Abstract
Background
Early recognition is a key factor to achieve improved outcomes for septic patients. Combinations of biomarkers, as opposed to single ones, may improve timely diagnosis and survival. We investigated the performance characteristics of sepsis biomarkers, alone and in combination, for diagnosis of verified bacterial sepsis using Sepsis-2 and Sepsis-3 criteria, respectively.
Methods
Procalcitonin (PCT), neutrophil-lymphocyte count ratio (NLCR), C-reactive protein (CRP), and lactate were determined in a total of 1,572 episodes of adult patients admitted to the emergency department on suspicion of sepsis. All sampling were performed prior to antibiotic administration. Discriminant analysis was used to construct two composite biomarkers consisting of linear combinations of the investigated biomarkers, one including three selected biomarkers (i.e., NLCR, CRP, and lactate), and another including all four (i.e., PCT, NLCR, CRP, and lactate). The diagnostic performances of the composite biomarkers as well as the individual biomarkers were compared using the area under the receiver operating characteristic curve (AUC).
Results
For diagnosis of bacterial sepsis based on Sepsis-3 criteria, the AUC for PCT (0.68; 95% CI 0.65–0.71) was comparable to the AUCs for the both composite biomarkers. Using the Sepsis-2 criteria for bacterial sepsis diagnosis, the AUC for the NLCR (0.68; 95% CI 0.65–0.71) but not for the other single biomarkers, was equal to the AUCs for the both composite biomarkers. For diagnosis of severe bacterial sepsis or septic shock based on the Sepsis-2 criteria, the AUCs for both composite biomarkers were significantly greater than those of the single biomarkers (0.85; 95% CI 0.82–0.88 for the composite three-biomarker, and 0.86; 95% CI 0.83–0.89 for the composite four-biomarker).
Conclusions
Combinations of biomarkers can improve the diagnosis of verified bacterial sepsis in the most critically ill patients, but in less severe septic conditions either the NLCR or PCT alone exhibit equivalent performance.
Introduction
Sepsis is a life-threatening condition that arises when the host responds to an infection and damages its organs [1]. It is often difficult to distinguish between bacterial and non-bacterial aetiologies early in suspected sepsis. Clinical signs of sepsis such as tachycardia, leucocytosis, tachypnea, and pyrexia, often overlap with other non-infectious conditions in critically ill patients. Concurrently, it has been shown that prompt diagnosis and early administration of appropriate antibiotic therapy considerably improve the outcomes of septic patients [2–4]. The difficulty in distinguishing between bacterial and non-bacterial aetiologies is also a major cause of the misuse of antibiotics [5]. Inappropriate or prolonged use of antibiotics may lead to the emergence of antibiotic resistant bacteria [6–8] and to various adverse events [9–11], whereas antibiotic underuse due to delayed or missed diagnosis may result in worsened condition and medical complications [12–16].
There is an unmet need for diagnostic tools differentiating between bacterial and non-bacterial causes of sepsis. Although various biomarkers have been proposed, no single clinical or biological indicator of sepsis has gained general acceptance [17, 18]. The most widely studied biomarkers in patients with suspected bacterial sepsis are C-reactive protein (CRP) and procalcitonin (PCT). Both CRP and PCT are today routinely employed in clinical practice but have limited abilities to distinguish bacterial sepsis from other inflammatory conditions [19]. Even if PCT has an established role as biomarker in septic patients, the diagnostic accuracy of routine PCT measurements have been questioned because of inconsistent and variable results depending on the severity of illness and infection in the studied patient population [20–38]. Lactate is another biomarker frequently used as a biomarker in septic patients, however, it lacks specificity [39] as elevated lactate levels can be seen in a wide variety of conditions in addition to sepsis, e.g., cardiac arrest, trauma, seizure [40]. Zahorec et al. were the first to propose to use the ratio of neutrophil and lymphocyte count (NLCR) as an additional infection marker in clinical practice [41]. Previously, the NLCR has been found to correlate with disease severity [41–43] and has also been suggested as a predictor of bacteraemia [44–48]. Considering the complexity of sepsis, no single marker is good enough for precise diagnostics, but a combination of biomarkers could improve diagnosis [49] and treatment efficacy, and patient outcome.
In view of previously results and the paramount importance of timely diagnosis and accurate treatment of bacterial sepsis, we undertook the present study to determine the diagnostic value of PCT and the NLCR in comparisons with two conventional biomarkers, i.e., CRP and lactate, using a large sample size. In addition, we determined the discriminatory power of combining multiple biomarkers in diagnosis of adult patients suspected of having community-onset sepsis.
Materials and methods
Patients and study design
This study is part of a prospective observational study of community-onset severe sepsis and septic shock in adults conducted from September 2011 to June 2012 at Skaraborg Hospital, a secondary hospital with 640 beds, in the western region of Sweden. All patients ≥18 years consecutively admitted to the emergency department for suspicion of a community-onset sepsis were asked to participate in the study. Only those patients who gave their written informed consent were enrolled. The study was approved by the Regional Ethical Review Board of Gothenburg (376–11).
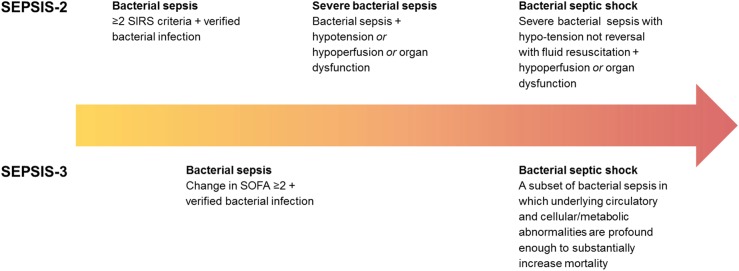
At the time of admission to the emergency department, signs and symptoms, clinical and laboratory data were collected and recorded. The sampling was performed according to routine hospital procedures and prior to administration of antibiotic therapy. All medical records were retrospectively reviewed by two senior specialists in infectious diseases (LL and GJ) to determine whether the patients fulfilled Sepsis-2 and Sepsis-3 criteria, respectively. According to Sepsis-2 criteria, bacterial sepsis was defined as verified bacterial infection and systemic inflammatory response syndrome (SIRS), whereas severe bacterial sepsis was defined as verified bacterial sepsis and sepsis-induced hypotension or tissue hypoperfusion or organ dysfunction according to the Swedish criteria (Fig 1; S1 Text) [50]. SIRS was defined as the occurrence of at least two of the following criteria: fever >38.0°C or hypothermia <36.0°C, tachypnea >20 breaths/min, tachycardia >90 beats/min, leucocytosis >12x109 cells/L or leucopenia <4x109 cells/L. According to Sepsis-3 criteria, bacterial sepsis was defined as bacterial infection-induced organ dysfunction characterized by a rise in total SOFA ≥2 (Fig 1) [1]. For both Sepsis-2 and Sepsis-3, verified bacterial infection was defined as a clinical infection and identification of relevant bacteria by culture, or as typical clinical symptoms, such as erysipelas. Bacteraemia was defined as a positive blood culture result.
Fig 1. Definitions of verified bacterial sepsis, severe bacterial sepsis, and bacterial septic shock according to Sepsis-2 and Sepsis-3, respectively.
SIRS, systemic inflammatory response syndrome; MAP, mean arterial pressure.
Biomarker measurements
CRP levels were measured with the ADVIA Chemistry Instrument (Siemens Healthcare Diagnostics Inc.) and plasma lactate on an ABL 800 Flex (Radiometer Medical ApS). NLCR were determined on an ADVIA 2120i (Siemens Healthcare Diagnostics Inc.), counting neutrophils and lymphocytes using the white blood cell differential methods to calculate the NLCR. CRP, lactate and NLCR were analysed on the same day as collected and as per manufacturer´s instructions. For the PCT measurements, samples were taken into sodium citrate tubes, centrifuged and plasma stored at -80°C until the end of study when all samples were retrospectively measured on a mini-VIDAS® (bioMérieux, France) as per the manufacturer’s instructions.
