, increase in fibrosis due to Dox administration might be a reparative response by which myocardium compensates for the loss of cells due to Dox induced apoptosis. We did not measure TGF-ß1 levels, however, resveratrol has been previously reported to prevent Dox induced increase in TGF- ß1 and conversion of fibroblasts to myofibroblasts [21,28]. We also found that prophylactic use of resveratrol was more effective than its therapeutic use in mitigating Dox induced fibrosis. Therefore, resveratrol may be used prophylactically as a possible adjuvant therapy to minimize cardio-toxic side effects of Doxorubicin in cancer patients. In conclusion, the outcome of this study will help in interpreting the results of ongoing RSV based clinical trials and carefully design future trials.
Acknowledgments
This work was supported by research grants from the Faculty of Medicine, Cairo University and Manitoba Medical Service Foundation.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by Faculty of Medicine, Cairo University and Manitoba Medical Service Foundation.
References
- 1.Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109: 3122–3131. doi: 10.1161/01.CIR.0000133187.74800.B9 [DOI] [PubMed] [Google Scholar]
- 2.Jain D. Cardiotoxicity of doxorubicin and other anthracycline derivatives. J Nucl Cardiol. 2000; 7:53–62. doi: 10.1067/mnc.2000.103324 [DOI] [PubMed] [Google Scholar]
- 3.Nitobe J, Yamaguchi S, Okuyama M, Nozaki N, Sata M, Miyamoto T et al. Reactive oxygen species regulate FLICE inhibitory protein (FLIP) and susceptibility to Fas-mediated apoptosis in cardiac myocytes. Cardiovasc Res. 2003;57: 119–128. [DOI] [PubMed] [Google Scholar]
- 4.Ammar HI, Saba S, Ammar RI, Elsayed LA, Ghaly WB, Dhingra S. Erythropoietin protects against doxorubicin-induced heart failure. Am J Physiol Heart Circ Physiol. 2011;301: 2413–2421. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulou LC, Theophilidis G, Thomopoulos GN, Tsiftsoglou AS. Structural and functional impairment of mitochondria in adriamycin-induced cardiomyopathy in mice: suppression of cytochrome c oxidase II gene expression. Biochem Pharmacol. 1999;57: 481–489. [DOI] [PubMed] [Google Scholar]
- 6.Liu B, Bai QX, Chen XQ, Gao GX, Gu HT. Effect of curcumin on expression of survivin, Bcl-2 and Bax in human multiple myeloma cell line. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007;15: 762–766. [PubMed] [Google Scholar]
- 7.Ueno M, Kakinuma Y, Yuhki K. Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. J Pharmacol Sci. 2006;101: 151–158. [DOI] [PubMed] [Google Scholar]
- 8.Youn HJ, Kim HS, Jeon MH, Lee JH, Seo YJ, Lee YJ, et al. Induction of caspase-independent apoptosis in H9c2 cardiomyocytes by adriamycin treatment. Mol Cell Biochem. 2005;270: 13–19. [DOI] [PubMed] [Google Scholar]
- 9.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annual Review of Immunology. 1997;15: 707–747. doi: 10.1146/annurev.immunol.15.1.707 [DOI] [PubMed] [Google Scholar]
- 10.de la Pompa JL, Timmerman LA, Takimoto H, Yoshida H, Elia AJ, Samper E et al. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature. 1998;392: 182–186. doi: 10.1038/32419 [DOI] [PubMed] [Google Scholar]
- 11.Ranger AM, Grusby MJ, Hodge MR, Gravallese EM, de la Brousse FC, Hoey T et al. The transcription factor NF-ATc is essential for cardiac valve formation. Nature. 1998;392: 186–190. doi: 10.1038/32426 [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharyya S, Deb J, Patra AK, Thuy Pham DA, Chen W, Vaeth M, et al. NFATc1 affects mouse splenic B cell function by controlling the calcineurin—NFAT signaling network. J Exp Med. 2011;208: 823–839. doi: 10.1084/jem.20100945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graef IA, Chen F, Chen L, Kuo A, Crabtree GR. Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell. 2001; 105: 863–875. [DOI] [PubMed] [Google Scholar]
- 14.Wang K, Zhang DL, Long B, An T, Zhang J, Zhou LY, et al. NFAT4-dependent miR-324-5p regulates mitochondrial morphology and cardiomyocyte cell death by targeting Mtfr1. Cell Death Dis. 2015; 6: e2007 doi: 10.1038/cddis.2015.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohammadshahi M, Haidari F, Soufi FG. Chronic resveratrol administration improves diabetic cardiomyopathy in part by reducing oxidative stress. Cardiol J, 2013: 39–46. doi: 10.5603/CJ.a2013.0051 [DOI] [PubMed] [Google Scholar]
