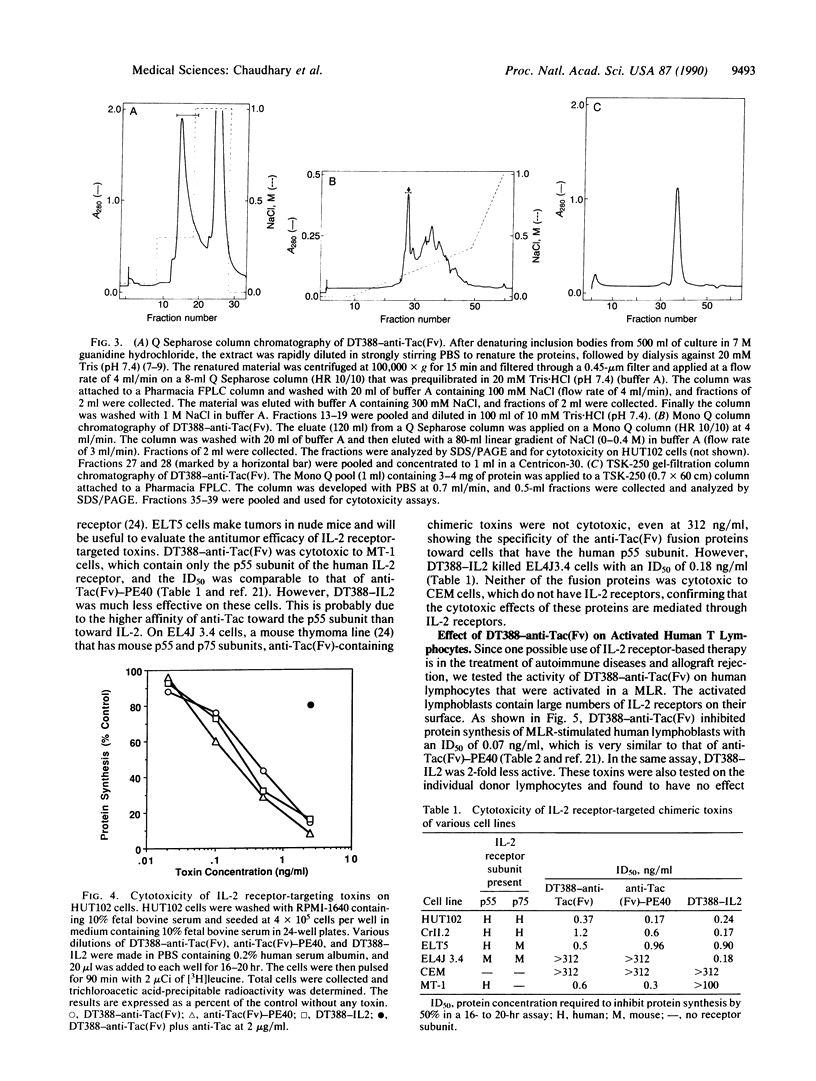
Abstract
To kill human or primate cells expressing the p55 subunit of the interleukin 2 receptor, we have constructed a single-chain immunotoxin. DNA sequences encoding the first 388 amino acids of diphtheria toxin (DT) were fused to DNA elements encoding the antigen-binding portion (variable region or Fv) of the anti-Tac monoclonal antibody. The antigen-binding portion consists of 116 amino acids of the heavy-chain variable region connected by a 15-amino acid linker to 106 amino acids of the variable region of the light chain. The single-chain immunotoxin DT388-anti-Tac(Fv) was expressed in Escherichia coli and found in inclusion bodies. The monomeric form was then purified to near homogeneity with a high yield (3-5 mg/liter). Monomeric DT388-anti-Tac(Fv) was highly cytotoxic to cell lines bearing the p55 subunit of the human interleukin 2 receptor but not to cells without this subunit. DT388-anti-Tac(Fv) was also very effective in killing proliferating human T cells produced in a mixed leukocyte reaction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batra J. K., FitzGerald D., Gately M., Chaudhary V. K., Pastan I. Anti-Tac(Fv)-PE40, a single chain antibody Pseudomonas fusion protein directed at interleukin 2 receptor bearing cells. J Biol Chem. 1990 Sep 5;265(25):15198–15202. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Case J. P., Lorberboum-Galski H., Lafyatis R., FitzGerald D., Wilder R. L., Pastan I. Chimeric cytotoxin IL2-PE40 delays and mitigates adjuvant-induced arthritis in rats. Proc Natl Acad Sci U S A. 1989 Jan;86(1):287–291. doi: 10.1073/pnas.86.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., FitzGerald D. J., Adhya S., Pastan I. Activity of a recombinant fusion protein between transforming growth factor type alpha and Pseudomonas toxin. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4538–4542. doi: 10.1073/pnas.84.13.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., Mizukami T., Fuerst T. R., FitzGerald D. J., Moss B., Pastan I., Berger E. A. Selective killing of HIV-infected cells by recombinant human CD4-Pseudomonas exotoxin hybrid protein. Nature. 1988 Sep 22;335(6188):369–372. doi: 10.1038/335369a0. [DOI] [PubMed] [Google Scholar]
- Chaudhary V. K., Queen C., Junghans R. P., Waldmann T. A., FitzGerald D. J., Pastan I. A recombinant immunotoxin consisting of two antibody variable domains fused to Pseudomonas exotoxin. Nature. 1989 Jun 1;339(6223):394–397. doi: 10.1038/339394a0. [DOI] [PubMed] [Google Scholar]
- FitzGerald D. J., Willingham M. C., Pastan I. Pseudomonas exotoxin--immunotoxins. Cancer Treat Res. 1988;37:161–173. doi: 10.1007/978-1-4613-1083-9_11. [DOI] [PubMed] [Google Scholar]
- Griffin T. W., Morgan A. C., Blythman H. E. Immunotoxin therapy: assessment by animal models. Cancer Treat Res. 1988;37:433–455. doi: 10.1007/978-1-4613-1083-9_24. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Minamoto S., Uchiyama T., Hardy R. R., Yamada G., Taniguchi T. Reconstitution of functional receptor for human interleukin-2 in mouse cells. Nature. 1985 Dec 5;318(6045):467–470. doi: 10.1038/318467a0. [DOI] [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Kiyokawa T., Shirono K., Hattori T., Nishimura H., Yamaguchi K., Nichols J. C., Strom T. B., Murphy J. R., Takatsuki K. Cytotoxicity of interleukin 2-toxin toward lymphocytes from patients with adult T-cell leukemia. Cancer Res. 1989 Jul 15;49(14):4042–4046. [PubMed] [Google Scholar]
