Abstract
WRKY transcription factors play pivotal roles in regulation of stress responses. This study identified 79 WRKY genes in potato (Solanum tuberosum). Based on multiple sequence alignment and phylogenetic relationships, WRKY genes were classified into three major groups. The majority of WRKY genes belonged to Group II (52 StWRKYs), Group III had 14 and Group I consisted of 13. The phylogenetic tree further classified Group II into five sub-groups. All StWRKY genes except StWRKY79 were mapped on potato chromosomes, with eight tandem duplication gene pairs and seven segmental duplication gene pairs found from StWRKY family genes. The expression analysis of 22 StWRKYs showed their differential expression levels under various stress conditions. Cis-element prediction showed that a large number of elements related to drought, heat and salicylic acid were present in the promotor regions of StWRKY genes. The expression analysis indicated that seven StWRKYs seemed to respond to stress (heat, drought and salinity) and salicylic acid treatment. These genes are candidates for abiotic stress signaling for further research.
Introduction
Transcription factors (TFs) participate in gene transcription regulatory networks that regulate gene expression in plants. In the plant genome, a large number of genes encode TFs[1].
The WRKY TF family, named because of containing the WRKY domain, exists widely in many organisms [2]. The WRKY domain is a highly conserved sequence of 60 amino acids [3], heptapeptide WRKYGQK which also has WRKYGKK, WKKYGQK, WRKYGQR, WRKYGEK and some other forms [4, 5], and a zinc finger structure. which is itself divided into two types, C2H2 and C2HC [6]. According to the number of WRKY domains and the type of zinc finger, the WRKY family is divided into three main groups [7]. Group I contains two WRKY domains located in the C- and N-terminus, respectively. The other two groups have just one WRKY domain. Groups I and II have the C2H2-type zinc finger, and only Group III has the C2HC-type. In Arabidopsis thaliana, Group II genes are also divided into five sub-groups: II-a, II-b, II-c, II-d and II [8].
The first WRKY TF, which was reported to be a DNA binding protein, SPF1, was cloned from sweet potato [9] and WRKY TF families have since identified [10]. Previous studies identified at least 72 WRKY family genes in Arabidopsis thaliana [8], about 100 in Populus [11], 59 in Vitis vinifera [12], 81 in Solanum lycopersicum [13] and 71 in Capsicum annuum [14]. Previous studies of WRKY family evolution showed that Group I was an ancestral group. The descent of Group II and III were origin from group I which were lack of an N-terminal WRKY domain [15].
Many studies have shown that WRKY gene family members are related to plant development processes, such as fiber development [16] and leaf senescence in cotton [17]. Some researchers showed that the WRKY gene family members were related to development of the embryo and anther [18, 19]. They were also played roles in plant stresses. WRKY genes can defend against infections by bacteria [20], fungi [11] and viruses [20] and provide resistance to cold [12], salt [21], drought [22] and wounding [23]. The research indicated that 11 OsWRKY genes were responses to salt, drought, cold and heat stresses by Qin [24]. Similarly, several GmWRKY genes through the transgenic Arabidopsis plants to improve the resistance in these stresses [25]. Identification of WRKY genes function in stresses could increase stress resistance to breed new cultivars.
There has been a little relevant research on the WRKY family in the potato. As one of the popular foods in the world, especially in Europe and America, many potatoes are consumed each year. To satisfy this demand, enhancing yield is increasingly important. Studies of planting microenvironment have shown that abiotic and biotic stresses are important factors restraining potato yield [26]. However, StWRKY1 has only been researched by Yogendra [27] and Shahzad [28]. And some WRKY genes related to arbuscular mycorrhizal potato root colonization have been reported [29]. In 2016, Yogendra showed the StWRKY8 was related to blight [30].As a stress-related gene family, the possibility of using them to improve the stress resistance and adaptation should be researched. This study aimed to survey the information about WRKY genes in S. tuberosum and the expression for several StWRKY genes under stress conditions.
Materials and methods
Database sequence searches
The potato protein and gene sequences were downloaded from the PGSC database (http://solanaceae.plantbiology.msu.edu/pgsc_download.shtml). The Arabidopsis WRKY and S. lycopersicum WRKY locus numbers were acquired from papers by Eulgem [8] and Huang [13], respectively. The WRKY protein sequences were obtained from the TAIR (https://www.arabidopsis.org/tools/bulk/sequences/index.jsp) and plantTFDB (http://planttfdb.cbi.pku.edu.cn/) websites.
AtWRKY and SlWRKY protein sequences were used as queries to search the Solanum tuberosum WRKY family members using BLASTP [31]. To achieve accurate results, the E value was set at 10. Then the website NCBI-CDD (http://www.ncbi.nlm.nih.gov/cdd) was used to analyze the conserved sequences and to remove sequences that lacked WRKY annotation. The software cluster X [32, 33] was used to ensure existence of the WRKYGQK conserved domain or similar sequences.
Mapping potato WRKY genes on chromosomes
Relevant information on all identified StWRKYs was found on the PGSC website (http://solanaceae.plantbiology.msu.edu/index.shtml). The software MapChart2.30 was used to map the gene locus on chromosomes. Tandem duplication of StWRKYs was identified according the method of Cheng [3].Two or more WRKY genes within a distancs of 100 kb were regarded as tandem duplications [3, 34]. Information on segmental duplications was sourced from the PGDD website (http://chibba.agtec.uga.edu/duplication/), and segmental duplications were displayed using Circos software.
Constructing the phylogenetic tree and classification of WRKY members
To build the StWRKY phylogenetic tree and classify them into groups required information on some other plants’ WRKY proteins. The Arabidopsis, C. annuum and S. lycopersicum WRKY proteins were acquired from other research [8, 13, 35]. All of these protein sequences were assessed with Clustal X software and complete alignment performed. Then, the phylogenetic tree was constructed by Test Neighbor-Joining Tree Method [36] using MEGA6.0 software. DNAMAN software was used to analyze sequence alignments and identify StWRKY.
Structures of WRKY members
The exon-intron structures of potato WRKY genes were determined using the GSDS2.0 website (http://gsds.cbi.pku.edu.cn/index.php) [37]. The motifs were analyzed using the MEME website (http://meme-suite.org/index.html) [4]. In total, 20 motifs were searched per member.
Plant growth conditions and treatments
The potato material was a sequenced double-haploid variety, DM1-3-516-R44. Virus-free seedlings were grown in a plant incubator at 25±1°C under a 10 000 Lx in light/dark for 16/8 h with solid Murashige & Skoog Basal Medium with Vitamins (MS, USA) culture medium for 4 weeks. Then the seedling transferred into MS liquid medium.
Potential heat-, drought- and salt- related WRKY genes in potato were identified in potato. 150 mM NaCl was added for salt treatment, and 260 mM Mannitol was added for drought-treatment. The seedlings were maintained at 35°C for heat-treatment. For the biotic stress, salicaylic said (SA) was sprayed on potato leaf surfaces after growing in greenhouse for 4 weeks. Bring the treatment-plants into culture dish with SA liquid (10 μM in water) filter paper. All processing materials were collected at 3 and 24 h.
Quantitative real-time PCR (qRT-PCR) analysis
After 3 h and 24 h plant shoot were harvested and total RNA was isolated from the various treated samples using the Plant RNA Purification Reagent (Invitrogen, USA) And cDNA was synthesized using thePrimeScript reagent kit (Takara, Japan). In general, the final concentration of cDNA was diluted five-fold for use. Primer Premier 5 software designed the gene-specific primers for qRT-PCR reactions. Bio-Rad Real-Quantitative real-time PCR analysis Time System (CFX96, USA) was used to analysis which was based on three technical replicates. The expression levels of gene from the diverse treatment were normalized using a reference gene, elongation factor 1-a (ef1a)[38]. The relative expression levels were analyzed from the method of Haoli Ma [39].
Cis-element analysis of StWRKY genes
The gene upstream sequences (approximately 1500bp) of 22 StWRKYs were searched in the Phytozome 12 website (https://phytozome.jgi.doe.gov/pz/portal.html), and cis-elements were acquired from the plantCARE website (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). The mainly focused in ABRE (cis-acting element involved in the abscisic acid responsiveness), HSE (cis-acting element involved in heat stress responsiveness), MBS (MYB binding site involved in drought-inducibility), TC-rich repeats (cis-acting element involved in defense and stress responsiveness), TCA-element (cis-acting element involved in salicylic acid responsiveness) and W-box (WRKY binding site) [35].