Statistical analysis
The continuous variables were expressed as the median and interquartile range due to non-normal distribution (p <0.05 for Kolmogorov-Smirnov´s test for all continuous variables). The categorical variables were summarised as frequencies and percentages. Mann-Whitney U tests were performed for comparisons of continuous variables in different patient groups. Adjustment of p values for multiple comparisons were made using the Benjamini and Hochberg method. For the diagnostic evaluation of single biomarkers, the performance characteristics for several cut-off points were recorded. The different cut-offs were selected for each biomarker based on previously reported findings as well as recommendations for clinical practice in Sweden [44, 51–53]. Two composite biomarkers were constructed using linear discriminant analysis (S2 Text). One composite biomarker consisted of a linear combination of three selected biomarkers, and the other one included all four biomarkers. The three-marker combination consisted of CRP, lactate, and NLCR, and were primarily chosen as they are routinely employed in our hospital and can easily be integrated in daily practice without extra costs. A comparison of the diagnostic accuracy of the biomarkers, alone and in combination, was made using receiver operating characteristics curves (ROC) analyses by calculating the area under the curve (AUC). For comparison of AUCs, DeLong's test for two correlated ROC curves was used [54, 55]. All tests were two-sided, and p <0.05 was considered statistically significant. The statistical analyses were performed using IBM SPSS Statistics version 24.0 (IBM Corp., United States), R version 3.2.3 (R Foundation for Statistical Computing, Austria), and MATLAB R2016a (The Mathworks Inc., United States).
Results
Patients
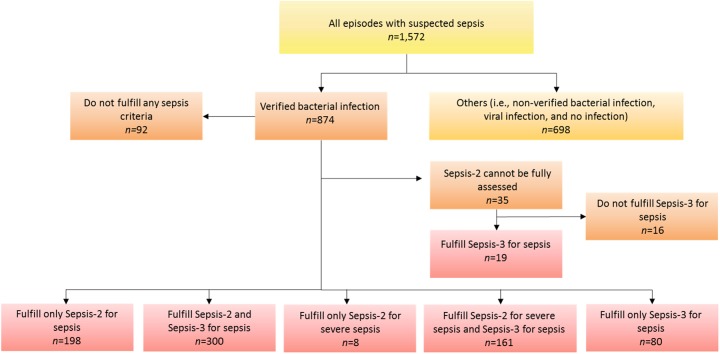
A total of 1,572 episodes of adult patients suspected with sepsis admitted to the emergency department at Skaraborg Hospital were enrolled. Of these, 874 episodes (55.6%) had a verified bacterial infection (Fig 2), whereas the remaining 698 episodes did not. Six-hundred and sixty-seven episodes (42.4%) fulfilled the Sepsis-2 criteria for bacterial sepsis, whereof 169 episodes (10.8%) also fulfilled the criteria for severe bacterial sepsis or septic shock. For 35 of the episodes having a bacterial infection, at least one of four SIRS criteria was missing and thus not possible to evaluate based on the Sepsis-2 criteria. Five-hundred and sixty episodes (35.6%) fulfilled the Sepsis-3 criteria for bacterial sepsis. Patient characteristics by categories are summarised in Table 1, whereas the levels of the biomarkers by patient categories are shown in Fig 3.
Fig 2. Distribution of 1,572 episodes of adult patients with suspected sepsis depending on presence of bacterial infection and whether the criteria for Sepsis-2 and/or Sepsis-3 were fulfilled.
Table 1. Patient characteristics at admission.
| Sepsis-2 | Sepsis-3 | |||||
|---|---|---|---|---|---|---|
| Characteristic | All episodes suspected with sepsis (n = 1,572) | Othersa (n = 698) | Bacterial infection (n = 874) | Bacterial sepsisb (n = 667) | Severe bacterial sepsis/septic shock (n = 169) | Bacterial sepsisc (n = 560) |
| Age (years) | 71 (58–81) | 70 (56–80) | 73 (59–81) | 73 (60–82) | 76 (68–84) | 77 (67–85) |
| Sex (female) | 697 (44.3%) | 297 (42.6%) | 400 (45.8%) | 305 (45.7%) | 76 (45.0%) | 254 (45.4%) |
| Systolic blood pressure (mmHg) | 134 (118–150) | 138 (120–154) | 131 (115–150) | 131 (114–150) | 120.0 (104–142) | 130 (111–150) |
| Respiratory rate (breaths/min) | 24 (20–28) | 23 (18–28) | 24 (20–28) | 24 (22–30) | 28 (24–32) | 25 (21–30) |
| Oxygen saturation (%) | 95 (92–97) | 95 (92–97) | 95 (92–97) | 95 (92–96) | 93 (88–96) | 94 (90–96) |
| Heart rate (beats/min) | 96 (83–109) | 96 (82–108) | 97 (84–110) | 102 (91–114) | 108 (94–121) | 99 (85–113) |
| Body temperature (oC) | 37.9 (37.1–38.6) | 37.8 (37.0–38.5) | 38.0 (37.2–38.7) | 38.3 (37.5–38.8) | 38.2 (37.4–38.8) | 38.0 (37.1–38.7) |
| Haemoglobin (g/L) | 131 (118–142) | 132 (119–144) | 130 (117–141) | 130 (118–141) | 130 (117–143) | 128 (116–140) |
| LPK (x109 cells/L) | 12.0 (8.7–15.6) | 11.1 (7.8–14.5) | 12.5 (9.4–16.5) | 13.6 (10.3–17.7) | 14.7 (10.5–19.3) | 13.1 (9.8–17.6) |
| CRP (mg/L) | 102 (43–174) | 85 (31–152) | 114 (51–191) | 114 (51–198) | 109 (43–216) | 126 (54–204) |
| PCT (ng/mL) | 0.20 (0.06–1.07) | 0.13 (0.05–0.49) | 0.31 (0.09–1.87) | 0.37 (0.10–2.17) | 1.17 (0.24–12.40) | 0.51 (0.13–4.07) |
| P-lactate (mmol/L) | 1.70 (1.30–2.31) | 1.63 (1.27–2.20) | 1.77 (1.30–2.40) | 1.81 (1.37–2.49) | 3.30 (2.00–4.20) | 1.90 (1.44–2.70) |
| NLCR | 9.6 (5.4–16.8) | 7.8 (4.4–13.0) | 11.5 (6.5–19.9) | 13.1 (7.9–22.1) | 18.4 (11.2–31.2) | 13.0 (8.0–22.4) |
| ICU (yes) | 111 (7.1%) | 52 (7.4%) | 59 (6.8%) | 52 (7.8%) | 46 (27.2%) | 59 (10.5%) |
| 28 days survival | 1489 (94.7%) | 650 (93.1%) | 839 (96.0%) | 634 (95.1%) | 144 (85.2%) | 525 (93.8%) |
| Blood culture (positive) | 197 (12.5%) | 0 (0%) | 197 (22.5%) | 155 (23.2%) | 77 (45.6%) | 154 (27.5%) |
Data presented as median (interquartile range) or number (percentage) of episodes. CRP, C-reactive protein; ICU, intensive care unit; LPK, leukocyte particle concentration; NLCR, neutrophil-lymphocyte count ratio; PCT, procalcitonin.
a Including episodes having possible but non-verified bacterial infections, viral infections, and non-infectious diseases.
b Including all episodes fulfilling the Sepsis-2 criteria for bacterial sepsis irrespective severity (i.e., sepsis, severe sepsis, and septic shock).
c Including all episodes fulfilling the Sepsis-3 criteria for bacterial sepsis irrespective severity (i.e., sepsis and septic shock).
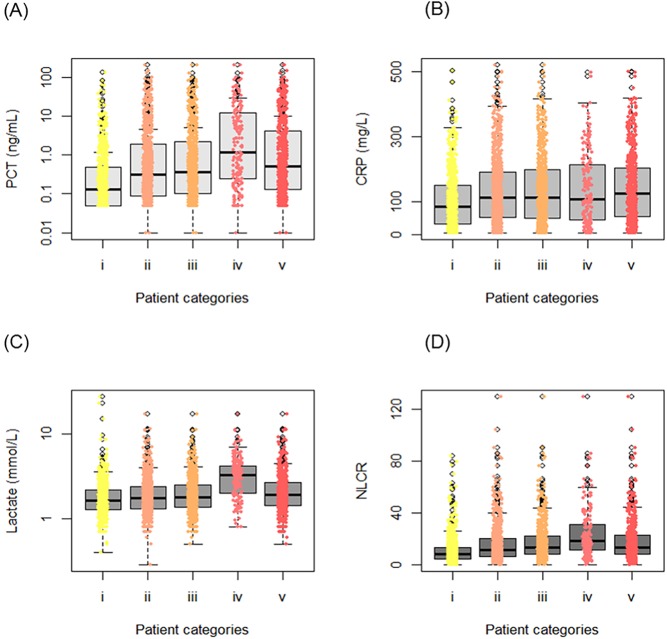
Fig 3. Comparisons of levels of single biomarkers stratified by patient categories.
i. Others (i.e., non-verified bacterial infection, viral infection, and no infection) (n = 698); ii. Verified bacterial infection (n = 874); iii. Verified bacterial sepsis (Sepsis-2) (n = 667); iv. Verified severe bacterial sepsis/septic shock (Sepsis-2) (n = 169); v. Verified bacterial sepsis (Sepsis-3) (n = 560). (A) Procalcitonin (PCT), (B) C-reactive protein (CRP), (C) Lactate, (D) Neutrophil-lymphocyte count ratio (NLCR).
