Abstract
In the past decade novel agents are on the market for non-small cell lung cancer adenocarcinoma based on pharmacogenomics. The epidermal growth factor receptor mutation, anaplastic lymphoma kinase and programmed death-ligand 1 investigation is necessary in the everyday clinical practice for the oncologic patient. Immunotherapy is nowadays the novel therapy for advanced stage non-small cell lung cancer with two agents nivolumab and pembrolizumab. In the current case series we will present adverse effects from our centers and comment on the treatment and follow-up of the patients.
Keywords: NSCLC, Immunotherapy, Adenocarcinoma, Nivolumab, Colitis, Pneumonitis, Pericarditis
1. Introduction
During the past ten years a bloom of novel therapies has been observed for non-small cell lung cancer [1], [2]. In specific based on the pharmacogenomics of the cancer novel targeted drugs are on the market [3]. The epidermal growth factor mutation (EGFR) and the anaplastic lymphoma kinase (ALK) should be first investigated in non-small lung cancer (NSCLC) adenocarcinoma. Moreover; nowadays programmed death-ligand 1 (PD-L1) has to be also investigated as in the case of >50% overexpression the patient can receive immunotherapy (pembrolizumab) as first line treatment. The programmed death-ligand 1 (PD-L1) overexpression can be investigated also in squamous cell NSCLC. In the case where both EGFR and >50% PD-L1 is observed then the patient should start its first line with tyrosine kinase inhibitors (TKIs) [4]. The cost of immunotherapy still remains high when compared to the standard non-specific cytotoxic therapy [5], however; the progression free survival is higher and less adverse effects for the patients. We present two cases with adverse effects due to immunotherapy administration and comment on them.
2. Case report 1
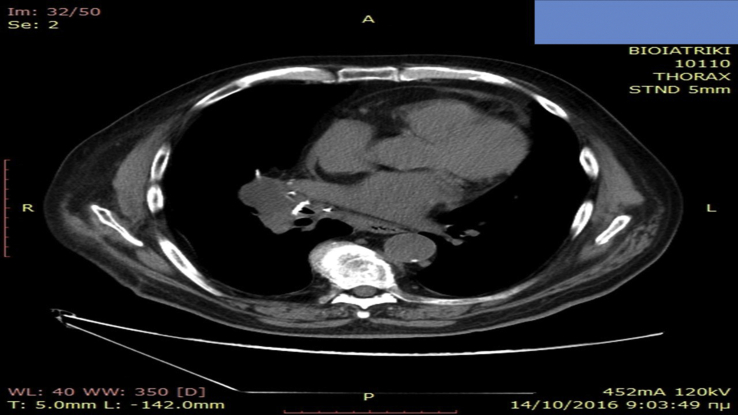
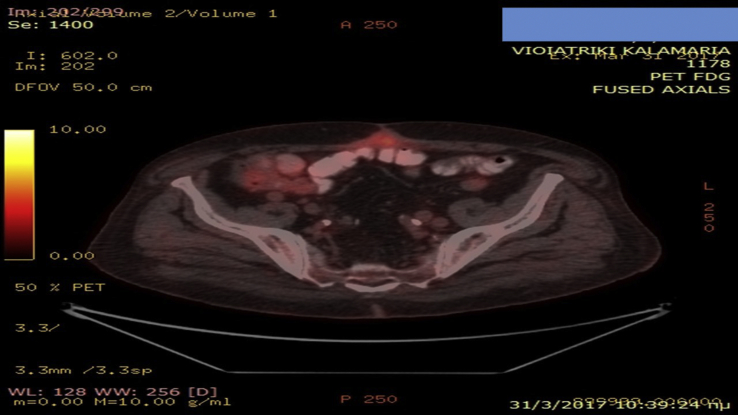
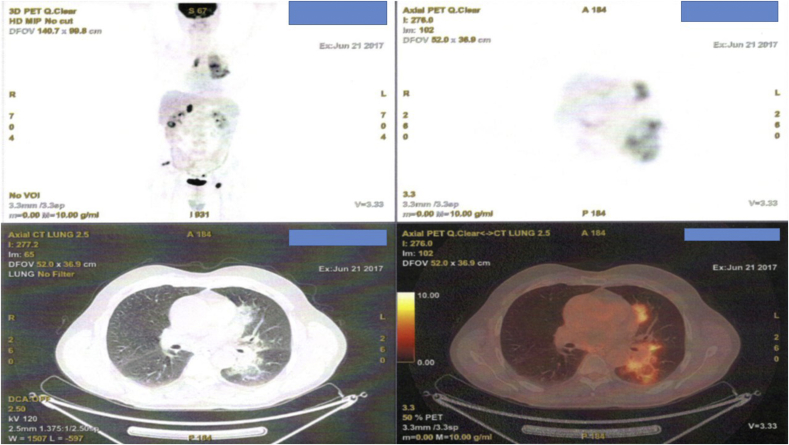