- 16.Movahed A, Yu L, Thandapilly SJ, Louis XL, Thomas N. Resveratrol protects adult cardiomyocytes against oxidative stress mediated cell injury. Arch Biochem Biophys. 2012;527: 74–80. doi: 10.1016/j.abb.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 17.Usta E, Mustafi M, Walker T. Resveratrol suppresses apoptosis in intact human cardiac tissue—in vitro model simulating extracorporeal circulation. J Cardiovasc Surg. 2011;52: 399–309. [PubMed] [Google Scholar]
- 18.Moreira AC, Branco AF, Sampaio SF, Cunha-Oleviera T, Martins TR, Holy J, et al. Mitochondrial apoptosis-inducing factor is involved in doxorubicin-induced toxicity on H9c2 cardiomyoblasts. Biochim Biophys Acta. 2014; 1842: 2468–2478. doi: 10.1016/j.bbadis.2014.09.015 [DOI] [PubMed] [Google Scholar]
- 19.Zhang C, Feng Y, Qu S, Wei X, Zhu H, Luo Q, et al. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc Res. 2011;90: 538–545. doi: 10.1093/cvr/cvr022 [DOI] [PubMed] [Google Scholar]
- 20.Kim TY, Chung HM, Lee KH, Kim NM, Kim MS, Park BR et al. The Cardioprotective Effects of Resveratrol via Anti-Apoptosis in Hypoxic Injury of Myocardial Cells. Korean. Circulation J. 2007;37: 408–413. [Google Scholar]
- 21.Arafa MH, Mohammad NS, Atteia HH, Abd-Elaziz HR. Protective effect of resveratrol against doxorubicin-induced cardiac toxicity and fibrosis in male experimental rats. J Physiol Biochem. 2014;70: 701–711. doi: 10.1007/s13105-014-0339-y [DOI] [PubMed] [Google Scholar]
- 22.Gu J, Song ZP, Gui DM, Hu W, Chen YG, Zhang DD. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in lymphoma nude mice by heme oxygenase-1 induction. Cardiovasc Toxicol. 2012;12: 341–349. doi: 10.1007/s12012-012-9178-7 [DOI] [PubMed] [Google Scholar]
- 23.Tatlidede E, Sehirli O, Velioğlu-Oğünc A, Cetinel S, Yegen BC, Yarat A et al. Resveratrol treatment protects against doxorubicin-induced cardiotoxicity by alleviating oxidative damage. Free Radic Res. 2009;43: 195–205. doi: 10.1080/10715760802673008 [DOI] [PubMed] [Google Scholar]
- 24.Dolinsky VW, Soltys CL, Rogan KJ, Chan AY, Nagendaran J, Wang S et al. Resveratrol prevents pathological but not physiological cardiac hypertrophy. J Mol Med (Berl). 2015;93: 413–425. [DOI] [PubMed] [Google Scholar]
- 25.Anita YM, Chan K, Dolinsky VM, Violett B, Baksh S, Light PE et al. Resveratrol Inhibits Cardiac Hypertrophy via AMP-activated Protein Kinase and Akt. J Biol Chem. 2008;283: 24194–24201. doi: 10.1074/jbc.M802869200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sin TK, Tam BT, Yung BY, Yip SP, Chan LW, Wong CS et al. Resveratrol protects against doxorubicin-induced cardiotoxicity in aged hearts through the SIRT1-USP7 axis. J Physiol. 2015;593: 1887–1899. doi: 10.1113/jphysiol.2014.270101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu J, Song ZP, Gui DM, Hu W, Chen YG, Zhang DD. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in lymphoma nude mice by heme oxygenase-1 induction. Cardiovasc Toxicol. 2012;12: 341–349. doi: 10.1007/s12012-012-9178-7 [DOI] [PubMed] [Google Scholar]
- 28.Osman AM, Al-Harthi SE, AlArabi OM, Elshal MF, Ramadan WS, Alaama MN et al. Chemosensetizing and cardioprotective effects of resveratrol in doxorubicin- treated animals. Cancer Cell Int. 2013;13: 52 doi: 10.1186/1475-2867-13-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smoliga JM, Vang O, Baur JA. Challenges of translating basic research into therapeutics: resveratrol as an example. J Gerontol A Biol Sci Med Sci. 2012; 67:158–167. doi: 10.1093/gerona/glr062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zordoky BN, Robertson IM, Dyck JR. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim Biophys Acta. 2015;1852: 1155–1177. doi: 10.1016/j.bbadis.2014.10.016 [DOI] [PubMed] [Google Scholar]
- 31.Sterba M, Popelova O, Vavrova A, Jirkovsky E, Kovarilkova P, Gersl V et al. Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection Antioxid Redox Signal. 2013;18: 899–29. doi: 10.1089/ars.2012.4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res. 2012;32:537–541. doi: 10.1016/j.nutres.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 33.Tomé-Carneiro J, Larrosa M, González-Sarrías A, Tomás-Barberán FA, García-Conesa MT, Espín E J. Resveratrol and clinical trials: The crossroad from In vitro studies to human evidence. Current Pharma Design. 2013;19; 6064–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magyar K, Halmosi R, Palfi A, Feher G, Czopf L, Fulop A et al. Szabados, Cardioprotection by resveratrol: a human clinical trial in patients with stable coronary artery disease, Clin. Hemorheol. Microcirc. 2012;50:179–187. [DOI] [PubMed] [Google Scholar]