Results
Identification of StWRKY family genes
To identify the WRKY TF genes in potato, the nonredundant proteins were used to identify the WRKY family members through BLASTP search. A total of 761 unique sequences had high similarity with Arabidopsis WRKY genes and 147 unique sequences had high similarity with tomato WRKY genes. Following the analysis for NCBI-CDD website, 129 sequences were acquired as the putative WRKY members. However, this result contained the non-representative sequences. Furthermore, WRKY family members should contain a conserved heptapeptide [40], but sequences alignment showed that some sequences did not have a WRKYGQK or WRKYGQK-like conserved domain [4, 40]. Following ClustalX analysis of full-length protein sequences, 79 unique sequences were acquired and relevant information obtained (Table 1). Although the WRKYGQK motif was a highly conserved domain, but 12 genes were also existed a part of variations in amino acids (Table 1).
Table 1. Identification of WRKY genes in potato.
| NAME | Gene locus | chromosome Location | WRKY domain | Group | PI | Mw (KD) | Protein length(aa) | CDS length(bp) | Extron | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conserved heptapeptide | Zinc-finger type | Domain number | |||||||||
| StWRKY01 | PGSC0003DMG400011457 | chr01 | WRKYGQK | C2H2 | 1 | IIc | 4.97 | 37.28 | 339 | 1020 | 3 |
| StWRKY02 | PGSC0003DMG400009014 | chr01 | WRKYGQK | C2H2 | 1 | IIe | 5.2 | 27.22 | 244 | 735 | 3 |
| StWRKY03 | PGSC0003DMG400009051 | chr01 | WRKYGQK | C2H2 | 1 | IIc | 6.07 | 35.88 | 317 | 954 | 3 |
| StWRKY04 | PGSC0003DMG400031175 | chr01 | WRKYGQK | C2H2 | 1 | IIc | 8.16 | 27.50 | 251 | 756 | 4 |
| StWRKY05 | PGSC0003DMG400000064 | chr01 | WRKYGQK | C2H2 | 1 | IIe | 5.73 | 38.83 | 352 | 1059 | 3 |
| StWRKY06 | PGSC0003DMG400000211 | chr01 | WRKYGQK | C2HC | 1 | III | 5.64 | 38.87 | 346 | 1041 | 3 |
| StWRKY07 | PGSC0003DMG400029779 | chr01 | WRKYGQK | C2H2 | 1 | IIb | 8.14 | 45.94 | 413 | 1242 | 4 |
| StWRKY08 | PGSC0003DMG400015104 | chr02 | WRKYGQK | C2H2 | 1 | IIe | 5.16 | 46.16 | 413 | 1242 | 3 |
| StWRKY09 | PGSC0003DMG400022063 | chr02 | WRKYGQK | C2H2 | 1 | IIb | 7.02 | 55.21 | 496 | 1491 | 5 |
| StWRKY10 | PGSC0003DMG400025481 | chr02 | WRKYGQK | C2H2 | 1 | IId | 9.12 | 37.38 | 334 | 1005 | 3 |
| StWRKY11 | PGSC0003DMG402006935 | chr02 | WRKYGQK | C2H2 | 1 | IIb | 6.29 | 48.68 | 443 | 1332 | 3 |
| StWRKY12 | PGSC0003DMG400009703 | chr02 | WRKYGQK | C2H2 | 1 | IIc | 6.49 | 36.17 | 318 | 957 | 3 |
| StWRKY13 | PGSC0003DMG400028469 | chr02 | WRKYGQK | C2H2 | 1 | IIe | 5.94 | 33.57 | 299 | 900 | 3 |
| StWRKY14 | PGSC0003DMG400016441 | chr02 | WRKYGQK | C2H2 | 1 | IIb | 6.98 | 60.06 | 553 | 1662 | 6 |
| StWRKY15 | PGSC0003DMG400024961 | chr02 | WRKYGQK | C2H2 | 1 | IId | 9.70 | 36.01 | 324 | 975 | 3 |
| StWRKY16 | PGSC0003DMG400001434 | chr02 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | 6.13 | 50.16 | 451 | 1356 | 4 |
| StWRKY17 | PGSC0003DMG400020206 | chr02 | WRKYGQK | C2H2 | 1 | IIc | 9.52 | 16.45 | 141 | 426 | 2 |
| StWRKY18 | PGSC0003DMG401005575 | chr03 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | 6.56 | 57.08 | 516 | 1551 | 4 |
| StWRKY19 | PGSC0003DMG400020608 | chr03 | WRKYGQK | C2HC | 1 | III | 6.17 | 31.89 | 277 | 834 | 3 |
| StWRKY20 | PGSC0003DMG400041197 | chr03 | WRKYGQK | C2H2 | 1 | IIe | 8.46 | 28.69 | 249 | 750 | 3 |
| StWRKY21 | PGSC0003DMG400039175 | chr03 | WRKCGQK* | C2H2 | 1 | IIe | 8.89 | 28.66 | 249 | 750 | 3 |
| StWRKY22 | PGSC0003DMG400009103 | chr03 | WRKYGQK | C2HC | 1 | III | 5.90 | 40.31 | 356 | 1071 | 3 |
| StWRKY23 | PGSC0003DMG400044842 | chr03 | WRKYGMK* | C2H2 | 1 | IIe | 6.06 | 29.51 | 259 | 780 | 1 |
| StWRKY24 | PGSC0003DMG400034476 | chr03 | WKKHGSN* | C2H2 | 1 | IIe | 7.06 | 19.99 | 173 | 552 | 1 |
| StWRKY25 | PGSC0003DMG400018081 | chr03 | WRKYGQK | C2H2 | 1 | IIb | 6.82 | 58.45 | 525 | 1578 | 5 |
| StWRKY26 | PGSC0003DMG400019824 | chr03 | WRKYGQK | C2H2 | 1 | IIa | 8.74 | 39.76 | 355 | 1068 | 5 |
| StWRKY27 | PGSC0003DMG400040494 | chr04 | WRKYGQK | C2H2 | 1 | IIe | 8.86 | 35.67 | 311 | 936 | 5 |
| StWRKY28 | PGSC0003DMG401031196 | chr04 | WRKYGKK* | C2H2 | 1 | IIc | 9.47 | 16.12 | 138 | 417 | 3 |
| StWRKY29 | PGSC0003DMG400019706 | chr04 | WRKYGQK | C2H2 | 1 | IIc | 9.00 | 27.26 | 234 | 705 | 3 |
| StWRKY30 | PGSC0003DMG400031140 | chr04 | WRKYGKK* | C2H2 | 1 | IIc | 6.55 | 26.68 | 231 | 696 | 3 |
| StWRKY31 | PGSC0003DMG400007947 | chr04 | WRKYGQK | C2H2 | 1 | IId | 9.67 | 38.88 | 354 | 1065 | 3 |
| StWRKY32 | PGSC0003DMG400028335 | chr05 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | 8.05 | 55.46 | 508 | 1527 | 4 |
| StWRKY33 | PGSC0003DMG400028381 | chr05 | WRKYGQK | C2H2 | 1 | IIc | 5.34 | 35.28 | 326 | 981 | 3 |
| StWRKY34 | PGSC0003DMG400021895 | chr05 | WRKYGQK | C2H2 | 1 | IIc | 9.22 | 19.92 | 172 | 519 | 2 |
| StWRKY35 | PGSC0003DMG400036639 | chr05 | WRKYGQK | C2H2 | 1 | IIe | 9.53 | 37.44 | 330 | 993 | 3 |
| StWRKY36 | PGSC0003DMG400035855 | chr05 | WRKYGQK | C2H2 | 1 | IIe | 9.04 | 37.55 | 330 | 993 | 3 |
| StWRKY37 | PGSC0003DMG400033884 | chr05 | WRKYGQR* | C2HC | 1 | III | 6.25 | 32.92 | 294 | 885 | 3 |
| StWRKY38 | PGSC0003DMG401033880 | chr05 | WRKYGQK | C2HC | 1 | III | 5.83 | 37.37 | 331 | 996 | 3 |
| StWRKY39 | PGSC0003DMG400017990 | chr05 | WRKYGQK | C2HC | 1 | III | 5.48 | 34.06 | 301 | 906 | 3 |