Diagnostic performance of the biomarkers
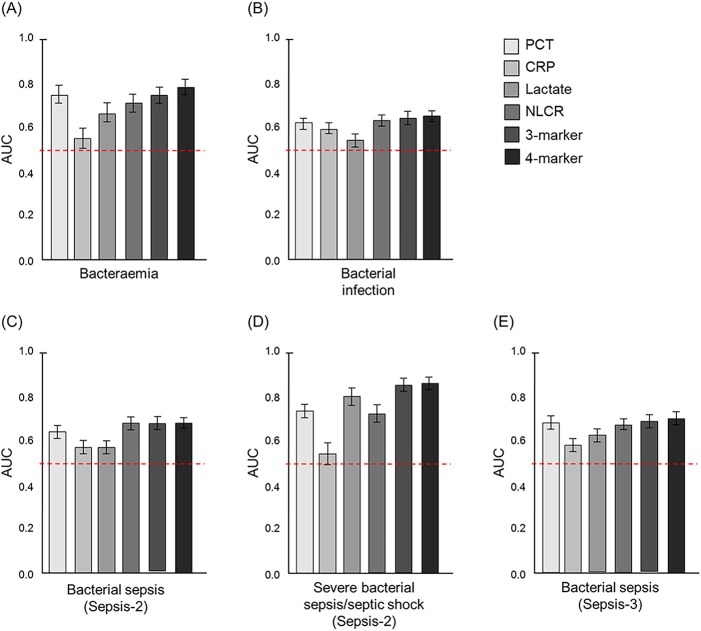
Levels of the various biomarkers between different patient groups were compared using Mann-Whitney U test. The biomarker levels in the 197 episodes with bacteraemia were compared with the 1,375 episodes without bacteraemia. All four biomarkers were significantly higher (all p < 0.01) in episodes with bacteraemia. The respective median levels were as follows: 1.70 and 0.17 ng/mL for PCT, 128.0 and 100.0 mg/L for CRP, 2.10 and 1.66 mmol/L for lactate, 16.6 and 8.8 for the NLCR (Fig 3). In the ROC curve analysis, PCT (AUC 0.74; 95% CI 0.70–0.78) showed the highest ability to diagnose bacteraemia among the single biomarkers (Fig 4A) and was significantly better than both CRP (p < 0.001; AUC 0.56; 95% CI 0.51–0.60) and lactate (p = 0.001; 0.66; 95% CI 0.61–0.70), but not the NLCR (p = 0.17; AUC 0.71; 95% CI 0.67–0.75). However, the composite four-biomarker had the highest AUC (0.78; 95% CI 0.74–0.81) and was significantly higher (all p < 0.001) than the composite three-biomarker (AUC 0.75; 95% CI 0.71–0.79) and all single biomarkers except PCT (p = 0.06).
Fig 4. AUC for the biomarkers evaluated in the present study.
Error bars represent 95% CI. The red dotted lines represent a reference line corresponding to AUC = 0.5. The three-biomarker consists of a combination of CRP, lactate, and NLCR, whereas the four-biomarker consists of a combination of PCT, CRP, lactate, and NLCR. (A) AUC for diagnosis of bacteraemia. (B) AUC for diagnosis of verified bacterial infection. (C) AUC for diagnosis of verified bacterial sepsis using Sepsis-2 criteria irrespective severity (i.e., sepsis, severe sepsis, and septic shock). (D) AUC for diagnosis of verified severe bacterial sepsis/septic shock using Sepsis-2 criteria. (E) AUC for diagnosis of verified bacterial sepsis using Sepsis-3 criteria irrespective severity (i.e., sepsis and septic shock). AUC, area under receiver operating caracteristic curve; CI, confidence interval; CRP, C reactive protein; NLCR, neutrophil-lymphocyte ratio; PCT, procalcitonin.
The biomarker levels in the 874 episodes having a verified bacterial infection were compared with the other 698 episodes, which did either have a non-verified bacterial infection or a viral infection or a non-infectious disease. All biomarker levels were significantly higher (all p < 0.01) in episodes having a bacterial infection (Table 1 and Fig 3). In the ROC curve analysis (Fig 4B), the NLCR showed the highest ability among the single biomarkers to discriminate between verified bacterial infections and others (AUC 0.63; 95% CI 0.61–0.66), but was not significantly better (p = 0.52) than PCT (AUC 0.62; 95% CI 0.60–0.65). The AUCs for both composite biomarkers were comparable to the NLCR and PCT (all p > 0.05); the three-biomarker had an AUC of 0.64 (95% CI 0.62–0.67) and the four-biomarker had an AUC of 0.64 (95% CI 0.61–0.67).
The biomarker levels in the 667 episodes fulfilling the Sepsis-2 criteria for verified bacterial sepsis, including episodes ranging from sepsis to septic shock, were compared with the remaining 870 episodes excluding 35 episodes unable to determine. There were significant differences between these two groups for all four biomarkers (all p < 0.001). The respective median levels were as follows: 0.37 and 0.13 ng/mL for PCT, 114.0 and 91.0 mg/L for CRP, 1.81 and 1.60 mmol/L for lactate, 13.1 and 7.7 for the NLCR (Fig 3). For diagnosing bacterial sepsis, the NLCR showed a significantly higher AUC (0.68; 95% CI 0.65–0.71) than PCT (p = 0.019; AUC 0.64; 95% CI 0.61–0.67), CRP (p < 0.001; AUC 0.57; 95% CI 0.54–0.60), and lactate (p < 0.001; AUC 0.57; 95% CI 0.54–0.60; Fig 4C). Both composite biomarkers showed an ability equal to that of the NLCR in diagnosis of bacterial sepsis (all p > 0.05).
Significant differences between the 169 episodes diagnosed as either severe bacterial sepsis or septic shock based on Sepsis-2 criteria and the 1,403 episodes that did not fulfil these criteria were found for all biomarkers (p < 0.001), except for CRP (p = 0.136). The respective median levels were as follows: 1.17 and 0.17 ng/mL for PCT, 109.0 and 101.0 mg/L for CRP, 3.30 and 1.61 mmol/L for lactate, 18.4 and 8.9 for the NLCR (Fig 3). When comparing the AUC in severe bacterial sepsis/septic shock for the biomarkers (Fig 4D), lactate had the highest AUC (0.81; 95% CI 0.77–0.85) among the single biomarkers and was significantly better than PCT (p = 0.01), the NLCR (p < 0.01) and CRP (p < 0.001). The composite three-biomarker (AUC 0.86; 95% CI 0.83–0.89) as well as the four-biomarker (0.85; 95% CI 0.82–0.88) were both significantly better than all single biomarkers (all p <0.05).
The 560 episodes fulfilling the Sepsis-3 criteria for verified bacterial sepsis were compared to the 1,012 episodes not fulfilling these criteria, and significant differences were found for all biomarkers (all p < 0.01). The respective median levels were as follows: 0.51 and 0.13 ng/mL for PCT, 126.0 and 91.0 mg/L for CRP, 1.90 and 1.60 mmol/L for lactate, 13.0 and 8.0 for the NLCR. In the ROC curve analysis (Fig 4E), PCT showed the highest ability among the single biomarkers to discriminate between episodes with verified bacterial sepsis and episodes not having a verified bacterial sepsis (AUC 0.68; 95% CI 0.65–0.71), but was not significantly better (p = 0.35) than the NLCR (AUC 0.67; 95% CI 0.64–0.69). The AUCs for both composite biomarkers were comparable to PCT (both p > 0.05); the three-biomarker had an AUC of 0.69 (95% CI 0.66–0.72) and the four-biomarker had an AUC of 0.70 (95% CI 0.67–0.73).