A 65 year old patient was diagnosed with squamous cell carcinoma in 2014 stage IIa at that time. Two years after during follow-up he presented disease relapse in the site of the surgery (lobectomy) (Fig. 1). He received four cycles of carbo/pax doublet and remained under follow-up. Three months after he presented in the outpatient cabinet with head ache and bone metastasis was diagnosed with scintigraphy bone scan. Programmed death-ligand 1 (PD-L1) was investigated and the expression was 0%. However; nivolumab was initiated due to the toxic adverse effects presented during the first line with neutropenia. Nivolumab 150 mg/15 days was initiated and after three administrations bowel rupture was observed and the patient had an emergency surgery and colostomy was performed. After three months without any therapy the surgeons decided to make an anastomosis of the bowel. A PET-CT was performed in order to make restaging (Fig. 2). In the site of the anastomosis as it can be observed there is an area that retain Hi 18-FDG, however; it was considered to be due to the inflammation of the area. The patient during the three months had stable disease in the thorax and the bone pain was manageable with mild painkillers. Zoledronic acid was initiated along with a platinum doublet.
Fig. 1.
Disease relapse with CT of the thorax.
Fig. 2.
PET-CT with hi FDG retention in the site of anastomosis.
3. Case report 2
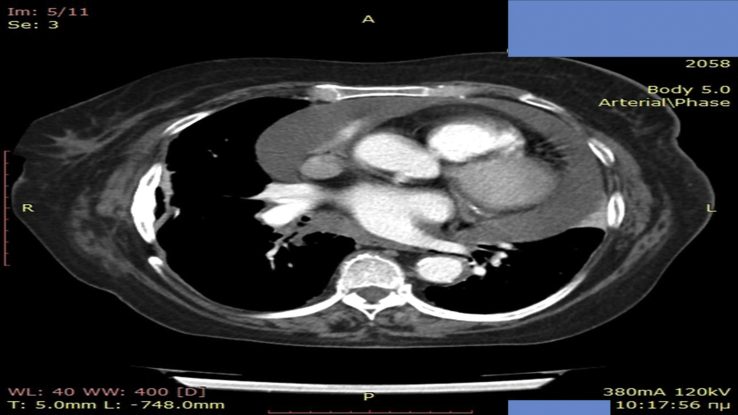
A 60 year patient was diagnosed with adenocarcinoma with bronchoscopy and he was stage IV due to bone metastasis. He was EGFR and ALK negative and PD-L1 0%. He received four cycles with carboplatin AUC 6 and permetrexed 500 × BSA. He had stable disease for 3 months until relapse and nivolumab was initiated as second line treatment. During restaging after four months of treatment with immunotherapy pericarditis was observed and aspiration was performed (Fig. 3). The cytology report was negative for disease relapse and the pericarditis was attributed to the immunotherapy, as it is known that orogonitis is considered an adverse effect of immunotherapy. Immunotherapy stopped, and the patient received third line chemotherapy with a doublet of carbo plus gemcitabine along with methylprednisolone tablets 2 × 16 mg daily for 1 week with the appropriate tapering during a thirty day period.
Fig. 3.
Pericarditis with CT of the thorax.