| StWRKY40 | PGSC0003DMG400027208 | chr05 | WRKYGQK | C2H2 | 1 | IIc | 9.01 | 28.14 | 250 | 753 | 3 |
| StWRKY41 | PGSC0003DMG400023360 | chr05 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | 6.84 | 51.90 | 457 | 1374 | 3 |
| StWRKY42 | PGSC0003DMG400005329 | chr06 | WRKYGQK | C2H2 | 1 | IId | 9.62 | 39.95 | 355 | 1068 | 3 |
| StWRKY43 | PGSC0003DMG400008776 | chr06 | WRKYGQK | C2HC | 1 | III | 5.94 | 38.15 | 347 | 1044 | 4 |
| StWRKY44 | PGSC0003DMG400027582 | chr06 | WRKYGQK | C2HC | 1 | III | 8.06 | 26.02 | 223 | 672 | 3 |
| StWRKY45 | PGSC0003DMG400016769 | chr06 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | 6.90 | 59.57 | 534 | 1605 | 5 |
| StWRKY46 | PGSC0003DMG400028520 | chr06 | WRKYGQK | C2H2 | 1 | IIa | 8.37 | 39.79 | 360 | 1083 | 4 |
| StWRKY47 | PGSC0003DMG400006155 | chr07 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | 6.55 | 46.54 | 422 | 1269 | 4 |
| StWRKY48 | PGSC0003DMG400015015 | chr07 | WRKYGQK | C2H2 | 1 | IIb | 6.09 | 60.26 | 557 | 1674 | 5 |
| StWRKY49 | PGSC0003DMG400020432 | chr07 | WRKYGQK | C2H2 | 1 | IIe | 5.51 | 28.19 | 256 | 771 | 4 |
| StWRKY50 | PGSC0003DMG400017349 | chr07 | WRKYGQK | C2H2 | 1 | IIc | 6.26 | 36.29 | 319 | 960 | 3 |
| StWRKY51 | PGSC0003DMG400022290 | chr07 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | 7.05 | 64.91 | 596 | 1791 | 6 |
| StWRKY52 | PGSC0003DMG400022143 | chr07 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | 6.01 | 81.04 | 748 | 2247 | 5 |
| StWRKY53 | PGSC0003DMG400009530 | chr08 | WRKYGQK | C2H2 | 1 | IId | 9.62 | 36.33 | 334 | 1005 | 3 |
| StWRKY54 | PGSC0003DMG400005835 | chr08 | WRKYGQK | C2HC | 1 | III | 5.69 | 40.74 | 360 | 1083 | 3 |
| StWRKY55 | PGSC0003DMG400005836 | chr08 | WRKYGQK | C2HC | 1 | III | 6.89 | 34.07 | 305 | 918 | 3 |
| StWRKY56 | PGSC0003DMG400008188 | chr08 | WRKYGKK* | C2H2 | 1 | IIc | 4.79 | 14.65 | 128 | 387 | 2 |
| StWRKY57 | PGSC0003DMG402007388 | chr08 | WRKYGQK | C2H2 | 1 | IIa | 8.84 | 28.72 | 253 | 762 | 4 |
| StWRKY58 | PGSC0003DMG400007387 | chr08 | WRKYGQK | C2H2 | 1 | IIa | 5.70 | 29.74 | 261 | 786 | 4 |
| StWRKY59 | PGSC0003DMG400012318 | chr08 | WRKYGQK | C2H2 | 1 | IIe | 5.57 | 35.04 | 307 | 924 | 3 |
| StWRKY60 | PGSC0003DMG400012317 | chr08 | WRKYGQK | C2H2 | 1 | IIc | 9.13 | 26.17 | 228 | 687 | 2 |
| StWRKY61 | PGSC0003DMG400012160 | chr08 | WRKYGQK | C2HC | 1 | III | 5.40 | 38.57 | 339 | 1020 | 3 |
| StWRKY62 | PGSC0003DMG400011633 | chr09 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | 7.05 | 45.47 | 408 | 1227 | 4 |
| StWRKY63 | PGSC0003DMG400029207 | chr09 | WRKYGQK | C2HC | 1 | III | 5.63 | 33.46 | 293 | 882 | 3 |
| StWRKY64 | PGSC0003DMG400015076 | chr09 | WRKYGQK | C2H2 | 1 | IId | 9.65 | 39.10 | 346 | 1041 | 3 |
| StWRKY65 | PGSC0003DMG400011271 | chr10 | WIKYGEN/WRKYGQK | C2H2 | 2 | I | 5.07 | 75.77 | 697 | 2094 | 5 |
| StWRKY66 | PGSC0003DMG401010558 | chr10 | WRKYGQK | C2H2 | 1 | IIe | 8.36 | 23.24 | 208 | 627 | 3 |
| StWRKY67 | PGSC0003DMG400019884 | chr10 | WRKYGQK | C2HC | 1 | III | 5.55 | 33.52 | 290 | 873 | 3 |
| StWRKY68 | PGSC0003DMG400019408 | chr10 | WRKYGQK | C2H2 | 1 | IIe | 4.73 | 26.70 | 238 | 717 | 3 |
| StWRKY69 | PGSC0003DMG400008391 | chr10 | WRKYGQK | C2HC | 1 | III | 8.94 | 25.43 | 218 | 657 | 3 |
| StWRKY70 | PGSC0003DMG400010987 | chr10 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | 9.51 | 52.05 | 467 | 1404 | 5 |
| StWRKY71 | PGSC0003DMG400007788 | chr12 | WRKYGKK* | C2H2 | 1 | IIc | 6.56 | 37.68 | 334 | 1005 | 3 |
| StWRKY72 | PGSC0003DMG400037700 | chr12 | WHKCGQK* | C2H2 | 1 | IIe | 8.11 | 37.51 | 331 | 996 | 3 |
| StWRKY73 | PGSC0003DMG402028822 | chr12 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | 6.62 | 60.48 | 549 | 1650 | 4 |
| StWRKY74 | PGSC0003DMG400029815 | chr12 | WRKYGQK/WRKYGQK | C2H2 | 2 | I | 6.37 | 65.80 | 611 | 1836 | 6 |
| StWRKY75 | PGSC0003DMG400028633 | chr12 | WRKYGQK | C2H2 | 1 | IIa | 9.52 | 24.70 | 217 | 654 | 4 |
| StWRKY76 | PGSC0003DMG400023196 | chr12 | WRKYGKK* | C2H2 | 1 | IIc | 8.28 | 24.90 | 213 | 642 | 3 |
| StWRKY77 | PGSC0003DMG400016957 | chr12 | WRKYGKK* | C2H2 | 1 | IIc | 9.35 | 14.57 | 124 | 375 | 2 |
| StWRKY78 | PGSC0003DMG400029371 | chr12 | WRKYGQK | C2H2 | 1 | IId | 9.61 | 34.03 | 312 | 939 | 3 |
| StWRKY79 | PGSC0003DMG400033177 | chr00 | WRKYGKK* | C2H2 | 1 | IIc | 9.23 | 12.10 | 102 | 309 | 2 |
Notes:
* WRKY conserved sequence is WRKYGQK-like that is indicated by asterisk.
Chr00 means this sequence has not located.
The average StWRKY proteins length was 338 aa and in the range of 102 aa (StWRKY79) to 748 aa (StWRKY52). Information on gene loci number, chromosome location, WRKYGQK conserved heptapeptide, zinc-finger motif type, number of WRKY domains, isolelectrical point (PI), molecular weight (Mw) and number of exons are given in Table 1.
Chromosomal location of StWRKY members and analyze the gene duplication
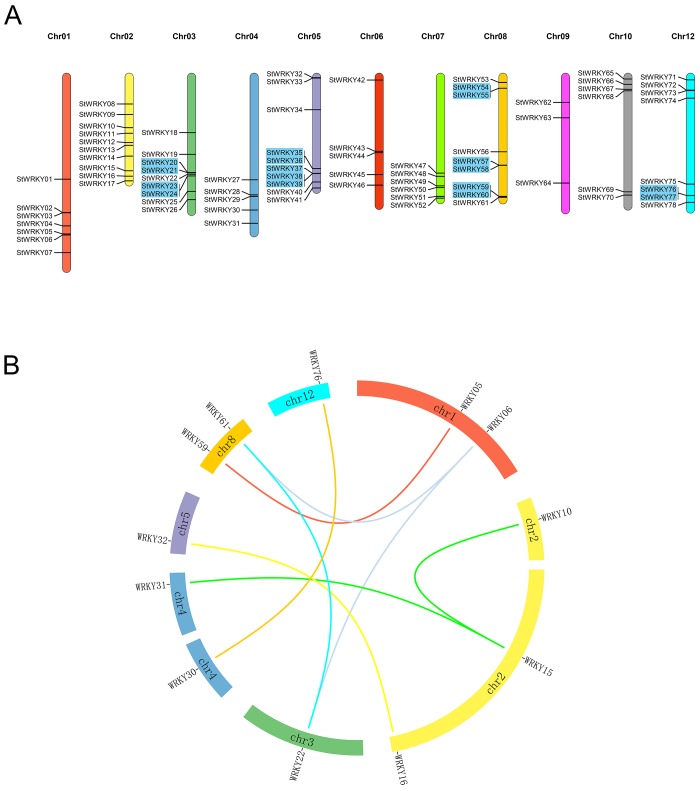
Mapchart software was used to determine the location of the gene on each chromosome (Fig 1A). According to the position of genes in chromosomes, we were named these WRKY genes from 1 to 79. The map showed that chromosomes contained all StWRKYs, except StWRKY79. Two shorter chromosomes, the chromosome 2 (48.61 Mb) and the chromosome 5 (52.07 Mb) had 10 genes, respectively. Only 3 genes were occurred in the chromosome 9. And none located in the chromosome 11. The event of genes located closely in some chromosomes.