The computed specificities, sensitivities, accuracy, diagnostic odds ratios, as well as positive and negative predictive values of the single biomarkers at selected cut-off values for diagnosis of verified bacterial sepsis using Sepsis-2 criteria are shown in Table 2; for diagnosis of verified severe bacterial sepsis/septic shock using Sepsis-2 criteria are shown in Table 3; for diagnosis of verified bacterial sepsis using Sepsis-3 criteria are shown in Table 4. Complete performance characteristics of the single biomarkers at additional cut-off values are presented in Supporting Information (S1–S3 Tables).
Table 2. Performance characteristics of single biomarkers for diagnosing verified bacterial sepsis using Sepsis-2 criteriaa.
| Biomarker (cut-off) | Sensitivity (95% CI) | Specificity (95% CI) | Accuracy (95% CI) | DOR (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|
| PCT (2.0 ng/mL) | 26.4% (23.0–29.7) | 88.6% (86.5–90.7) | 61.6% (59.2–64.0) | 2.79 (2.13–3.66) | 64.0% (58.3–70.0) | 61.0% (58.4–63.8) |
| PCT (10.0 ng/mL) | 11.1% (8.7–13.5) | 96.4% (95.2–97.7) | 59.4 (57.0–61.9) | 3.38 (2.19–5.20) | 70.5% (61.8–79.2) | 58.6% (56.0–61.1) |
| CRP (20 mg/L) | 88.1% (85.7–90.6) | 14.5% (12.1–16.8) | 46.4% (43.9–49.0) | 1.26 (0.93–1.70) | 44.1% (41.4–46.8) | 61.4% (54.7–68.1) |
| CRP (100 mg/L) | 57.1% (53.3–60.9) | 52.3% (49.9–56.6) | 54.9% (52.4–57.4) | 1.51 (1.23–1.86) | 48.3% (44.8–51.8) | 61.8% (58.3–65.3) |
| Lactate (2.5 mmol/L) | 24.9% (21.5–28.2) | 82.7% (80.1–85.2) | 57.4% (54.9–59.9) | 1.58 (1.22–2.03) | 52.6% (47.0–58.2) | 58.7% (55.9–61.5) |
| Lactate (3.5 mmol/L) | 11.9% (9.4–14.4) | 94.4% (92.8–95.9) | 58.4% (55.9–60.9) | 4.84 (2.69–8.71) | 62.1% (53.6–70.6) | 58.0% (55.4–60.6) |
| NLCR (3.0) | 95.9% (94.4–97.4) | 13.2% (10.9–15.5) | 49.0% (46.5–51.6) | 3.54 (2.29–5.45) | 45.8% (43.2–48.4) | 80.7% (74.2–87.3) |
| NLCR (10.0) | 64.3% (60.6–67.9) | 64.0% (60.8–67.2) | 64.1% (61.7–66.6) | 3.20 (2.59–3.96) | 57.8% (54.2–61.3) | 70.1% (66.9–73.3) |
CRP, C-reactive protein; DOR, diagnostic odds ratio; NLCR, neutrophil-lymphocyte count ratio; NPV, negative predictive value; PCT, procalcitonin; PPV, predictive positive value.
aIncluding all episodes fulfilling the Sepsis-2 criteria for bacterial sepsis irrespective severity (i.e., sepsis, severe sepsis, and septic shock).
Table 3. Performance characteristics of single biomarkers for diagnosing verified severe bacterial sepsis/bacterial septic shock using Sepsis-2 criteria.
| Biomarker (cut-off) | Sensitivity (95% CI) | Specificity (95% CI) | Accuracy (95% CI) | DOR (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|
| PCT (2.0 ng/mL) | 46.8% (39.2–54.3) | 85.5% (83.7–87.4) | 81.3% (79.4–83.3) | 5.19 (3.70–7.27) | 28.1% (22.9–33.4) | 92.3% (91.6–94.4) |
| PCT (10.0 ng/mL) | 26.6% (20.0–33.3) | 95.5% (94.4–96.6) | 88.1% (86.4–89.7) | 7.68 (5.02–1.74) | 41.7% (32.4–51.0) | 91.5% (90.1–92.9) |
| CRP (20 mg/L) | 87.3% (82.2–92.4) | 13.5% (11.7–15.3) | 21.4% (19.4–23.5) | 1.07 (0.66–1.74) | 10.8% (9.1–12.5) | 89.9% (85.7–94.0) |
| CRP (100 mg/L) | 56.4% (48.8–63.9) | 49.4% (46.7–52.0) | 50.1% (47.6–52.6) | 1.26 (0.91–1.74) | 11.8% (9.5–14.0) | 90.4% (88.3–92.5) |
| Lactate (2.5 mmol/L) | 66.5% (59.2–73.7) | 85.1% (83.2–87.0) | 83.1% (81.2–85.0) | 11.36 (7.94–16.23) | 35.3% (30.0–40.6) | 95.4% (94.2–96.6) |
| Lactate (3.5 mmol/L) | 67.1% (61.4–72.7) | 96.5% (95.5–97.5) | 91.7% (90.3–93.0) | 56.23 (38.15–82.89) | 79.0% (73.7–84.4) | 93.7% (92.5–95.0) |
| NLCR (3.0) | 96.4% (93.6–99.2) | 10.0% (8.4–11.6) | 19.4% (17.4–21.3) | 2.98 (1.29–6.95) | 11.5% (9.9–13.2) | 95.8% (92.5–99.1) |
| NLCR (10.0) | 79.0% (72.9–85.2) | 55.6% (53.0–58.2) | 58.2% (55.7–60.6) | 4.73 (3.21–6.96) | 17.8% (15.1–20.6) | 95.6% (94.2–97.0) |
CRP, C-reactive protein; DOR, diagnostic odds ratio; NLCR, neutrophil-lymphocyte count ratio; NPV, negative predictive value; PCT, procalcitonin; PPV, predictive positive value.
Table 4. Performance characteristics of single biomarkers for diagnosing verified bacterial sepsis using Sepsis-3 criteriaa.
| Biomarker (cut-off) | Sensitivity (95% CI) | Specificity (95% CI) | Accuracy (95% CI) | DOR (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|
| PCT (2.0 ng/mL) | 32.1% (28.3–36.0) | 89.5% (87.6–91.4) | 69.1% (66.8–71.4) | 4.05 (3.10–5.29) | 62.9% (57.3–68.5) | 70.5% (68.0–72.9) |
| PCT (10.0 ng/mL) | 15.4% (12.4–18.3) | 97.4% (96.5–98.4) | 68.2% (65.9–70.5) | 6.88 (4.38–10.81) | 76.8% (69.0–84.6) | 67.5% (65.1–69.9) |
| CRP (20 mg/L) | 88.4% (85.8–91.1) | 14.4% (12.2–16.5) | 40.8% (38.4–43.3) | 1.28 (0.93–1.75) | 36.4% (33.9–39.0) | 69.0% (62.8–75.4) |
| CRP (100 mg/L) | 59.7% (55.6–63.8) | 53.3% (50.2–56.4) | 55.6% (53.1–58.1) | 1.69 (1.37–2.09) | 41.5% (38.1–44.9) | 70.4% (67.2–73.7) |
| Lactate (2.5 mmol/L) | 29.5% (25.7–33.4) | 84.2% (81.9–86.5) | 64.6% (62.2–67.1) | 2.23 (1.73–2.87) | 51.0% (45.4–56.5) | 68.2% (65.6–70.9) |
| Lactate (3.5 mmol/L) | 14.9% (11.9–18.0) | 95.3% (94.0–96.6) | 66.6% (64.2–68.9) | 3.54 (2.43–5.18) | 63.8% (55.4–72.1) | 66.8% (64.3–69.3) |
| NLCR (3.0) | 95.1% (93.3–96.9) | 11.7% (9.7–13.7) | 41.5% (39.1–44.0) | 2.58 (1.67–3.97) | 37.5% (35.0–40.0) | 81.1% (74.7–87.5) |
| NLCR (10.0) | 64.7% (60.8–68.7) | 60.8% (57.9–63.9) | 62.2% (59.8–64.6) | 2.85 (2.30–3.54) | 47.9% (44.3–51.5) | 75.6% (72.6–78.6) |
CRP, C-reactive protein; DOR, diagnostic odds ratio; NLCR, neutrophil-lymphocyte count ratio; NPV, negative predictive value; PCT, procalcitonin; PPV, predictive positive value.
aIncluding all episodes fulfilling the Sepsis-3 criteria for bacterial sepsis irrespective severity (i.e., sepsis and septic shock).