- Laurent G., Frankel A. E., Hertler A. A., Schlossman D. M., Casellas P., Jansen F. K. Treatment of leukemia patients with T101 ricin A chain immunotoxins. Cancer Treat Res. 1988;37:483–491. doi: 10.1007/978-1-4613-1083-9_27. [DOI] [PubMed] [Google Scholar]
- Lorberboum-Galski H., Barrett L. V., Kirkman R. L., Ogata M., Willingham M. C., FitzGerald D. J., Pastan I. Cardiac allograft survival in mice treated with IL-2-PE40. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1008–1012. doi: 10.1073/pnas.86.3.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R., Bishai W., Borowski M., Miyanohara A., Boyd J., Nagle S. Genetic construction, expression, and melanoma-selective cytotoxicity of a diphtheria toxin-related alpha-melanocyte-stimulating hormone fusion protein. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8258–8262. doi: 10.1073/pnas.83.21.8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., FitzGerald D. Pseudomonas exotoxin: chimeric toxins. J Biol Chem. 1989 Sep 15;264(26):15157–15160. [PubMed] [Google Scholar]
- Pastan I., Willingham M. C., FitzGerald D. J. Immunotoxins. Cell. 1986 Dec 5;47(5):641–648. doi: 10.1016/0092-8674(86)90506-4. [DOI] [PubMed] [Google Scholar]
- Siegall C. B., Chaudhary V. K., FitzGerald D. J., Pastan I. Cytotoxic activity of an interleukin 6-Pseudomonas exotoxin fusion protein on human myeloma cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9738–9742. doi: 10.1073/pnas.85.24.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Vallera D. A., Myers D. E. Immunotoxins containing ricin. Cancer Treat Res. 1988;37:141–159. doi: 10.1007/978-1-4613-1083-9_10. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Fulton R. J., May R. D., Till M., Uhr J. W. Redesigning nature's poisons to create anti-tumor reagents. Science. 1987 Nov 20;238(4830):1098–1104. doi: 10.1126/science.3317828. [DOI] [PubMed] [Google Scholar]
- Williams D. P., Parker K., Bacha P., Bishai W., Borowski M., Genbauffe F., Strom T. B., Murphy J. R. Diphtheria toxin receptor binding domain substitution with interleukin-2: genetic construction and properties of a diphtheria toxin-related interleukin-2 fusion protein. Protein Eng. 1987 Dec;1(6):493–498. doi: 10.1093/protein/1.6.493. [DOI] [PubMed] [Google Scholar]
- Williams D. P., Snider C. E., Strom T. B., Murphy J. R. Structure/function analysis of interleukin-2-toxin (DAB486-IL-2). Fragment B sequences required for the delivery of fragment A to the cytosol of target cells. J Biol Chem. 1990 Jul 15;265(20):11885–11889. [PubMed] [Google Scholar]
- Youle R. J., Greenfield L., Johnson V. G. Genetic engineering of immunotoxins. Cancer Treat Res. 1988;37:113–122. doi: 10.1007/978-1-4613-1083-9_8. [DOI] [PubMed] [Google Scholar]