Fig 1. Located the StWRKY members in potato chromosomes and analyzed their duplications.
(A) The location of StWRKY members on potato chromosomes and the tandem duplication. The blue boxes indicate these members are tandem duplications. And StWRKY79 is not located in any chromosomes. (B) The segmental duplication about StWRKY members. The different color blocks indicate the part of potato chromosomes. The thick lines with the same colors about chromosomes mean the tandem duplications and the fine lines are connected the segmental duplication pairs and different colors means different duplication.
Through identification of tandem duplication was based on Cheng’s method and these closely genes were termed a cluster [3]. StWRKY genes formed in clusters on chromosome 3, 5, 8 and 12. A total of 8 clusters (17 genes) confirmed in blue boxes (Fig 1A). The research of the database which downloaded from PGSC website showed seven parts from StWRKY genes with segmental duplications (15 genes) (Fig 1B) and these were distributed on seven chromosomes.
Phylogenetic analysis and classification of StWRKY genes
The defining characteristics of the WRKY family are the WRKYGQK domain and zinc-finger structure. However, different group owned their specific components. The detail structures about WRKY domain and zinc finger type could be displayed by multiple sequence alignment (Fig 2). A total of 13 genes contained two WRKYGQK domains were divided into one group, whereas the zinc finger had slight differences, one was CX4CX22HXH and another was CX4CX23HXH. The heptapeptide WRKYGQK co-located in C- and N-terminal, respectively. Thus, Group I also could be separated into I-C and I-N [41], even though they were be in same gene. The other part was constructed by 14 genes which could be easily differentiated by presence of special zinc finger, CX7CX24HXC [42]. The remaining genes (52 genes) formed the last part, a large genes containing one WRKY domain and an adjacent CX5CX23HXH zinc finger structure [8]. Interestingly, four StWRKYs which did not contain a whole zinc finger motif structure also belonged to the WRKY family.
Fig 2. Comparison of the protein sequences from 79 StWRKY proteins.
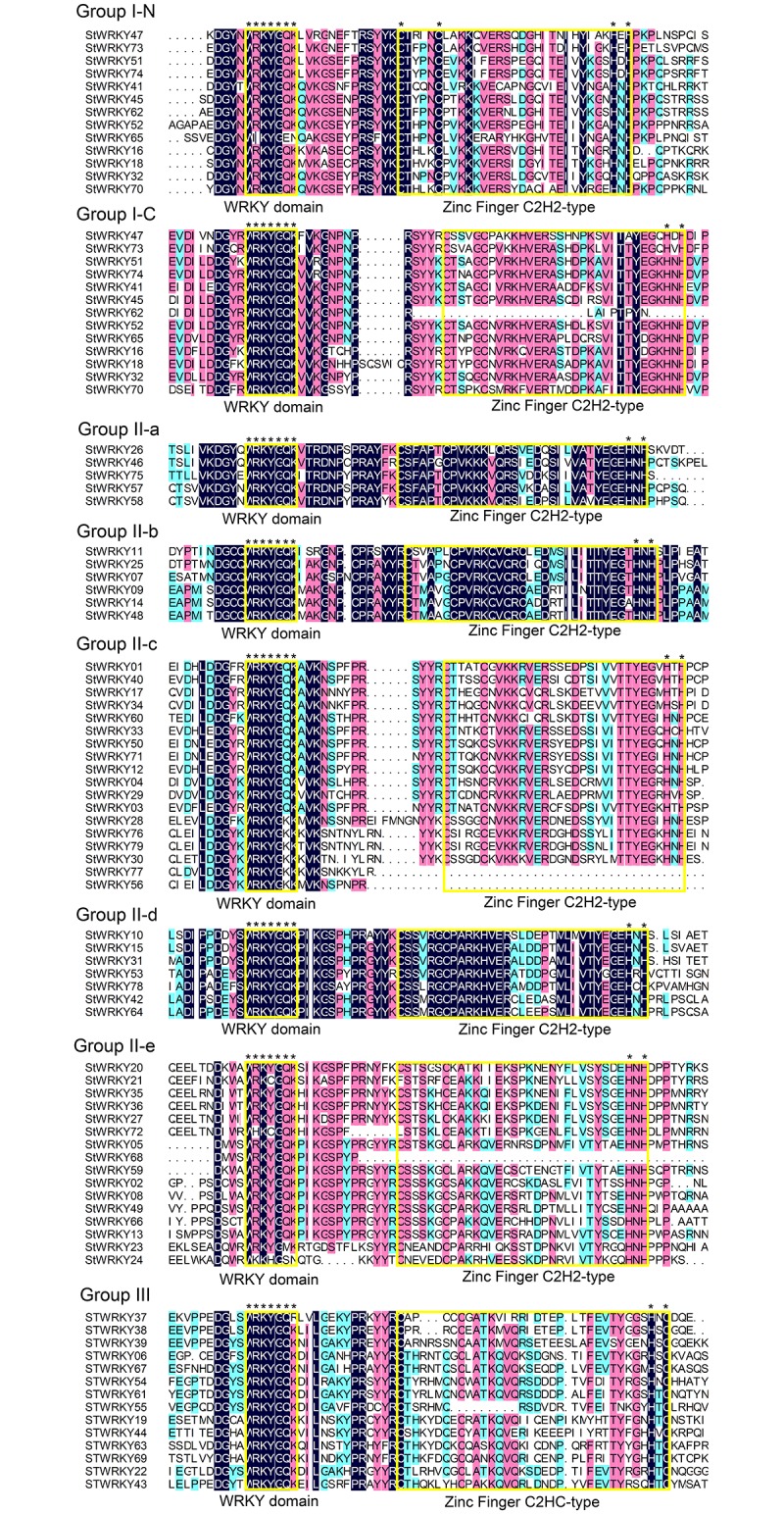
Alignment was using software DNAMAN. The N or C indicates the N-terminal and the C-terminal, respectively. The amino acids forming the zinc-finger motif and WRKY domain are covered in yellow box. The asterisk indicated the main conversed sequences.
In order to acquire a detailed classification, four kinds of plant WRKY members were used to construct the phylogenetic tree: 54 WRKY proteins from A. thaliana, 53 WRKY proteins from C. annuum, 77 from S. lycopersicum and 79 from S. tuberosum (Fig 3). Following others categories, Group II was classified into five sub-groups: Group II-a (5 members), II-b (6), II-c (18), II-d (7), and II-e (16). Group II-a and II-b, and Group II-d and II-e, were clustered in a branch, respectively. The classification was the same as found in other higher plants, especially in dicotyledons [8].
Fig 3. Phylogenetic tree builds for all WRKY proteins from 4 plants.
Arabidopsis, C. annuum, S. lycopersicum and S. tuberosum, totally 261 protein sequences build the tress using the Neighbor-Joining method. Different regions distinguish the groups.