Discussion
Over the years, numerous studies have been performed investigating the clinical usefulness of biomarkers in diagnosis, prognosis, staging, and monitoring of sepsis [34, 56, 57]. Many studies have thus focused on the use of single biomarkers although the interest in multimarker approaches in sepsis diagnostics has increased, especially in the search for novel sepsis biomarkers using high-throughput methods for screening of patient samples [58–61]. A weakness in many previous biomarkers studies is the use of small sample size of patients which may lead to inconsistent results [56]. In the present study, we have investigated the diagnostic value of known biomarkers, i.e., PCT, the NLCR, CRP and lactate, alone as well as in combination using a large sample consisting of 1,572 episodes of adult patients suspected with sepsis.
Recently, the criteria for sepsis were updated [1] and the definition of sepsis was changed from a systemic inflammatory response syndrome caused by an infection (Sepsis-2) to a life-threatening organ dysfunction due to a dysregulated host response to infection (Sepsis-3). Changing the criteria may ultimately also affect the performance of diagnostic biomarkers. In the present study, we found that CRP at a cut-off of 20 mg/mL had the highest sensitivity (88%) for bacterial sepsis as defined by Sepsis-3 criteria, whereas both PCT at a cut-off 10.0 ng/mL and lactate at a cut-off of 4.0 mmol/L showed high specificity (97%). Considering the AUCs, all single biomarkers except for the NLCR performed slightly better in diagnosing bacterial sepsis based on Sepsis-3 criteria than Sepsis-2 criteria (Fig 4). PCT showed a moderate AUC (0.68), but was nonetheless the single biomarker with the highest AUC and comparable to the AUCs for the composite variables (Fig 4E). However, more studies based on the updated Sepsis-3 criteria are needed to further assess the diagnostic performance of these biomarkers.
The NLCR has been reported to correlate with the severity of disease [41–43] and has gained interest as a predictor of survival in various clinical circumstances ranging from oncological patients to patients with cardiovascular diseases [62–67]. In the context of sepsis, the NLCR has previously been described as a predictor of bacteraemia [44–48]. In this study, the AUC for predicting positive blood culture results based on the NLCR was moderate (0.71) and in line with previous studies reporting AUCs of 0.73 [44] and 0.77 [48]. In the ROC curve analysis for detection of bacterial sepsis based on Sepsis-2 criteria, the NLCR showed a moderate AUC (0.68), nonetheless significantly higher than all the other single biomarkers (Fig 4C). The diagnostic discriminatory power is usually improved when combining information from several biomarkers [68, 69], but here it can be observed that the NLCR had an ability equal to both composite biomarkers for diagnosing bacterial sepsis. The NLCR also demonstrated a high sensitivity (96%, Tables 2 and 3) at a cut-off of 3.0 for diagnosis of bacterial sepsis as well as severe bacterial sepsis or septic shock using Sepsis-2 criteria. This suggests that low NLCR values (<3.0) can be used to exclude bacteria as the etiological cause in critically ill patients.
PCT has been investigated in many studies previously and the outcomes have been contradicting [20–38]. One reason could be that many studies have had relatively small sample sizes [70–72] which may undermine the reliability of the results [73]. In addition, only a minority of the previous studies have formally excluded patients treated with antibiotics before inclusion. This may result in false-negative results due to possible antibiotic treatment before inclusion, leading to underestimation of the effect. Besides small sample sizes and antibiotic treatment before inclusion, other factors such as differences in target population, type of clinical setting, cut-off value, disease prevalence, assay type, and disease severity could also have contributed to the conflicting results in previous studies. In the present study, PCT showed a high specificity (96%, Tables 2 and 3) at a cut-off of 10.0 ng/mL for diagnosis of bacterial sepsis and severe bacterial sepsis or septic shock using Sepsis-2 criteria. This means that patients with a PCT concentration of 10 ng/mL or higher, are likely to have bacterial sepsis. The numerous factors that may affect the diagnostic performance of a biomarker and thereby contributing to contradicting results also make it difficult to directly compare outcomes between studies. However, the sensitivities for PCT in sepsis diagnostics described in the present study (Table 2) are generally lower compared with previous findings [35, 37]. This is consistent with the reflection made in a review by Kibe et al., who noted that larger studies tend to find lower estimates of PCT sensitivity than smaller studies [73]. For instance, using a cut-off of 0.5 ng/mL for PCT generated a sensitivity of 44% (S1 Table) whereas the sensitivities reported by others usually are in the range 60–90% [74–78]. Also the sensitivity of 26% at a cut-off of 2.0 ng/mL (S1 Table) is considerably lower than described in other studies where the sensitivity typically ranges between 65% and 97% [71, 79, 80].
The results from the present study suggest lactate as the single best-performing biomarker in diagnosing severe bacterial sepsis and septic shock based on Sepsis-2 criteria (Table 3). Using a cut-off of 3.5 mmol/L for lactate results in a specificity of 97%, an accuracy of 92% and a DOR of 56.23. This result is not surprising as lactate >3.5 mmol/L is included in the criteria for severe sepsis (S1 Text) and might therefore be due to incorporation bias. Although lactate is widely used for early detection of sepsis, elevated levels of lactate are not considered specific for diagnosis of sepsis [40]. Lactate has proven more valuable as prognostic sepsis biomarker as elevated lactate levels have been associated with high mortality in several studies [81–83]. Both composite biomarkers had significantly higher AUCs than all the single biomarkers including lactate in diagnosing severe bacterial sepsis and septic shock (Fig 4D) which further suggest the need of a joint interpretation of several biomarkers in sepsis diagnostics.
The present study demonstrate that combinations of biomarkers appears to be a useful approach to improve the diagnostic accuracy for bacterial sepsis, irrespectively of using Sepsis-2 or Sepsis-3 criteria (Fig 4). For all diagnostic categories investigated, except for bacterial sepsis defined according Sepsis-2 criteria, the four-biomarker had a higher AUC than all single biomarkers. A major challenge in using multimarker approaches is the translation of several measurements into one composite variable. In this study, we employed different methods (e.g., linear discriminant analysis, logistic regression, and additive composite variable based on normalized values) to combine several biomarkers into one variable. As the diagnostic ability were found to be equivalent for all three types of composite variables, we chose to only report the results of those composite biomarkers developed using linear discriminant analysis as a linear combination of variable can easily be adopted for clinical use. Other drawbacks of multimarker approaches may include high cost and less availability in comparison with single biomarker as the NLCR for example, and thus limiting their utility, especially in resource-limited settings. In the future we foresee that much more complex multimarker combinations will be developed for diagnostic purposes and thus will probably require digital support for the interpretation.
Several limitations of this study merit consideration. First, there is no gold standard test by which to diagnose bacterial sepsis. We therefore used a strict definition of bacterial sepsis and only included episodes with verified bacterial infection, i.e., either clinical infection with a positive culture or typical clinical symptoms such as erysipelas. However, many patients do have bacterial infections without being microbiologically proven. So most probably a part of the episodes categorised as ‘others’, i.e., non-verified bacterial infections, viral infections and no infections, had a bacterial infection and this may have negatively affected the diagnostic performance. Secondly, this study was performed prior the introduction of the new sepsis definition, Sepsis-3 [1]. We assessed the diagnostic accuracy of the biomarkers using this new definition as well, but were only able to assess it for the diagnosis of bacterial sepsis, not bacterial septic shock, based on available data. Finally, it is probably an oversimplification to use linear models for combining biomarkers. The diagnostic accuracy might be further improved by using non-linear approaches.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability
All data is available from the Zenodo database (https://doi.org/10.5281/zenodo.823967).
