Abstract
Background
The therapeutic approach of gastric cancer strictly depends on TNM staging mainly provided by CT and PET/CT. However, the lymph node size criterion as detected by MDCT causes a poor differential diagnosis between reactive and metastatic enlarged lymph nodes with low specificity values.
Our study aims to compare 320-row CT Net enhancement and fluorine-18 fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography (F-FDG PET/CT) SUV for N staging of gastric cancer.
Materials and methods
45 patients with histologically proven gastric cancer underwent CT and F-FDG PET/CT. Two radiologists in consensus evaluated all images and calculated the CT Net enhancement and F-FDG PET/CT SUV for N staging, having the histological findings as the reference standard. CT and F-FDG PET/CT sensitivity, specificity, diagnostic accuracy, positive and negative predictive values (PPV and NPV) were evaluated and compared by using the Mc Nemar test.
Results
The histological examination revealed nodal metastases in 29/45 cases (64%). CT Net enhancement obtained sensitivity, specificity, accuracy, PPV and NPV of 90%, 81%, 87%, 90% and 81%, respectively. F-FDG PET/CT SUV obtained sensitivity, specificity, accuracy, PPV and NPV of 66%, 88%, 73%, 90% and 58%, respectively. No statistically significant difference between the two imaging modalities was found (p = 0.1).
Conclusion
CT Net enhancement represents an accurate tool for N staging of gastric cancer and could be considered as the CT corresponding quantitative parameter of F-FDG PET/CT SUV. It could be applied in the clinical practice for differentiating reactive lymph nodes from metastatic ones improving accuracy and specificity of CT.
Keywords: CT, Gastric cancer, N staging, Net enhancement, PET CT
Highlights
-
•
Gastric cancer N staging represents a diagnostic challenge for patient management.
-
•
CT and PET-TC play a crucial role in this field.
-
•
CT has a high sensitivity and a low specificity.
-
•
Disease over-staging causes ineffective care when patient categorized as palliative is excluded from curative treatment.
-
•
The proposed new 3D CT software with quantitative data for N staging improves CT specificity.
1. Introduction
Gastric cancer represents one of the most common cancer in the world and a major cause of morbidity and mortality [1], [2], [3]. The diagnosis of gastric cancer is provided by endoscopy associated with biopsy [2]. The therapeutic approach mainly bases on cancer staging which provides crucial information in terms of tumor resectability and patient prognosis. Cancer staging includes primary tumor local extension, lymph node involvement and distant metastases assessment [3], [4], [5]. The most widely used diagnostic technique for preoperative staging of gastric cancer is represented by multi-detector computed tomography (MDCT) [3], [5], [6], [7], [8], [9], [10]. An overall MDCT diagnostic accuracy of 94% for T staging has been reported applying specific software of image reconstructions with values of 96%, 96%, 98% and 100%, respectively for T1, T2, T3 and T4 stages [3]. Despite the recent advances in the field of pre-operative CT imaging of gastric cancer, N staging still remains controversial with lower accuracy value as compared with T staging [5], [6], [11]. As regard with gastric cancer M staging, PET and MDCT show overall accuracies respectively of 88 and 82% for detecting distant metastases [11].
The debated role of MDCT for N staging of gastric cancer is mainly related to the high frequency of microscopic tumor involvement in small-sized lymph nodes and the poor differential diagnosis between reactive and metastatic enlarged lymph nodes with low specificity values [12], [13], [14]. The recent use of metabolic information provided by fluorine-18 fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography (18F-FDG PET/CT) has improved the role of imaging in the field of cancer diagnosis, staging, therapeutic response and recurrence as suggested by the National Comprehensive Cancer Network and The European Society for Medical Oncology. However, also the role of 18F-FDG PET/CT in the staging of gastric cancer is still controversial because of its relatively low sensitivity [12], [13], [14].
The aim of our study is to compare the diagnostic accuracy of quantitative and qualitative parameters respectively provided by net enhancement (NE) value and by vessel probe (VP)/3D analysis reconstructions on 320- row CT for N staging of gastric cancer and to compare the obtained results with 18F-FDG PET –CT quantitative parameters.
2. Patients
Between September 2013 and March 2015, forty-five consecutive patients (18 women and 27 men; mean age: 58.6 y; age range: 35–80 y) with endoscopic and histological diagnosis of gastric cancer, underwent 320-row MDCT examination within 8 days after conventional endoscopy.