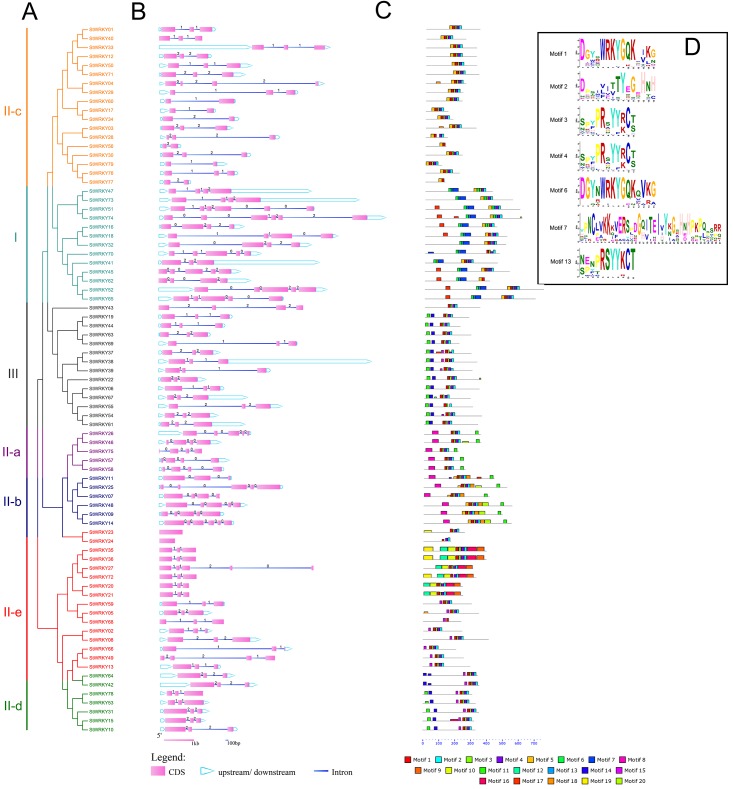
Structures of StWRKY gene family
To better understand the similarity and diversity of gene motifs in different genes, 20 motifs were examined within genes using the MEME website (Fig 4C and 4D). Motif 1 and 6 were WRKYGQK or WRKYGQK-like domains and were broadly distributed in every StWRKY gene. Motif 6, 7 and 13 together were formed the main structure of N-terminal WRKY domain, and motif 1–4 comprised the C-terminal WRKY domain. Almost every gene contained motif 1–4. In addition, 13 genes had both motifs 1 and 6 [35]. The WRKY family was divided into 3 parts following the motifs species and combinations. The members in first part had motif 6, 7 and 13 and these members comprised a group. In the other group, there were 14 StWRKY genes which had variation in motif 2 compared with other members, the last amino acid residue H was instead C. This transformation led to the change of zinc finger type from C2H2 to CH2C. The 52 members belonged to a group was different with others (Fig 4A and 4C). The division of these members was same as phylogenetic tree.
Fig 4. Structure analyzes StWRKY members.
The WRKY members are divided into 7 groups. (A) Phylogenetic tree of StWRKY members. Neighbor-Joining tree constructed with WRKY proteins in potato. (B) Intron-exon structure of StWRKY genes. The purple blocks indicate CDS, the black lines indicate introns and the blue blocks indicated upstream or downstream. (C) Distribution of conserved motifs of StWRKYs. (D) The sequences of 7 main motifs.
The concept of intron–exon was first introduced due to the discovery that transcription sequences were usually longer than gene sequences [41]. Analysis using the GSDS2.0 website showed that almost every gene contained at least one intron in StWRKY genes and most genes usually had 1–5 introns. However, two members (StWRKY23 and StWRKY24) had no introns. About one-half of StWRKYs possessed two introns. Furthermore, six members just had one intron, and 26 had more than three. The analysis showed that introns of StWRKY members had three phases (Fig 4B): 0, 1 and 2. Phases 1 and 2 contained more than a half of the WRKY genes. 18 genes had phase 0 only. Just StWRKY38 gene had downstream sequence and StWRKY52 gene had upstream sequence through the analysis.
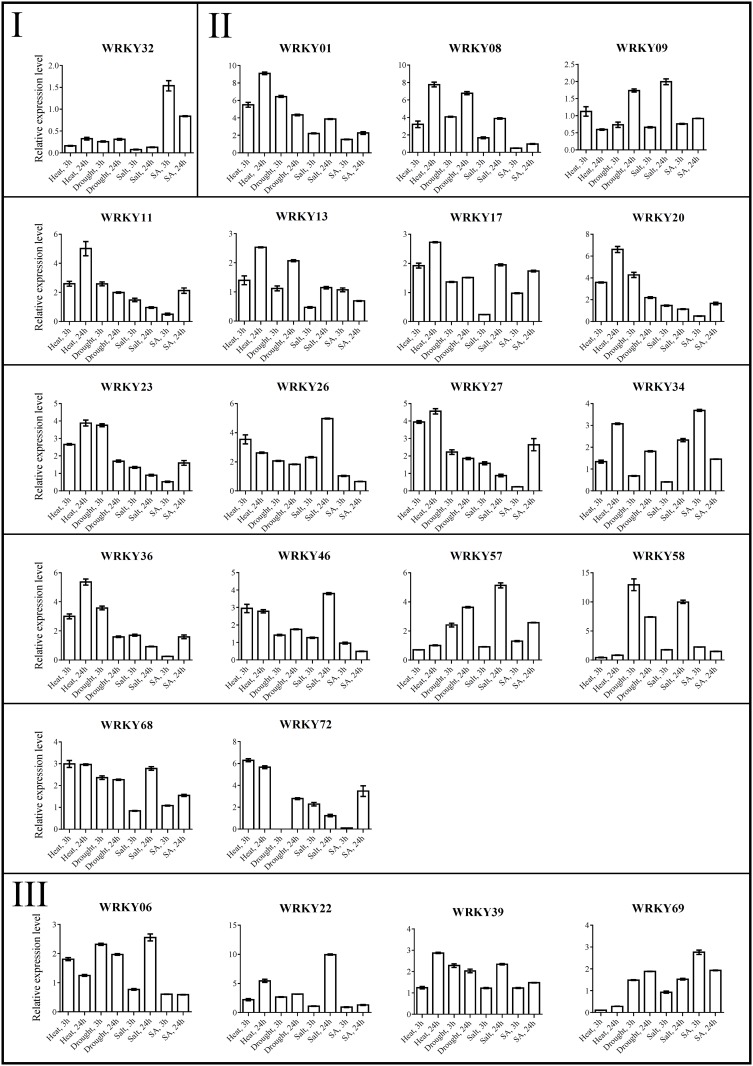
Expression analysis of StWRKY genes in response to stresses
To demonstrate the function of WRKY genes and obtain information for future research, qRT-PCR analysis was used to reveal the expression of WRKY genes under stress. Using information from RNA-seq analysis of the WRKY genes by Massa [43], 22 genes were selected for analysis in our research (Fig 5). Drought, salt, heat (35°C) and SA treatments were the stress conditions.
Fig 5. The expression pattern of 22 StWRKYs in four different stresses.
S3 and S24 mean the salt treatment at 3 h and 24 h. H is Heat shorthand, D indicates Drought, and SA means salicylic acid. The data lower than 1.0 represents the down-regulated and higher 1.0 is up-regulated. The grey box indicates the StWRKY72 cannot show detectable expression under drought-treatment in 3 h.
Analysis of the 22 genes under the 4 stresses showed (Fig 6) that only 2 genes (StWRKY01 and StWRKY39) were up-regulation under each stress. The overwhelming majority of genes under SA stress had low expression only StWRKY34 under 3 h treatment and StWRKY72 under 24 h treatment had highest expression in these genes, and they all had three-fold expression compared with control. Under abiotic stresses (heat, drought and salt), almost all genes were up-regulated in 3 h or 24 h treatment exceptStWRKY32. Under the different stress treatments, StWRKY58 had highest expression under drought and salt stresses, with twelve-fold and nearly ten-fold than control, respectively. StWRKY01 and StWRKY22 also had nearly ten-fold and nine-fold higher than control under the heat and salt treatment, respectively. Moreover, individual WRKY gene could respond more than one stress factor, simultaneously. That is to say, one gene could have diverse function under different stresses. StWRKY01, 08, 22 and 26 responded to the abiotic stresses. StWRKY20, 27, 36 and 72 were induced by drought and heat stresses. Similar conditions have also occurred in other plants AtWRKY25 and AtWRKY33 responded concurrently under heat and salt treatments [44] and GmWRKY53 was induced by cold, salt and drought treatments [45].
Fig 6. Expression analyzes 22 StWRKY genes under different stresses.
SA means salicylic acid. The data lower than 1.0 represent the down-regulation and higher 1.0 is up-regulation. The StWRKY72 cannot show detectable expression under drought-treatment in 3 h, so the database is missing.
It is common knowledge that potato originated in low-temperature environments in the Andes Mountains, and so it is suited to cold locations. Conversely, warm environments could be a negative factor in plant development.
Thus, heat treatment was used to search for regulating genes in the WRKY family. For example, overexpressing OsWRKY11 under the control of HSP101 promoter enhanced heat tolerance [46]. In our study, only 4 of 22 were down-regulated following 3 h and 24 h under heat treatment. Seventeen of 22 genes enhanced their expression. Of these, almost all of genes showed higher expression after 24 h and only StWRKY09 turned down-regulated by 24 h. StWRKY32, 58 and 69 were down-regulated at both times. StWRKY01, 08, 20 and 22 were significantly up-regulation after 24 h treatment compared with 3 h.