- 35.Fujitaka K, Otani H, Jo F, Jo H, Nomura E, Iwasaki M et al. Modified resveratrol Longevinex improves endothelial function in adults with metabolic syndrome receiving standard treatment. Nutr Res. 2011;31:842–847. doi: 10.1016/j.nutres.2011.09.028 [DOI] [PubMed] [Google Scholar]
- 36.Wong RH, Berry NM, Coates AM, Buckley JD, Bryan J, Kunz I et al. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults, J Hypertens. 2013;31:1819–1827. doi: 10.1097/HJH.0b013e328362b9d6 [DOI] [PubMed] [Google Scholar]
- 37.Thazhath SS, Wu T, Bound MJ, Checklin HL, Standfield S, Jones KL. et al. Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: a randomized controlled trial. Am J Clin Nutr 2016;103:66–70. doi: 10.3945/ajcn.115.117440 [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Chen Q, Qi H, Wang C, Wang C, Zhang J et al. Doxorubicin-induced systemic Inflammation is driven by upregulation of Toll-like receptor TLR4 and endotoxin leakage. Cancer Res. 2016;28. [DOI] [PubMed] [Google Scholar]
- 39.Hadi N, Yousif NG, Al-amran FG, Huntei NK, Mohammad BI, Ali SJ. Vitamin E and telmisartan attenuates doxorubicin induced cardiac injury in rat through down regulation of inflammatory response. BMC Cardiovascular Disorders. 2012;12: 63 doi: 10.1186/1471-2261-12-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer retrospective analysis of the Childhood Cancer Survivor Study cohort. Bmj. 2009; 339: b4606 doi: 10.1136/bmj.b4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abu-Khalaf MM, Juneja V, Chung GG. Long-term assessment of cardiac function after dose-dense and -intense sequential doxorubicin (A) paclitaxel (T) and cyclophosphamide (C) as adjuvant therapy for high risk breast cancer. Breast Cancer Res Treat. 2007;104: 341–349. doi: 10.1007/s10549-006-9413-7 [DOI] [PubMed] [Google Scholar]
- 42.Park DW, Baek K, Kim JR, Lee JJ, Ryu SH, Chin BR, et al. Resveratrol inhibits foam cell formation via NADPH oxidase 1-mediated reactive oxygen species and monocyte chemotactic protein-1. Exp Mol Med. 2009; 41: 171–179. doi: 10.3858/emm.2009.41.3.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Qiang, Si Liang-Yi. Resveratrol role in cardiovascular and metabolic health and potential mechanisms of action. Nutr Res. 2012;32: 648–658. doi: 10.1016/j.nutres.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 44.Clementi ME, Giardina B, Di Stasio E. Doxorubicin-derived metabolites induce release of cytochrome C and inhibition of respiration on cardiac isolated mitochondria. Anticancer Res. 2003;23: 2445–2450. [PubMed] [Google Scholar]
- 45.Berthiaume JM, Wallace KB. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biology and Toxicology. 2007; 23:15–25. doi: 10.1007/s10565-006-0140-y [DOI] [PubMed] [Google Scholar]
- 46.Kalivendi SV, Konorev EA, Cunningham S, Vanamala SK, Kaji EH, Joseph J et al. Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: role of mitochondrial reactive oxygen species and calcium. Biochem J. 2005;389: 527–539. doi: 10.1042/BJ20050285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gopinath S, Vanamala SK, Gujrati M, Klopfenstein JD, Dinh DH, Rao JS. Doxorubicin-mediated apoptosis in glioma cells requires NFAT3. Cell Mol Life Sci. 2009;66: 3967–3678. doi: 10.1007/s00018-009-0157-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aramburu J, Drews-Elger K, Estrada-Gelonch A, Minguillion J, Morancho B, Santiago V et al. Regulation of the hypertonic stress response and other cellular functions by the rel-like transcription factor NFAT5. Biochem Pharmacol. 2006;72: 1597–1604. doi: 10.1016/j.bcp.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 49.Ito T, Fujio Y, Takahashi K, Azuma J. Degradation of NFAT5, a Transcriptional Regulator of Osmotic Stress-related Genes, Is a Critical Event for Doxorubicin-induced Cytotoxicity in Cardiac Myocytes. J Biol Chem. 2007;282: 1152–1160. doi: 10.1074/jbc.M609547200 [DOI] [PubMed] [Google Scholar]
- 50.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes & Dev. 2003;18: 2205–2232. [DOI] [PubMed] [Google Scholar]
- 51.Tsimokha AS, Mittenberg AG, Kulichkova VA, Kozhukharova IV, Gause LN, Konstantinova IM. Changes in composition and activities of 26S proteasomes under the action of doxorubicin—apoptosis inductor of erythroleukemic K562 cells. Cell Biol Int. 2007;31: 338–48. doi: 10.1016/j.cellbi.2007.01.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.