In all cases 18F-FDG-PET/CT scan was performed within 18 days after CT and before any treatment. Written informed consent was obtained from all patients for the diagnostic procedures. Pathological staging was established according to the 7th edition of the American Joint Committee on Cancer Staging guidelines, and histologic types were classified according to the World Health Organization classifications [15]. Surgery was performed within 15 days after imaging (mean interval time of 8 days). 39 out of 45 patients underwent radical surgical treatment (total or partial gastrectomy), while in the remaining 6 patients palliative gastrectomy was performed. No patient underwent preoperative chemotherapy or radiotherapy. CT and 18F-FDG PET/CT results were compared with operative and pathological data, having the latest as reference standard.
3. CT protocol
CT scans were performed with patients in the supine position from the lung apices to the pubic symphysis, before and after intravenous injection of 1.5 mL/kg of iopamidol (Iomeron 400; Bracco, Milan; Italy) at 3 mL/s through the antecubital vein with an automatic power injector.
Before CT examination, gastric wall distension was obtained by the intramuscular injection of 20 mg of scopolamine-N-butyl bromide (Buscopan, Boehringer Ingelhein Japan, Tokyo, Japan) and by ingestion of 400–600 ml of water, after 12 h of fasting.
A 320-row CT scanner (Aquilion One, Toshiba, Nasu, Japan) was used with the following protocol: detector collimation 320 × 0.5 mm, rotation time 0.5 s, mean kV/mAs: 120/200; in all cases, the volumetric acquisition was performed during the venous phase 55 s after contrast material injection. Radiation dose to patients was modulated for each study by means of the volume CT dose index, which was calculated by the CT scanner.
4. 18F-FDG PET/CT protocol
Images were acquired by using a Discovery LSA PET/CT device (GE Healthcare, Waukesha, WI) combining a PET (advance nI) with 16-slice CT scanner (light speed plus). All patients had a capillary blood glucose of <160 mg/mL and fasted for at least 8 h before 18F-FDG injection. Gastric distension was obtained by drinking 500 mL of water before images acquisition. The image acquisition was performed 50 min after intravenous injection of 4.6 MBq/kg of 18F-FDG. The CT scan was carried out without contrast material injection from the neck to the pubic symphysis with the following CT protocol: mean kV/mAs:120 kV/340 mA; slice thickness: 3.75 mm, tube rotation time: 0.8 ms, collimation field of view: 50 cm. The CT data were used for the attenuation correction of PET scanning, performed immediately after CT scans.
The PET acquisition was obtained in caudal-cranial direction; PET was reconstructed with a matrix of 128128, ordered subset expectation maximum iterative reconstruction algorithm (2 iterations, 28 subsets), 8 mm Gaussian filter, and 50 cm field of view.
5. Image analysis
All CT data were transferred to a workstation (HP XW8600, Minnetonka, US) equipped with a dedicated software for image reconstructions (Vitrea Fx, Vital Images, Minneapolis, Minnesota, US). Two radiologists with more than 10 years' experience in the field of CT imaging and blinded to the histological results of peri-gastric lymph nodes evaluated CT images in consensus using a threshold net enhancement value of 40 HU for differentiating reactive lymph nodes from metastatic ones.
CT images were evaluated by applying the Vessel Probe (VP) in MPR reconstructions and the automatic 3D analysis software which respectively provided peri-gastric lymph nodes qualitative and quantitative data [3], [16].
Multi-Planar-Reformatting (MPR) and Vessel Probe (VP) in MPR mode programs were used for the qualitative analysis of peri-gastric lymph nodes including shape (round, oval or irregular) and edges (regular or irregular). By clicking on the lymph node site in simple transverse images, VP in MPR mode automatically generates a reference line along the major axis of lymph node and displays the best views in multiple curved planes [3], [16].
Automatic 3D analysis software was used for the quantitative analysis of peri-gastric lymphonodes. It was applied for measuring lymph node diameters and density by selecting a region of interest (ROI) on the whole lymph node. Lymph node density values were calculated before and after contrast material injection and then Net enhancement (NE) was calculated by subtracting pre-contrast density from the density obtained by post-contrast values, as already reported in other experiences with regard to other anatomical districts [17]. The overall image analysis was performed within 15 min for patient.
Two nuclear physicians with more than 8 years' experience in the field of PET-CT imaging and blinded to the histological results of peri-gastric lymph nodes evaluated 18F-FDG PET-CT images in consensus. 18F-FDG PET/CT was considered positive for lymph node involvement for any increased 18F-FDG uptake in at least 1 lymph node.