Drought is usually related to salinity and they have a combined impact on plant growth, development and productivity. Thus, understanding drought and salinity regulations is important for yield. The WRKY family is well known to respond to a variety of stresses [47]. Almost all genes were up-regulated in 24 h of drought treatment except for StWRKY32. Only StWRKY09, 32 and 34 were down-regulated at 3 h. StWRKY72 could not show detectable expression. Significantly enhanced expression occurred for StWRKY58.
Following salt treatment, thirteen of 22 genes were up-regulated after 3 h of treatment and seventeen of 22 genes were up-regulated after 24 h. The highest expression genes were StWRKY22 and 58 in 24 h treatments. However, up-regulation of the WRKY family genes in potato showed little increase after 3 h treatment. After 24 h treatment, the expression increased and only five of 22 genes (StWRKY11, 23, 27, 32 and 36) were lower than controls. Interestingly, StWRKY32 always maintained down-regulation and showed less than one-tenth of control expression. In these 22 genes, the expression of StWRKY22 and StWRKY58 were greatly enhanced till 24 h treatment.
SA can be a key signaling molecule in response to biotic stresses. Infection of plants causes rapid increase of SA level that leads to the expression of genes encoding the pathogen proteins and the activating disease resistance [48]. That was why SA was chosen as a stress treatment in this search. The genes expression showed that nearly all genes were the same as control, and some genes were even down-regulated. Only seven of 22 genes were up-regulated. The highest expression was just below four-fold compared with control. Interestingly, StWRKY72 was highly up-regulated under 24 h treatment, but had lower expression at 3 h, its homologous genes were down-regulated at both times [32, 49].
Cis-elements in the promoter regions of StWRKY genes
Regulatory elements are essential to control gene expression [50]. To further understand the response of StWRKY genes under the various stress treatments, the cis-elements in 22 StWRKY genes which were used for qRT-PCR analysis was predicted their elements which were related with various stress in promoter regions. The 1500-bp upstream promoter regions were searched in PlantCARE, and the elements were showed in S1 Fig, including ABRE element (ABA responsiveness) found in eight promoter regions of selected StWRKY genes. Interestingly, StWRKY57 contained six ABRE elements and this gene can respond to the abscisic acid (ABA) signaling pathway. MBS element (drought-inducibility) occurred in the promoter region of 12 genes. Previous research has shown two signaling pathways in response to drought stress: ABA-dependent and ABA-independent [51]. Thus, two elements, ABRE and MBS could respond to drought stress. The HSE element (heat stress responsiveness) existed in the promoter area of 17 StWRKYs, and of these StWRKY58 had more HSEs (about 7) and may be related to heat stress resistance. TC-rich repeats element (cis-acting element involved in defense and stress responsiveness) was the most element in the promoter region of 22 StWRKYs, with about 18 genes containing this element. Fourteen promoters had a TCA-element (SA responsiveness) and only 10 promoters showed a W-box element (WRKY binding site).
Discussion
The WRKY TFs are among the most important regulatory network members in plants and play pivotal roles in abiotic [52] and biotic stress responses. [53]. Considerable research has been performed on the functions of WRKY TFs, and some WRKY genes have been cloned to analyze their function [54, 55]. However, there is little relevant information from the world’s fourth most-important staple crop, potato. [29].
A total of 79 genes were found to encode the WRKY family. The majority of sequences were located on chromosomes, and only one of the StWRKY genes, StWRKY79 was not located in any chromosome according to the data from the internet. That would proof that the potato whole-genome sequencing was not complement. In previous research, several genes were also not located in any chromosomes [13, 56].
Research in A. thaliana has shown that duplication might affect gene family size and distribution. Tandem duplication influences expansion and segmental duplication influences evolution and functional prediction [57]. The StWRKY genes showed 15 duplications: eight tandem and seven segmental, the CaWRKY genes showed 11 tandem clusters [35], AdWRKY genes had four tandem clusters and seven segmental duplications, and AiWRKY genes had six tandem clusters and 10 segmental duplications [58]. Thus, a number of other higher plants showed WRKYs with duplications.
Usually, WRKY genes can be divided into three main groups, and later research in higher plants showed that WRKY Group II consisted of five sub-groups [10]. WRKY genes may also have four major lineages comprising sub-groups II-a and II-b, sub-groups II-d and II-e, sub-group II-c and Group I, and Group III alone [56]. This conclusion was confirmed by Rinerson’s research [36]. Wang [42] reported that Group III genes could influence disease resistance [59] and promoted biology evolution and good adaptability [10]. We found that 14 WRKY genes in potato belonged to Group III.
The other mainly evidence concerning WRKY family classification was the variety of motifs. Almost all WRKY genes contained the conserved WRKYGQK domain. The zinc finger structures are an important part of the WRKY TFs, as clearly shown by motifs research (Fig 4C and 4D). Each group was constituted of different motifs. Group I had two WRKY domains, motifs 1 and 6. Almost every gene in Group I contained by motifs 6, 13 and 7 from N-terminal and motifs 5, 1 (6), 3 (13), 4 and 2 from C-terminal. Others contained motifs 1‒4, which was the main structure of the WRKY family. Motifs 11 and 14 together appeared in Group III. Sub-groups II-a and II-b contained motifs 8 and 11, sub-groups II-d and II-e contained motif 15, and sub-group II-c contained motif 5 these could show the characteristics of each group. This distribution further illustrated the relationships with each group.
Comparing DNA sequences and transcription sequences, DNA sequences possessed more amino acids. So gene models consisted of two parts of genome sequences [60]: the exon, which results in protein, and the intron, which is lost in transcription sequences. Although introns are not translated they contain many elements to regulate gene expression [61]. The introns were located between codons named phase 0, and after the first or second nucleotides of codons named phase 1 or 2. These two kinds of introns were found in nine StWRKY genes. Interestingly, eight genes belonged to Group I. Analysis of tandem duplication genes showed that they also had same intron-exon structures.
The WRKY TF family occurs widely and has been researched in many plants. Flowering plants have a larger WRKY family, and these TFs have important functions [37]. Various stresses affect the yield and the WRKY family responds to many of these stresses [62].
Many studies have investigated salt, heat and other abiotic stresses and the response of WRKY genes [54, 62]. Biotic stresses [63] are also of wide concern and salicylic acid, abscisic acid, methyl jasmonate treatments can be used to represent biotic stresses [55]. In this study, salt-, heat-, drought- and SA-response mechanisms were the main research objects.
Under the three abiotic stresses, heat, salt and drought, StWRKY32 was all down-regulated, but in Brachypodium distachyon [32], Fragaria vesca [49] and C. annuum [35], its homologs BdWRKY61, FvWRKY24 and CaWRKY3 were up-regulated under heat and drought, and unchanged under salt stress, compared with controls. StWRKY58 was up-regulated with salt and drought treatments and down-regulated with heat, its homologs BdWRKY14, FvWRKY46 and CaWRKY10 were all down-regulated. In different plants the WRKY genes might have different functions. Interestingly, StWRKY22 expression was most enhanced after 24 h of salt treatment, and its homologs in soybean GmWRKY96 and in A. thaliana AtWRKY40 were also reported to show enhanced expression under salt treatment [1, 4]. StWRKY01 was up-regulated similarly to the homolog gene BdWRKY16. Thus, StWRKY22 and StWRKY58 had high expression revealing their importance in response to salt stress, StWRKY01 was relevant to heat resistance and StWRKY58 also had hightest expression under drought treatment.
More genes responded to drought stress compared with the other treatments. The initial analyses suggest that StWRKY genes may form a core component of drought stress response. StWRKY01 and StWRKY58 are candidate genes for further functional research and their roles in heat or drought stress signaling should be verified.
Conclusions
We found a total of 79 genes of the WRKY TF family located in 11 of 12 chromosomes of potato. Phylogenetic analysis divided these genes into three groups, with five sub-groups comprising Group II. The potato virus-free seedlings were treated with heat, salinity, drought and SA, and StWRKY gene expression monitored. qRT-PCR of 22 StWRKY genes in response to stresses, and database analysis, confirmed strong responses of WRKY genes, especially to drought and heat. Thus, we identified some interesting candidate WRKY genes for future functional analysis.
Supporting information
(DOC)
(DOC)
The cycle from outside-to-inside indicated different elements, ABRE, HSE, MBS, TC-rich repeats, TCA-element and W-box. The different color represented the various genes. And the different numbers in each boxes meant the quantity of cis-elements.
(TIF)
Acknowledgments
We thank the Northwest Agriculture Forestry University and State Key Laboratory of Crop Stress Biology for Arid Areas. This work was financially supported by the Research funding for special professor of Northwest Agriculture and Forestry University (grant Nos. Z111021202).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was financially supported by the Research funding for special professor of Northwest Agriculture and Forestry University (grant Nos. Z111021202).