4. Case report 3
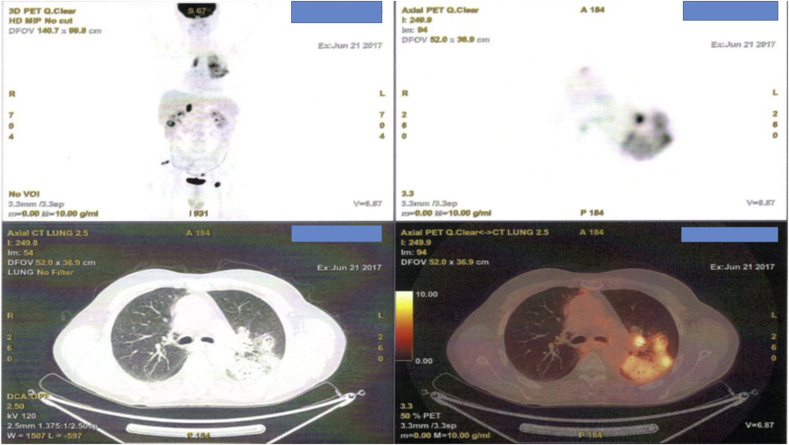
A 55 year old patient was diagnosed with adenocarcinoma with biopsy under CT guidance. He was diagnosed with stage IV disease due to positive pleural effusion. He was EGFR and ALK negative, however; he had PD-L1 65%. Pembrolizumab 200 mg every 21 days was initiated. Unfortunately after four months of immunotherapy administration restaging with PET-CT demonstrated pneumonitis (Fig. 4, Fig. 5). Immunotherapy was stopped and the patient received second line chemotherapy with a doublet of carbo plus a taxane derivative along with methylprednisolone tablets 2 × 16 mg daily for 1 week with the appropriate tapering during a thirty day period.
Fig. 4.
PET-CT images after a four month administration of pembrolizumab administration.
Fig. 5.
PET-CT images after a four month administration of pembrolizumab administration.
5. Discussion
Immunotherapy is considered a mild therapy when compared to the non-specific cytotoxic agents. However; there are still some side effects to consider such orogonitis, pneumonitis, psoriasis, diabetes mellitus and transient thyroid malfunction [6], [7], [8], [9]. Myasthenia gravis was also recently observed [10]. Nivolumab is licensed as second line therapy for advanced non-small cell lung cancer, while pembrolizumab can be used as first line treatment in the case of PD-L1 >50% and as second line when PD-L1 >1% [11]. Nivolumab based on a recent publication could be considered as first line treatment in the case of PD-L1 >5% as it has a more safe profile when compared to chemotherapy [12]. Targeted therapy with tyrosine kinase inhibitors (TKIs) or “targeted” immunotherapy based on the PD-L1 expression, in every case less adverse effects certainly should be considered first for every patient. The performance status of the patient plays an important role in for the treatment efficacy of immunotherapy, while it is not necessary for the use of TKIs. Re-biopsy might be also considered in the future for every patient after disease relapse because the tissue genome is certainly affected by the use of therapy [13], [14], [15], [16]. In the case of immunotherapy close follow-up of the patients is necessary on order to efficiently cop with the adverse effects of immune therapy. The percentage of adverse effects is the same in both immunotherapy drugs [17]. Patients should be frequently checked for transient thyreotoxicosis, since this adverse effect is mostly frequently to occur [18]. Patients' hormone levels should be carefully checked and if necessary treatment should be administered.
Conflict of interest
None to declare.
References
- 1.Domvri K., Zarogoulidis P., Darwiche K., Browning R.F., Li Q., Turner J.F., Kioumis I., Spyratos D., Porpodis K., Papaiwannou A., Tsiouda T., Freitag L., Zarogoulidis K. Molecular targeted drugs and biomarkers in NSCLC, the evolving role of individualized therapy. J. Cancer. 2013;4(9):736–754. doi: 10.7150/jca.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarogoulidis K., Zarogoulidis P., Darwiche K., Boutsikou E., Machairiotis N., Tsakiridis K., Katsikogiannis N., Kougioumtzi I., Karapantzos I., Huang H., Spyratos D. Treatment of non-small cell lung cancer (NSCLC) J. Thorac. Dis. 2013;5(Suppl 4):S389–S396. doi: 10.3978/j.issn.2072-1439.2013.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domvri K., Darwiche K., Zarogoulidis P., Zarogoulidis K. Following the crumbs: from tissue samples, to pharmacogenomics, to NSCLC therapy. Transl. Lung Cancer Res. 2013;2(4):256–258. doi: 10.3978/j.issn.2218-6751.2012.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reck M., Rodriguez-Abreu D., Robinson A.G., Hui R., Csoszi T., Fulop A., Gottfried M., Peled N., Tafreshi A., Cuffe S., O'Brien M., Rao S., Hotta K., Leiby M.A., Lubiniecki G.M., Shentu Y., Rangwala R., Brahmer J.R., K.- Investigators Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 5.Huang M., Lou Y., Pellissier J., Burke T., Liu F.X., Xu R., Velcheti V. Cost effectiveness of pembrolizumab vs. Standard-of-care chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in the United States. PharmacoEconomics. 2017 doi: 10.1007/s40273-017-0527-z. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwama S., Arima H. Clinical practice and mechanism of endocrinological adverse events associated with immune checkpoint inhibitors. Nihon Rinsho Men'eki Gakkai kaishi Jpn. J. Clin. Immunol. 2017;40(2):90–94. doi: 10.2177/jsci.40.90. [DOI] [PubMed] [Google Scholar]