Volume of interest (VOI) was drawn semi-automatically on the high 18F-FDG uptake area incorporating each target lesion in the 3 axes of PET images. Semi-quantitative analysis was performed calculating maximum and mean standardized uptake values (SUVmax and SUVmean), using the maximum and mean activity values within each VOI with the highest radioactivity concentration, normalized to the injected dose, and patient's body weight. A SUV threshold value of 3.6 was used to determine metastatic lymph nodes.
6. Statistical analysis
CT and PET-CT images were compared with histological findings, having the latest as the reference standard. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and diagnostic accuracy of CT and PET-CT for detecting and characterizing of lymph nodes were calculated basing on qualitative (morphological features) and quantitative (diameter, NE and SUV values) parameters. The overall diagnostic potential of the two imaging techniques was compared by using Mc Nemar test. Any statistically significant difference in terms of sensitivity, specificity and accuracy between the two imaging tools was also calculated by using the comparison of proportions. A P value of <0.05 was considered statistically significant.
The obtained results were graphically represented by ROC analysis.
All calculations were performed by using NCSS2007® statistical software (Kaysville, Utah,US).
7. Results
Among the 45 patients, the primary gastric tumors were located on the gastric body in 26 cases (58%), on the fundus-cardia in 12 (27%) and the remaining 7 (15%) on the gastric antrum.
The histological examination revealed intestinal type adenocarcinoma in all cases and nodal metastases in 29 out of 45 cases (64%). In particular, 18 N1 cases and 11 N2 were identified (Table 1).
Table 1.
Demographic features of the enrolled patients with tumor site indication and definitive N staging.
| Patients | Women | Men | Mean age | Age range | Gastric body | Fundus-cardia | Antrum | N0 | N1 | N2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 45 | 18 | 27 | 58.6 y | 35-80 y | 26 | 12 | 7 | 16 | 18 | 11 |
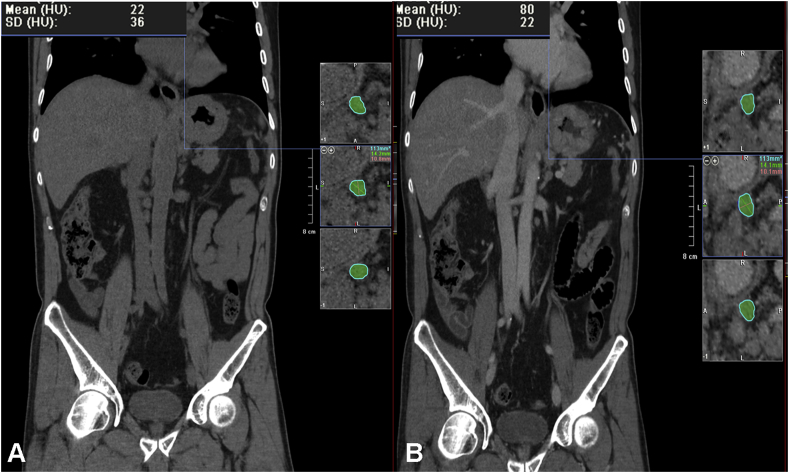
CT Net enhancement obtained sensitivity, specificity, diagnostic accuracy, positive predictive and negative predictive values respectively of 90%, 81%, 87%, 90% and 81%. In particular, in 29 cases a NE value of >40 HU was found (mean value ± standard deviation, 48 ± 2.4 HU) staged as N1 in 18 cases and N2 in the remaining 11 (Fig. 1). The mean short axis diameter of the detected lymph nodes was 1.6 cm (range 0.6÷2 cm). In 16 cases a NE value of <40 HU was found (mean value ± standard deviation, 31 ± 1.9 HU), classified as N0 in all cases. The mean short axis diameter of the detected lymph nodes was 1.1 cm (range 0.5÷1.3 cm). By comparing the CT data with the histological ones, 26 true positives, 13 true negatives, 3 false positives and 3 false negative occurred in our series.
Fig. 1.
CT images on the coronal plane with Net enhancement value provided by 3D Analysis CT software and Vessel Probe reconstructions showing a case of N2 staged gastric cancer with Net enhancement of 58 HU. A. Unenhanced scan. B. Enhanced CT scan on the portal venous phase.