Funding Statement
This study was supported by research grants from the Swedish Knowledge Foundation (grant no20130307, www.kks.se), regional funds in Västra Götaland (www.vgregion.se), internal Clinical Research Fund Skaraborg Hospital (http://www.vgregion.se/s/skaraborgs-sjukhus/om-skaraborgs-sjukhus/forskning-och-utveckling/beredningsgrupp/), and internal Unilabs R&D Fund (www.unilabs.se). The funding bodies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama. 2016;315(8):801–10. Epub 2016/02/24. doi: 10.1001/jama.2016.0287 ; PubMed Central PMCID: PMCPMC4968574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. Epub 2013/01/31. doi: 10.1007/s00134-012-2769-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar A, Ellis P, Arabi Y, Roberts D, Light B, Parrillo JE, et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest. 2009;136(5):1237–48. Epub 2009/08/22. doi: 10.1378/chest.09-0087 . [DOI] [PubMed] [Google Scholar]
- 4.Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med. 2017;376(23):2235–44. Epub 2017/05/23. doi: 10.1056/NEJMoa1703058 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig JC, Williams GJ, Jones M, Codarini M, Macaskill P, Hayen A, et al. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: prospective cohort study of 15 781 febrile illnesses. Bmj. 2010;340:c1594 Epub 2010/04/22. doi: 10.1136/bmj.c1594 ; PubMed Central PMCID: PMCPmc2857748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13(12):1057–98. Epub 2013/11/21. doi: 10.1016/S1473-3099(13)70318-9 . [DOI] [PubMed] [Google Scholar]
- 7.Schechner V, Temkin E, Harbarth S, Carmeli Y, Schwaber MJ. Epidemiological interpretation of studies examining the effect of antibiotic usage on resistance. Clin Microbiol Rev. 2013;26(2):289–307. Epub 2013/04/05. doi: 10.1128/CMR.00001-13 ; PubMed Central PMCID: PMCPmc3623381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watkins RR, Bonomo RA. Overview: Global and Local Impact of Antibiotic Resistance. Infectious disease clinics of North America. 2016;30(2):313–22. Epub 2016/05/22. doi: 10.1016/j.idc.2016.02.001 . [DOI] [PubMed] [Google Scholar]
- 9.Trubiano J, Phillips E. Antimicrobial stewardship's new weapon? A review of antibiotic allergy and pathways to 'de-labeling'. Current opinion in infectious diseases. 2013;26(6):526–37. Epub 2013/10/16. doi: 10.1097/QCO.0000000000000006 ; PubMed Central PMCID: PMCPmc3862073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surawicz CM, McFarland LV. Pseudomembranous colitis: causes and cures. Digestion. 1999;60(2):91–100. Epub 1999/03/30. doi: 7633. . [DOI] [PubMed] [Google Scholar]
- 11.Bruniera FR, Ferreira FM, Saviolli LR, Bacci MR, Feder D, da Luz Goncalves Pedreira M, et al. The use of vancomycin with its therapeutic and adverse effects: a review. European review for medical and pharmacological sciences. 2015;19(4):694–700. Epub 2015/03/11. . [PubMed] [Google Scholar]
- 12.Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis. 2003;36(11):1418–23. Epub 2003/05/27. doi: 10.1086/375057 . [DOI] [PubMed] [Google Scholar]
- 13.Meehan TP, Fine MJ, Krumholz HM, Scinto JD, Galusha DH, Mockalis JT, et al. Quality of care, process, and outcomes in elderly patients with pneumonia. Jama. 1997;278(23):2080–4. Epub 1997/12/24. . [PubMed] [Google Scholar]
- 14.Proulx N, Frechette D, Toye B, Chan J, Kravcik S. Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM: monthly journal of the Association of Physicians. 2005;98(4):291–8. Epub 2005/03/12. doi: 10.1093/qjmed/hci047 . [DOI] [PubMed] [Google Scholar]
- 15.Van Zuijlen DA, Schilder AG, Van Balen FA, Hoes AW. National differences in incidence of acute mastoiditis: relationship to prescribing patterns of antibiotics for acute otitis media? Pediatr Infect Dis J. 2001;20(2):140–4. Epub 2001/02/27. . [DOI] [PubMed] [Google Scholar]
- 16.Zasowski EJ, Claeys KC, Lagnf AM, Davis SL, Rybak MJ. Time Is of the Essence: The Impact of Delayed Antibiotic Therapy on Patient Outcomes in Hospital-Onset Enterococcal Bloodstream Infections. Clin Infect Dis. 2016;62(10):1242–50. Epub 2016/03/06. doi: 10.1093/cid/ciw110 ; PubMed Central PMCID: PMCPmc4845789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14(1):R15 Epub 2010/02/11. doi: 10.1186/cc8872 ; PubMed Central PMCID: PMC2875530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sankar V, Webster NR. Clinical application of sepsis biomarkers. J Anesth. 2013;27(2):269–83. Epub 2012/10/31. doi: 10.1007/s00540-012-1502-7 . [DOI] [PubMed] [Google Scholar]
- 19.O'Grady NP, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC, et al. Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36(4):1330–49. Epub 2008/04/02. doi: 10.1097/CCM.0b013e318169eda9 . [DOI] [PubMed] [Google Scholar]
- 20.Hoenigl M, Raggam RB, Wagner J, Prueller F, Grisold AJ, Leitner E, et al. Procalcitonin fails to predict bacteremia in SIRS patients: a cohort study. International journal of clinical practice. 2014;68(10):1278–81. Epub 2014/06/06. doi: 10.1111/ijcp.12474 . [DOI] [PubMed] [Google Scholar]
- 21.Jeong S, Park Y, Cho Y, Kim HS. Diagnostic utilities of procalcitonin and C-reactive protein for the prediction of bacteremia determined by blood culture. Clin Chim Acta. 2012;413(21–22):1731–6. Epub 2012/07/05. doi: 10.1016/j.cca.2012.06.030 . [DOI] [PubMed] [Google Scholar]
- 22.Kim MH, Lim G, Kang SY, Lee WI, Suh JT, Lee HJ. Utility of procalcitonin as an early diagnostic marker of bacteremia in patients with acute fever. Yonsei medical journal. 2011;52(2):276–81. Epub 2011/02/15. doi: 10.3349/ymj.2011.52.2.276 ; PubMed Central PMCID: PMCPmc3051230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakajima A, Yazawa J, Sugiki D, Mizuguchi M, Sagara H, Fujisiro M, et al. Clinical utility of procalcitonin as a marker of sepsis: a potential predictor of causative pathogens. Internal medicine (Tokyo, Japan). 2014;53(14):1497–503. Epub 2014/07/18. . [DOI] [PubMed] [Google Scholar]
- 24.Oussalah A, Ferrand J, Filhine-Tresarrieu P, Aissa N, Aimone-Gastin I, Namour F, et al. Diagnostic Accuracy of Procalcitonin for Predicting Blood Culture Results in Patients With Suspected Bloodstream Infection: An Observational Study of 35,343 Consecutive Patients (A STROBE-Compliant Article). Medicine. 2015;94(44):e1774 Epub 2015/11/12. doi: 10.1097/MD.0000000000001774 ; PubMed Central PMCID: PMCPmc4915876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leli C, Ferranti M, Marrano U, Al Dhahab ZS, Bozza S, Cenci E, et al. Diagnostic accuracy of presepsin (sCD14-ST) and procalcitonin for prediction of bacteraemia and bacterial DNAaemia in patients with suspected sepsis. J Med Microbiol. 2016;65(8):713–9. Epub 2016/05/14. doi: 10.1099/jmm.0.000278 . [DOI] [PubMed] [Google Scholar]
- 26.Mat-Nor MB, Md Ralib A, Abdulah NZ, Pickering JW. The diagnostic ability of procalcitonin and interleukin-6 to differentiate infectious from noninfectious systemic inflammatory response syndrome and to predict mortality. Journal of critical care. 2016;33:245–51. Epub 2016/02/07. doi: 10.1016/j.jcrc.2016.01.002 . [DOI] [PubMed] [Google Scholar]