- 7.van Kooten M.J., van den Berg G., Glaudemans A., Hiltermann T.J.N., Groen H.J.M., Rutgers A., Links T.P. Transient thyrotoxicosis during nivolumab treatment. Neth. J. Med. 2017;75(5):204–207. [PubMed] [Google Scholar]
- 8.Ruiz-Banobre J., Perez-Pampin E., Garcia-Gonzalez J., Gomez-Caamano A., Baron-Duarte F.J., Lopez-Lopez R., Vazquez-Rivera F. Development of psoriatic arthritis during nivolumab therapy for metastatic non-small cell lung cancer, clinical outcome analysis and review of the literature. Lung Cancer. 2017;108:217–221. doi: 10.1016/j.lungcan.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Godwin J.L., Jaggi S., Sirisena I., Sharda P., Rao A.D., Mehra R., Veloski C. Nivolumab-induced autoimmune diabetes mellitus presenting as diabetic ketoacidosis in a patient with metastatic lung cancer. J. Immunother. Cancer. 2017;5:40. doi: 10.1186/s40425-017-0245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makarious D., Horwood K., Coward J.I.G. Myasthenia gravis: an emerging toxicity of immune checkpoint inhibitors. Eur. J. Cancer. 2017;82:128–136. doi: 10.1016/j.ejca.2017.05.041. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra J., Jabbour S.K., Aisner J. Current state of immunotherapy for non-small cell lung cancer. Transl. Lung Cancer Res. 2017;6(2):196–211. doi: 10.21037/tlcr.2017.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbone D.P., Reck M., Paz-Ares L., Creelan B., Horn L., Steins M., Felip E., van den Heuvel M.M., Ciuleanu T.E., Badin F., Ready N., Hiltermann T.J.N., Nair S., Juergens R., Peters S., Minenza E., Wrangle J.M., Rodriguez-Abreu D., Borghaei H., Blumenschein G.R., Jr., Villaruz L.C., Havel L., Krejci J., Corral Jaime J., Chang H., Geese W.J., Bhagavatheeswaran P., Chen A.C., Socinski M.A., CheckMate I. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N. Engl. J. Med. 2017;376(25):2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarogoulidis P., Gaga M., Huang H., Darwiche K., Rapti A., Hohenforst-Schmidt W. Tissue is the issue and tissue competition. Re-biopsy for mutation T790: where and why? Clin. Transl. Med. 2017;6(1):6. doi: 10.1186/s40169-017-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorantes-Heredia R., Ruiz-Morales J.M., Cano-Garcia F. Histopathological transformation to small-cell lung carcinoma in non-small cell lung carcinoma tumors. Transl. Lung Cancer Res. 2016;5(4):401–412. doi: 10.21037/tlcr.2016.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takegawa N., Hayashi H., Iizuka N., Takahama T., Ueda H., Tanaka K., Takeda M., Nakagawa K. Transformation of ALK rearrangement-positive adenocarcinoma to small-cell lung cancer in association with acquired resistance to alectinib. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016;27(5):953–955. doi: 10.1093/annonc/mdw032. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto S., Ikushima S., Ono R., Awano N., Kondo K., Furuhata Y., Fukumoto K., Kumasaka T. Transformation to small-cell lung cancer as a mechanism of acquired resistance to crizotinib and alectinib. Jpn. J. Clin. Oncol. 2016;46(2):170–173. doi: 10.1093/jjco/hyv173. [DOI] [PubMed] [Google Scholar]
- 17.Leventakos K., Mansfield A.S. Advances in the treatment of non-small cell lung cancer: focus on nivolumab, pembrolizumab, and atezolizumab. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2016;30(5):397–405. doi: 10.1007/s40259-016-0187-0. [DOI] [PubMed] [Google Scholar]
- 18.Orlov S., Salari F., Kashat L., Walfish P.G. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J. Clin. Endocrinol. Metab. 2015;100(5):1738–1741. doi: 10.1210/jc.2014-4560. [DOI] [PubMed] [Google Scholar]