PET-CT SUV examination obtained sensitivity, specificity, diagnostic accuracy, positive predictive and negative predictive values respectively of 66%, 88%, 73%, 90% and 58%. In particular, 19 true positives, 14 true negatives, 2 false positives and 10 false negative occurred in our series. In particular, in 21 cases a mean SUV value of 3.6 was found and staged as N1 in 15 cases and N2 in the remaining 6 (Fig. 2). In 24 cases no lymph node uptake was found and they were classified as N0 (Table 2).
Fig. 2.
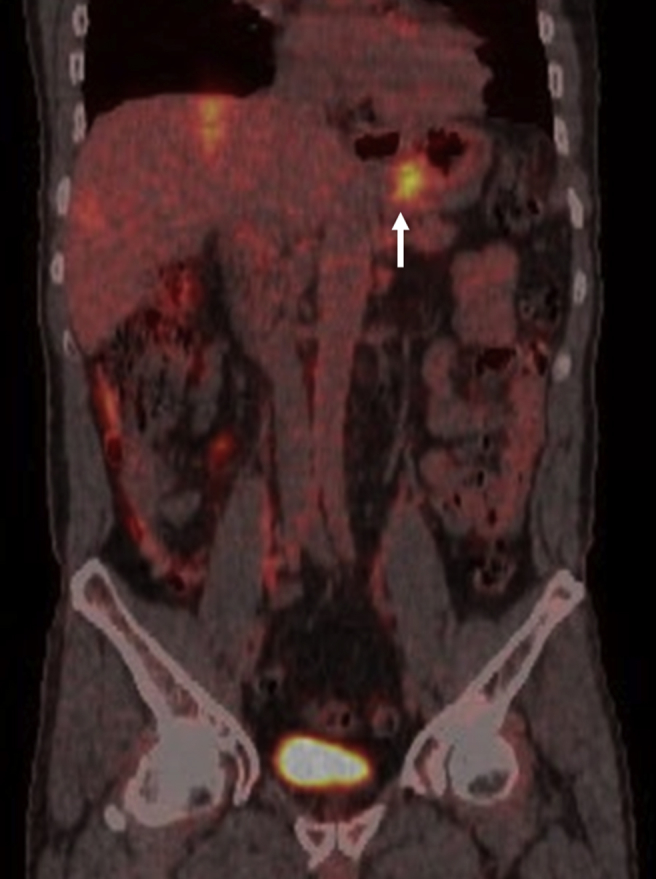
Same case of Fig. 1. PET-CT scan showing the N2 staged gastric cancer with perigastric nodal involvement (arrow) and a mean SUV of 4.
Table 2.
CT and PET-CT N staging compared with the histological data.
| N0 | N1 | N2 | |
|---|---|---|---|
| CT | 16 | 18 | 11 |
| PET-CT | 24 | 15 | 6 |
| Histology | 16 | 18 | 11 |
Mc Nemar test showed no statistically significant difference between the two imaging modality diagnostic potential (p = 0.1; 95% confidence interval 0.06÷15.98).
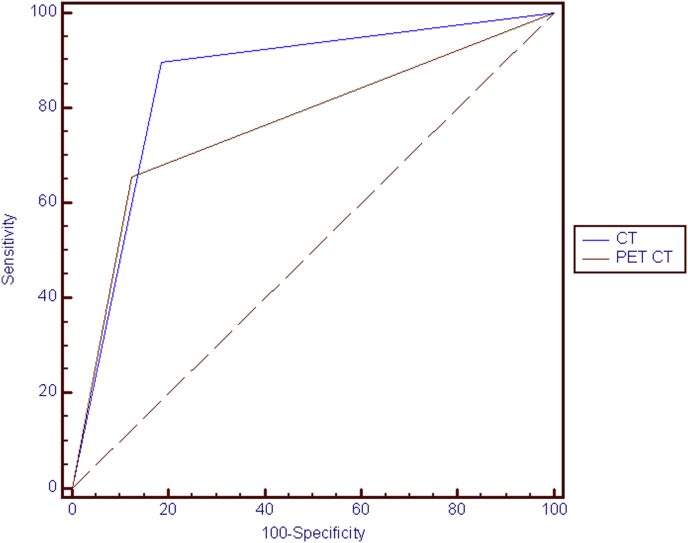
By using the comparison of proportion, no statistically significant difference was found in terms of diagnostic accuracy and specificity with p values respectively of p = 0.09; 95% confidence interval −4.06÷31.33 and p = 0.36; 95% confidence interval −9.61÷23.41, as shown by ROC analysis (Fig. 3).