References
- 1.Li MY, Xu ZS, Tian C, Huang Y, Wang F, Xiong AS. Genomic identification of WRKY transcription factors in carrot (Daucus carota) and analysis of evolution and homologous groups for plants. Sci Rep. 2016;6:23101 doi: 10.1038/srep23101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C, Deng P, Chen L, Wang X, Ma H, Hu W, et al. A wheat WRKY transcription factor TaWRKY10 confers tolerance to multiple abiotic stresses in transgenic tobacco. PLoS One. 2013;8(6):e65120 doi: 10.1371/journal.pone.0065120 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y, Yao ZP, Ruan MY, Ye QJ, Wang RQ, Zhou GZ, et al. In silico identification and characterization of the WRKY gene superfamily in pepper (Capsicum annuum L.). Genetics and molecular research: GMR. 2016;15(3). doi: 10.4238/gmr.15038675 . [DOI] [PubMed] [Google Scholar]
- 4.Yu Y, Wang N, Hu R, Xiang F. Genome-wide identification of soybean WRKY transcription factors in response to salt stress. Springerplus. 2016;5(1):920 doi: 10.1186/s40064-016-2647-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okay S, Derelli E, Unver T. Transcriptome-wide identification of bread wheat WRKY transcription factors in response to drought stress. Mol Genet Genomics. 2014;289(5):765–81. doi: 10.1007/s00438-014-0849-x . [DOI] [PubMed] [Google Scholar]
- 6.Song Y, Jing SJ, Yu DQ. Research progress on function analysis of rice WRKY genes. Rice Science. 2010;17(1):60–72. [Google Scholar]
- 7.Yin G, Xu H, Xiao S, Qin Y, Li Y, Yan Y, et al. The large soybean (Glycine max) WRKY TF family expanded by segmental duplication events and subsequent divergent selection among subgroups. BMC Plant Biology. 2013;13(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5(5):199–206. . [DOI] [PubMed] [Google Scholar]
- 9.Ulker B, Somssich IE. WRKY transcription factors: from DNA binding towards biological function. Curr Opin Plant Biol. 2004;7(5):491–8. doi: 10.1016/j.pbi.2004.07.012 . [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Wang L. The WRKY transcription factor superfamily: its origin in eukaryotes and expansion in plants. BMC Evol Biol. 2005;5:1 doi: 10.1186/1471-2148-5-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang Y, Duan Y, Yin J, Ye S, Zhu J, Zhang F, et al. Genome-wide identification and characterization of the Populus WRKY transcription factor family and analysis of their expression in response to biotic and abiotic stresses. J Exp Bot. 2014;65(22):6629–44. doi: 10.1093/jxb/eru381 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Zhu W, Fang L, Sun X, Su L, Liang Z, et al. Genome-wide identification of WRKY family genes and their response to cold stress in Vitis vinifera. BMC Plant Biol. 2014;14:103 doi: 10.1186/1471-2229-14-103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang S, Gao Y, Liu J, Peng X, Niu X, Fei Z, et al. Solanum lycopersicum. Mol Genet Genomics. 2012;287(6):495–513. doi: 10.1007/s00438-012-0696-6 . [DOI] [PubMed] [Google Scholar]
- 14.Diao WP, Snyder JC, Wang SB, Liu JB, Pan BG, Guo GJ, et al. Genome-Wide Identification and Expression Analysis of WRKY Gene Family in Capsicum annuum L. Frontiers in Plant Science. 2016;7(806):211. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Brand LH, Fischer NM, Harter K, Kohlbacher O, Wanke D. Elucidating the evolutionary conserved DNA-binding specificities of WRKY transcription factors by molecular dynamics and in vitro binding assays. Nucleic Acids Res. 2013;41(21):9764–78. doi: 10.1093/nar/gkt732 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding M, Chen J, Jiang Y, Lin L, Cao Y, Wang M, et al. Genome-wide investigation and transcriptome analysis of the WRKY gene family in Gossypium. Mol Genet Genomics. 2015;290(1):151–71. doi: 10.1007/s00438-014-0904-7 . [DOI] [PubMed] [Google Scholar]
- 17.Dou L, Zhang X, Pang C, Song M, Wei H, Fan S, et al. Genome-wide analysis of the WRKY gene family in cotton. Mol Genet Genomics. 2014;289(6):1103–21. doi: 10.1007/s00438-014-0872-y . [DOI] [PubMed] [Google Scholar]
- 18.Ma J, Zhang D, Shao Y, Liu P, Jiang L, Li C. Genome-wide analysis of the WRKY transcription factors in aegilops tauschii. Cytogenet Genome Res. 2014;144(3):243–53. doi: 10.1159/000370172 . [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Wang M, Zhang X, Hao B, Kaushik SK, Pan Y. WRKY gene family evolution in Arabidopsis thaliana. Genetica. 2011;139(8):973–83. doi: 10.1007/s10709-011-9599-4 . [DOI] [PubMed] [Google Scholar]
- 20.Sarris PF, Duxbury Z, Huh SU, Ma Y, Segonzac C, Sklenar J, et al. A Plant Immune Receptor Detects Pathogen Effectors that Target WRKY Transcription Factors. Cell. 2015;161(5):1089–100. doi: 10.1016/j.cell.2015.04.024 . [DOI] [PubMed] [Google Scholar]
- 21.Fan X, Guo Q, Xu P, Gong Y, Shu H, Yang Y, et al. Transcriptome-wide identification of salt-responsive members of the WRKY gene family in Gossypium aridum. PLoS One. 2015;10(5):e0126148 doi: 10.1371/journal.pone.0126148 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tripathi P, Rabara RC, Rushton PJ. A systems biology perspective on the role of WRKY transcription factors in drought responses in plants. Planta. 2014;239(2):255–66. doi: 10.1007/s00425-013-1985-y . [DOI] [PubMed] [Google Scholar]
- 23.Kopecki Z, Luchetti MM, Adams DH, Strudwick X, Mantamadiotis T, Stoppacciaro A, et al. Collagen loss and impaired wound healing is associated with c-Myb deficiency. J Pathol. 2007;211(3):351–61. Epub 2006/12/08. doi: 10.1002/path.2113 . [DOI] [PubMed] [Google Scholar]
- 24.Qiu Y, Jing S, Jian FU, Lu LI, Diqiu YU. Cloning and analysis of expression profile of 13 WRKY genes in rice. Science Bulletin. 2004;49(20):2159–68. [Google Scholar]
- 25.Zhou QY, Tian AG, Zou HF, Xie ZM, Lei G, Huang J, et al. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants: Plant Biotechnol J; 2008. 486–503 p. doi: 10.1111/j.1467-7652.2008.00336.x [DOI] [PubMed] [Google Scholar]
- 26.Evers D, Legay S, Lamoureux D, Hausman JF, Hoffmann L, Renaut J. Towards a synthetic view of potato cold and salt stress response by transcriptomic and proteomic analyses. Plant Mol Biol. 2012;78(4–5):503–14. doi: 10.1007/s11103-012-9879-0 . [DOI] [PubMed] [Google Scholar]
- 27.Yogendra KN, Kumar A, Sarkar K, Li Y, Pushpa D, Mosa KA, et al. Transcription factor StWRKY1 regulates phenylpropanoid metabolites conferring late blight resistance in potato. Journal of Experimental Botany. 2015;66(22):7377–89. doi: 10.1093/jxb/erv434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shahzad R, Harlina PW, Xie CH, Ewas M, Nishawy E, Pan Z, et al. Overexpression of potato transcription factor (StWRKY1) conferred resistance to Phytophthora infestans and improved tolerance to water stress. Plant Omics. 2016;9(2):149–58. [Google Scholar]
- 29.Gallou A, Declerck S, Cranenbrouck S. Transcriptional regulation of defence genes and involvement of the WRKY transcription factor in arbuscular mycorrhizal potato root colonization. Funct Integr Genomics. 2012;12(1):183–98. doi: 10.1007/s10142-011-0241-4 . [DOI] [PubMed] [Google Scholar]
- 30.Yogendra KN, Dhokane D, Kushalappa AC, Sarmiento F, Rodriguez E, Mosquera T. StWRKY8 transcription factor regulates benzylisoquinoline alkaloid pathway in potato conferring resistance to late blight. Plant Science. 2016. [DOI] [PubMed] [Google Scholar]
- 31.Pan LJ, Jiang L. Identification and expression of the WRKY transcription factors of Carica papaya in response to abiotic and biotic stresses. Mol Biol Rep. 2014;41(3):1215–25. doi: 10.1007/s11033-013-2966-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen F, Zhu H, Li P, Jiang M, Mao W, Ong C, et al. Brachypodium distachyon. DNA Res. 2014;21(3):327–39. doi: 10.1093/dnares/dst060 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satapathy L, Singh D, Ranjan P, Kumar D, Kumar M, Prabhu KV, et al. Transcriptome-wide analysis of WRKY transcription factors in wheat and their leaf rust responsive expression profiling. Mol Genet Genomics. 2014;289(6):1289–306. doi: 10.1007/s00438-014-0890-9 . [DOI] [PubMed] [Google Scholar]