- 27.Anand D, Das S, Bhargava S, Srivastava LM, Garg A, Tyagi N, et al. Procalcitonin as a rapid diagnostic biomarker to differentiate between culture-negative bacterial sepsis and systemic inflammatory response syndrome: a prospective, observational, cohort study. Journal of critical care. 2015;30(1):218.e7–12. Epub 2014/09/30. doi: 10.1016/j.jcrc.2014.08.017 . [DOI] [PubMed] [Google Scholar]
- 28.Dou YH, Du JK, Liu HL, Shong XD. The role of procalcitonin in the identification of invasive fungal infection-a systemic review and meta-analysis. Diagnostic microbiology and infectious disease. 2013;76(4):464–9. Epub 2013/05/29. doi: 10.1016/j.diagmicrobio.2013.04.023 . [DOI] [PubMed] [Google Scholar]
- 29.Garnacho-Montero J, Huici-Moreno MJ, Gutierrez-Pizarraya A, Lopez I, Marquez-Vacaro JA, Macher H, et al. Prognostic and diagnostic value of eosinopenia, C-reactive protein, procalcitonin, and circulating cell-free DNA in critically ill patients admitted with suspicion of sepsis. Crit Care. 2014;18(3):R116 Epub 2014/06/07. doi: 10.1186/cc13908 ; PubMed Central PMCID: PMCPmc4229882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hur M, Kim H, Lee S, Cristofano F, Magrini L, Marino R, et al. Diagnostic and prognostic utilities of multimarkers approach using procalcitonin, B-type natriuretic peptide, and neutrophil gelatinase-associated lipocalin in critically ill patients with suspected sepsis. BMC Infect Dis. 2014;14:224 Epub 2014/04/26. doi: 10.1186/1471-2334-14-224 ; PubMed Central PMCID: PMCPmc4006080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain S, Sinha S, Sharma SK, Samantaray JC, Aggrawal P, Vikram NK, et al. Procalcitonin as a prognostic marker for sepsis: a prospective observational study. BMC research notes. 2014;7:458 Epub 2014/07/19. doi: 10.1186/1756-0500-7-458 ; PubMed Central PMCID: PMCPmc4105100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen JU, Hein L, Lundgren B, Bestle MH, Mohr TT, Andersen MH, et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med. 2011;39(9):2048–58. Epub 2011/05/17. doi: 10.1097/CCM.0b013e31821e8791 . [DOI] [PubMed] [Google Scholar]
- 33.Karlsson S, Heikkinen M, Pettila V, Alila S, Vaisanen S, Pulkki K, et al. Predictive value of procalcitonin decrease in patients with severe sepsis: a prospective observational study. Crit Care. 2010;14(6):R205 Epub 2010/11/17. doi: 10.1186/cc9327 ; PubMed Central PMCID: PMCPmc3219988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock—a systematic review and meta-analysis. Crit Care. 2013;17(6):R291 Epub 2013/12/18. doi: 10.1186/cc13157 ; PubMed Central PMCID: PMCPMC4056085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis. 2007;7(3):210–7. Epub 2007/02/24. doi: 10.1016/S1473-3099(07)70052-X . [DOI] [PubMed] [Google Scholar]
- 36.Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med. 2006;34(7):1996–2003. Epub 2006/05/23. doi: 10.1097/01.CCM.0000226413.54364.36 . [DOI] [PubMed] [Google Scholar]
- 37.Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(5):426–35. Epub 2013/02/05. doi: 10.1016/S1473-3099(12)70323-7 . [DOI] [PubMed] [Google Scholar]
- 38.van Nieuwkoop C, Bonten TN, van't Wout JW, Kuijper EJ, Groeneveld GH, Becker MJ, et al. Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: a prospective observational study. Crit Care. 2010;14(6):R206 Epub 2010/11/19. doi: 10.1186/cc9328 ; PubMed Central PMCID: PMCPmc3220019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clec'h C, Ferriere F, Karoubi P, Fosse JP, Cupa M, Hoang P, et al. Diagnostic and prognostic value of procalcitonin in patients with septic shock. Crit Care Med. 2004;32(5):1166–9. Epub 2004/06/12. . [DOI] [PubMed] [Google Scholar]
- 40.Fan SL, Miller NS, Lee J, Remick DG. Diagnosing sepsis—The role of laboratory medicine. Clin Chim Acta. 2016;460:203–10. Epub 2016/07/09. doi: 10.1016/j.cca.2016.07.002 ; PubMed Central PMCID: PMCPMC4980259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zahorec R. Ratio of neutrophil to lymphocyte counts—rapid and simple parameter of systemic inflammation and stress in critically ill. Bratislavske lekarske listy. 2001;102(1):5–14. Epub 2001/11/29. . [PubMed] [Google Scholar]
- 42.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. Epub 1985/10/01. . [PubMed] [Google Scholar]
- 43.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10. Epub 1996/07/01. . [DOI] [PubMed] [Google Scholar]
- 44.de Jager CP, van Wijk PT, Mathoera RB, de Jongh-Leuvenink J, van der Poll T, Wever PC. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care. 2010;14(5):R192 Epub 2010/11/03. doi: 10.1186/cc9309 ; PubMed Central PMCID: PMCPmc3219299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terradas R, Grau S, Blanch J, Riu M, Saballs P, Castells X, et al. Eosinophil count and neutrophil-lymphocyte count ratio as prognostic markers in patients with bacteremia: a retrospective cohort study. PLoS One. 2012;7(8):e42860 Epub 2012/08/23. doi: 10.1371/journal.pone.0042860 ; PubMed Central PMCID: PMCPmc3415420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loonen AJ, Bos MP, van Meerbergen B, Neerken S, Catsburg A, Dobbelaer I, et al. Comparison of pathogen DNA isolation methods from large volumes of whole blood to improve molecular diagnosis of bloodstream infections. PLoS One. 2013;8(8):e72349 Epub 2013/08/27. doi: 10.1371/journal.pone.0072349 ; PubMed Central PMCID: PMC3744477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ljungström L, Karlsson D, Pernestig A, Andersson R, Jacobsson G. Neutrophil to lymphocyte count ratio performs better than procalcitonin as a biomarker for bacteremia and severe sepsis in the emergency department. Critical Care. 2015;19(1):P66 doi: 10.1186/cc14146 [Google Scholar]
- 48.Loonen AJ, de Jager CP, Tosserams J, Kusters R, Hilbink M, Wever PC, et al. Biomarkers and molecular analysis to improve bloodstream infection diagnostics in an emergency care unit. PLoS One. 2014;9(1):e87315 Epub 2014/01/30. doi: 10.1371/journal.pone.0087315 ; PubMed Central PMCID: PMCPmc3903623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shapiro NI, Trzeciak S, Hollander JE, Birkhahn R, Otero R, Osborn TM, et al. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med. 2009;37(1):96–104. Epub 2008/12/04. doi: 10.1097/CCM.0b013e318192fd9d . [DOI] [PubMed] [Google Scholar]
- 50.Ljungström L, Steinum O, Brink M, Gårdlund B, Martner J, Sjölin J. Diagnostik och diagnoskodning av svår sepsis och septisk shock. Läkartidningen 2011;108(8):276–8. [PubMed] [Google Scholar]
- 51.Povoa P, Almeida E, Moreira P, Fernandes A, Mealha R, Aragao A, et al. C-reactive protein as an indicator of sepsis. Intensive Care Med. 1998;24(10):1052–6. Epub 1998/12/05. . [DOI] [PubMed] [Google Scholar]
- 52.Reny JL, Vuagnat A, Ract C, Benoit MO, Safar M, Fagon JY. Diagnosis and follow-up of infections in intensive care patients: value of C-reactive protein compared with other clinical and biological variables. Crit Care Med. 2002;30(3):529–35. Epub 2002/05/07. . [DOI] [PubMed] [Google Scholar]
- 53.Svenska Infektionsläkarföreningen Ps. Vårdprogram Svår sepsis och septisk chock. 2015.