- 34.Kumar K, Srivastava V, Purayannur S, Kaladhar VC, Cheruvu PJ, Verma PK. WRKY domain-encoding genes of a crop legume chickpea (Cicer arietinum): comparative analysis with Medicago truncatula WRKY family and characterization of group-III gene(s). DNA Res. 2016;23(3):225–39. doi: 10.1093/dnares/dsw010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diao WP, Snyder JC, Wang SB, Liu JB, Pan BG, Guo GJ, et al. Capsicum annuum L. Front Plant Sci. 2016;7:211 doi: 10.3389/fpls.2016.00211 . [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Rinerson CI, Rabara RC, Tripathi P, Shen QJ, Rushton PJ. The evolution of WRKY transcription factors. BMC Plant Biol. 2015;15:66 doi: 10.1186/s12870-015-0456-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo C, Guo R, Xu X, Gao M, Li X, Song J, et al. grape (Vitis vinifera L.). J Exp Bot. 2014;65(6):1513–28. doi: 10.1093/jxb/eru007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicot N, Hausman JF, Hoffmann L, Evers D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot. 2005;56(421):2907–14. doi: 10.1093/jxb/eri285 . [DOI] [PubMed] [Google Scholar]
- 39.Ma H, Cao X, Shi S, Li S, Gao J, Ma Y, et al. Genome-wide survey and expression analysis of the amino acid transporter superfamily in potato (Solanum tuberosum L.). Plant Physiology & Biochemistry. 2016;107:164–77. [DOI] [PubMed] [Google Scholar]
- 40.Zhou H, Li Y, Zhang Q, Ren S, Shen Y, Qin L, et al. Genome-Wide Analysis of the Expression of WRKY Family Genes in Different Developmental Stages of Wild Strawberry (Fragaria vesca) Fruit. PLoS One. 2016;11(5):e0154312 doi: 10.1371/journal.pone.0154312 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zou Z, Yang L, Wang D, Huang Q, Mo Y, Xie G. Gene Structures, Evolution and Transcriptional Profiling of the WRKY Gene Family in Castor Bean (Ricinus communis L.). PLoS One. 2016;11(2):e0148243 doi: 10.1371/journal.pone.0148243 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Feng L, Zhu Y, Li Y, Yan H, Xiang Y. Comparative genomic analysis of the WRKY III gene family in populus, grape, arabidopsis and rice. Biol Direct. 2015;10:48 doi: 10.1186/s13062-015-0076-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massa AN, Childs KL, Buell CR. Abiotic and Biotic Stress Responses in Solanum tuberosum Group Phureja DM1-3 516 R44 as Measured through Whole Transcriptome Sequencing. Plant Genome. 2013;6(3). [Google Scholar]
- 44.Li S, Fu Q, Chen L, Huang W, Yu D. Arabidopsis thaliana WRKY25, WRKY26, and WRKY33 coordinate induction of plant thermotolerance. Planta. 2011;233(6):1237–52. doi: 10.1007/s00425-011-1375-2 [DOI] [PubMed] [Google Scholar]
- 45.Zhou QY, Tian AG, Zou HF, Xie ZM, Lei G, Huang J, et al. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol J. 2008;6(5):486–503. doi: 10.1111/j.1467-7652.2008.00336.x . [DOI] [PubMed] [Google Scholar]
- 46.Wu X, Shiroto Y, Kishitani S, Ito Y, Toriyama K. Enhanced heat and drought tolerance in transgenic rice seedlings overexpressing OsWRKY11 under the control of HSP101 promoter. Plant Cell Rep. 2009;28(1):21–30. doi: 10.1007/s00299-008-0614-x . [DOI] [PubMed] [Google Scholar]
- 47.Phukan UJ, Jeena GS, Shukla RK. WRKY Transcription Factors: Molecular Regulation and Stress Responses in Plants. Front Plant Sci. 2016;7:760 doi: 10.3389/fpls.2016.00760 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie Z, Zhang ZL, Hanzlik S, Cook E, Shen QJ. Salicylic acid inhibits gibberellin-induced alpha-amylase expression and seed germination via a pathway involving an abscisic-acid-inducible WRKY gene. Plant Mol Biol. 2007;64(3):293–303. doi: 10.1007/s11103-007-9152-0 . [DOI] [PubMed] [Google Scholar]
- 49.Wei W, Hu Y, Han YT, Zhang K, Zhao FL, Feng JY. diploid woodland strawberry Fragaria vesca. Plant Physiol Biochem. 2016;105:129–44. doi: 10.1016/j.plaphy.2016.04.014 . [DOI] [PubMed] [Google Scholar]
- 50.Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Yves VDP, et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Research. 2002;30(1):325–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shinozaki K, Yamaguchi-Shinozaki K. Gene networks involved in drought stress response and tolerance. Journal of Experimental Botany. 2007;58(58):221–7. [DOI] [PubMed] [Google Scholar]
- 52.Zhou QY, Tian AG, Zou HF, Xie ZM, Lei G, Huang J, et al. Soybean GmWRKY13, 21, 54in transgenic Arabidopsis plants. Plant Biotechnol J. 2008;6(5):486–503. [DOI] [PubMed] [Google Scholar]
- 53.Yu F, Huaxia Y, Lu W, Wu C, Cao X, Guo X. GhWRKY15, a member of the WRKY transcription factor family identified from cotton (Gossypium hirsutum L.), is involved in disease resistance and plant development. BMC Plant Biol. 2012;12:144 doi: 10.1186/1471-2229-12-144 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niu CF, Wei W, Zhou QY, Tian AG, Hao YJ, Zhang WK, et al. Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Environ. 2012;35(6):1156–70. doi: 10.1111/j.1365-3040.2012.02480.x . [DOI] [PubMed] [Google Scholar]
- 55.Liu X, Song Y, Xing F, Wang N, Wen F, Zhu C. GhWRKY25, a group I WRKY gene from cotton, confers differential tolerance to abiotic and biotic stresses in transgenic Nicotiana benthamiana. Protoplasma. 2016;253(5):1265–81. doi: 10.1007/s00709-015-0885-3 . [DOI] [PubMed] [Google Scholar]
- 56.Goel R, Pandey A, Trivedi PK, Asif MH. Genome-Wide Analysis of the Musa WRKY Gene Family: Evolution and Differential Expression during Development and Stress. Front Plant Sci. 2016;7:299 doi: 10.3389/fpls.2016.00299 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cannon SB, Mitra A, Baumgarten A, Young ND, May G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004;4:10 doi: 10.1186/1471-2229-4-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song H, Wang P, Lin JY, Zhao C, Bi Y, Wang X. Peanut SA. Front Plant Sci. 2016;7:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang Y, Li MY, Wu P, Xu ZS, Que F, Wang F, et al. Members of WRKY Group III transcription factors are important in TYLCV defense signaling pathway in tomato (Solanum lycopersicum). BMC Genomics. 2016;17(1):788 doi: 10.1186/s12864-016-3123-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rogozin IB, Sverdlov AV, Babenko VN, Koonin EV. Analysis of evolution of exon-intron structure of eukaryotic genes. Briefings in bioinformatics. 2005;6(2):118–34. . [DOI] [PubMed] [Google Scholar]
- 61.Koralewski TE, Krutovsky KV. Evolution of exon-intron structure and alternative splicing. PLoS One. 2011;6(3):e18055 doi: 10.1371/journal.pone.0018055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Banerjee A, Roychoudhury A. WRKY proteins: signaling and regulation of expression during abiotic stress responses. ScientificWorldJournal. 2015;2015:807560 doi: 10.1155/2015/807560 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.López-Delgado HA, Mora-Herrera ME, Martínez-Gutiérrez R, Sánchez-Rojo S. Short and Long Term Effects of Salicylic Acid on Protection to Phytoplasma Associated Stress in Potato Plants 2013. 315–37 p. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
The cycle from outside-to-inside indicated different elements, ABRE, HSE, MBS, TC-rich repeats, TCA-element and W-box. The different color represented the various genes. And the different numbers in each boxes meant the quantity of cis-elements.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.