- 54.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. Epub 1988/09/01. . [PubMed] [Google Scholar]
- 55.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC bioinformatics. 2011;12:77 Epub 2011/03/19. doi: 10.1186/1471-2105-12-77 ; PubMed Central PMCID: PMCPmc3068975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Hou JH, Li Q, Chen KJ, Wang SN, Wang JM. Biomarkers for diagnosis of sepsis in patients with systemic inflammatory response syndrome: a systematic review and meta-analysis. SpringerPlus. 2016;5(1):2091 Epub 2016/12/29. doi: 10.1186/s40064-016-3591-5 ; PubMed Central PMCID: PMCPMC5153391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benz F, Roy S, Trautwein C, Roderburg C, Luedde T. Circulating MicroRNAs as Biomarkers for Sepsis. International journal of molecular sciences. 2016;17(1). Epub 2016/01/14. doi: 10.3390/ijms17010078 ; PubMed Central PMCID: PMCPMC4730322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ludwig KR, Hummon AB. Mass spectrometry for the discovery of biomarkers of sepsis. Molecular bioSystems. 2017. Epub 2017/02/17. doi: 10.1039/c6mb00656f . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma Y, Vilanova D, Atalar K, Delfour O, Edgeworth J, Ostermann M, et al. Genome-wide sequencing of cellular microRNAs identifies a combinatorial expression signature diagnostic of sepsis. PLoS One. 2013;8(10):e75918 Epub 2013/10/23. doi: 10.1371/journal.pone.0075918 ; PubMed Central PMCID: PMCPMC3797812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oved K, Cohen A, Boico O, Navon R, Friedman T, Etshtein L, et al. A novel host-proteome signature for distinguishing between acute bacterial and viral infections. PLoS One. 2015;10(3):e0120012 Epub 2015/03/19. doi: 10.1371/journal.pone.0120012 ; PubMed Central PMCID: PMCPMC4364938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McHugh L, Seldon TA, Brandon RA, Kirk JT, Rapisarda A, Sutherland AJ, et al. A Molecular Host Response Assay to Discriminate Between Sepsis and Infection-Negative Systemic Inflammation in Critically Ill Patients: Discovery and Validation in Independent Cohorts. PLoS medicine. 2015;12(12):e1001916 Epub 2015/12/10. doi: 10.1371/journal.pmed.1001916 ; PubMed Central PMCID: PMCPMC4672921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gibson PH, Croal BL, Cuthbertson BH, Small GR, Ifezulike AI, Gibson G, et al. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. American heart journal. 2007;154(5):995–1002. Epub 2007/10/31. doi: 10.1016/j.ahj.2007.06.043 . [DOI] [PubMed] [Google Scholar]
- 63.Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2008;34(1):55–60. Epub 2007/04/24. doi: 10.1016/j.ejso.2007.02.014 . [DOI] [PubMed] [Google Scholar]
- 64.Halazun KJ, Hardy MA, Rana AA, Woodland DCt, Luyten EJ, Mahadev S, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Annals of surgery. 2009;250(1):141–51. Epub 2009/06/30. doi: 10.1097/SLA.0b013e3181a77e59 . [DOI] [PubMed] [Google Scholar]
- 65.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. The Journal of thoracic and cardiovascular surgery. 2009;137(2):425–8. Epub 2009/02/03. doi: 10.1016/j.jtcvs.2008.05.046 . [DOI] [PubMed] [Google Scholar]
- 66.Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. The American journal of cardiology. 2008;102(6):653–7. Epub 2008/09/09. doi: 10.1016/j.amjcard.2008.05.006 . [DOI] [PubMed] [Google Scholar]
- 67.Wei B, Yao M, Xing C, Wang W, Yao J, Hong Y, et al. The neutrophil lymphocyte ratio is associated with breast cancer prognosis: an updated systematic review and meta-analysis. OncoTargets and therapy. 2016;9:5567–75. Epub 2016/09/24. doi: 10.2147/OTT.S108419 ; PubMed Central PMCID: PMCPmc5021064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kofoed K, Schneider UV, Scheel T, Andersen O, Eugen-Olsen J. Development and validation of a multiplex add-on assay for sepsis biomarkers using xMAP technology. Clin Chem. 2006;52(7):1284–93. Epub 2006/05/13. doi: 10.1373/clinchem.2006.067595 . [DOI] [PubMed] [Google Scholar]
- 69.Pernestig A, Ljungström, L., Karlsson, D. Multimarker approach for sepsis diagnostics. European Congress of Clinical Mircobiology and Infectious Diseases; Barcelona, Spain 2015.
- 70.Arkader R, Troster EJ, Lopes MR, Junior RR, Carcillo JA, Leone C, et al. Procalcitonin does discriminate between sepsis and systemic inflammatory response syndrome. Archives of disease in childhood. 2006;91(2):117–20. Epub 2005/12/06. doi: 10.1136/adc.2005.077446 ; PubMed Central PMCID: PMCPmc2082702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meynaar IA, Droog W, Batstra M, Vreede R, Herbrink P. In Critically Ill Patients, Serum Procalcitonin Is More Useful in Differentiating between Sepsis and SIRS than CRP, Il-6, or LBP. Crit Care Res Pract. 2011;2011:594645 Epub 2011/06/21. doi: 10.1155/2011/594645 ; PubMed Central PMCID: PMC3113363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naeini AE, Montazerolghaem S. Procalcitonin marker for sepsis diagnosis. Evaluating a rapid immuno-chromatografic test. Saudi medical journal. 2006;27(3):422–4. Epub 2006/03/15. . [PubMed] [Google Scholar]
- 73.Kibe S, Adams K, Barlow G. Diagnostic and prognostic biomarkers of sepsis in critical care. The Journal of antimicrobial chemotherapy. 2011;66 Suppl 2:ii33–40. Epub 2011/03/16. doi: 10.1093/jac/dkq523 . [DOI] [PubMed] [Google Scholar]
- 74.Aikawa N, Fujishima S, Endo S, Sekine I, Kogawa K, Yamamoto Y, et al. Multicenter prospective study of procalcitonin as an indicator of sepsis. Journal of infection and chemotherapy: official journal of the Japan Society of Chemotherapy. 2005;11(3):152–9. Epub 2005/07/02. doi: 10.1007/s10156-005-0388-9 . [DOI] [PubMed] [Google Scholar]
- 75.Al-Nawas B, Krammer I, Shah PM. Procalcitonin in diagnosis of severe infections. European journal of medical research. 1996;1(7):331–3. Epub 1996/04/18. . [PubMed] [Google Scholar]
- 76.Bossink AW, Groeneveld AB, Thijs LG. Prediction of microbial infection and mortality in medical patients with fever: plasma procalcitonin, neutrophilic elastase-alpha1-antitrypsin, and lactoferrin compared with clinical variables. Clin Infect Dis. 1999;29(2):398–407. Epub 1999/09/07. doi: 10.1086/520222 . [DOI] [PubMed] [Google Scholar]
- 77.Latour-Perez J, Alcala-Lopez A, Garcia-Garcia MA, Sanchez-Hernandez JF, Abad-Terrado C, Viedma-Contreras JA, et al. Diagnostic accuracy of sTREM-1 to identify infection in critically ill patients with systemic inflammatory response syndrome. Clinical biochemistry. 2010;43(9):720–4. Epub 2010/03/23. doi: 10.1016/j.clinbiochem.2010.03.001 . [DOI] [PubMed] [Google Scholar]
- 78.Oshita H, Sakurai J, Kamitsuna M. Semi-quantitative procalcitonin test for the diagnosis of bacterial infection: clinical use and experience in Japan. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2010;43(3):222–7. Epub 2011/02/05. doi: 10.1016/S1684-1182(10)60035-7 . [DOI] [PubMed] [Google Scholar]
- 79.Sakr Y, Burgett U, Nacul FE, Reinhart K, Brunkhorst F. Lipopolysaccharide binding protein in a surgical intensive care unit: a marker of sepsis? Crit Care Med. 2008;36(7):2014–22. Epub 2008/06/17. doi: 10.1097/CCM.0b013e31817b86e3 . [DOI] [PubMed] [Google Scholar]
- 80.Suprin E, Camus C, Gacouin A, Le Tulzo Y, Lavoue S, Feuillu A, et al. Procalcitonin: a valuable indicator of infection in a medical ICU? Intensive Care Med. 2000;26(9):1232–8. Epub 2000/11/23. . [DOI] [PubMed] [Google Scholar]
- 81.Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33(11):1892–9. Epub 2007/07/10. doi: 10.1007/s00134-007-0680-5 . [DOI] [PubMed] [Google Scholar]
- 82.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37(5):1670–7. Epub 2009/03/28. doi: 10.1097/CCM.0b013e31819fcf68 . [DOI] [PubMed] [Google Scholar]
- 83.Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Annals of emergency medicine. 2005;45(5):524–8. Epub 2005/04/28. doi: 10.1016/j.annemergmed.2004.12.006 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All data is available from the Zenodo database (https://doi.org/10.5281/zenodo.823967).