Fig. 3.
ROC analysis showing no statistically significant difference between CT and PET-CT diagnostic potential.
A statistically significant higher sensitivity for CT as compared with PET-CT was found with p = 0.006; 95% confidence interval 5.54–41.09 (Table 3).
Table 3.
CT and PET-CT performance results for N staging with corresponding p values obtained in our series.
| Sensitivity | Specificity | Diagnostic accuracy | |
|---|---|---|---|
| CT | 90 | 81 | 87 |
| PET-CT | 66 | 88 | 73 |
| P values | 0.006 | 0.36 | 0.09 |
8. Discussion
The efforts to improve pre-operative staging of gastric cancer have not jet allowed to overcome the discrepancy between pre-operative and post-operative assessments [11]. Pre-operative assessment mainly bases on such techniques as CT of abdomen and pelvis, chest imaging, pelvic ultrasound, PET, PET-CT, esophagogastroduodenoscopy (EGDS) and endoscopic ultrasound (EUS) as reported by Current National Comprehensive Cancer Network (NCCN) practice guidelines for gastric cancer [18], [19], [20], [21], [22], [23], [24], [25]. On the other hand, the post-operative assessment includes intra-operative and pathological findings. In about one third of patients treated for gastric cancer, the post-operative assessment differed from pre-operative staging and this leads to patient re-assignment into a different pathologic stage category post-operatively [11], [20], [21], [22], [23], [26], [27]. Therefore, the limitations of pre-operative staging could cause disease understaging with positive resection margins or unnecessary laparotomy if metastases are not identified or disease overstaging with ineffective care if a patient categorized as palliative is excluded from potentially curative treatment [11], [20], [21], [22], [23], [24], [25], [26], [27]. The crucial issue of cancer staging mainly concerns lymph node involvement and medical literature reported that neither abdominal US, MDCT, conventional MRI, nor PET could reliably confirm or exclude the presence of lymph node metastasis [27], [28], [29], [30], [31], [32]. In particular, MDCT main limitation is represented by size criterion, which does not allow a differential diagnosis between reactive and metastatic lymph nodes. In fact, metastatic lymph nodes are not always enlarged while enlarged lymph nodes are not always metastatic. The majority of literature studies reported a diameter of 8 mm for suggesting lymph node involvement, even if this measure is calculated on the short axis in some cases and the long axis in other cases. For this reason, in our series, node diameter ranged between 6 mm and 2 cm and size criterion was not used for differential diagnosis between pathological or reactive nodes.
The overall sensitivity and specificity values reported for MDCT are 77% and 78%, respectively, regardless of the number of detector used and the type of image reconstruction [11], [12], [13], [14].
Differently, FDG-PET-CT provides metabolic features of lymph nodes regardless of their size contributing to improve imaging specificity but is limited by several factors, including time post-FDG injection, tumor size, normo-glycemia, technical parameters by such cancer histotypes as mucinous cancers which show low FDG uptake. These factors cause a low sensitivity value for PET-CT, being 40% the worst value reported for differentiating N0 and N+ lymph nodes. On the other hand, it represents a highly specific tool in N staging in order to clarify true positive patients with the best specificity values of 98%. Therefore, from the data reported in the medical literature, PET-CT has the highest specificity and the lowest sensitivity while CT the highest sensitivity and the lowest specificity values [12].
For this reason, the National Comprehensive Cancer Network recently recommend 18F-FDG PET/CT as a useful preoperative diagnostic tool for gastric cancer staging [12].
However, the mean SUV noted for N staging can also be variable, ranging from 4.5 to 6.8, and mean SUVs may overlap between N stage categories [12]. In our series, a threshold SUV value of 3.6 was used for gastric N staging.
Our study aims to introduce a new CT quantitative parameter useful for improving its diagnostic performance and to compare the obtained results with PET-CT metabolic parameters in N staging of gastric cancer. It is represented by the Net enhancement with a threshold value of 40 HU as highly suggestive of metastatic lymph nodes [17].
By using this parameter, in our experience a sensitivity and specificity of 90% and 81% respectively was found. The specificity value is slightly higher as compared with the values reported in previous studies; however, a new important information could emerge from our results. In fact, no significant difference in terms of specificity between CT and PET-CT was found and this could probably be due to the NE introduction for N staging allowing to differentiate benign form malignant nodes with an important reduction of false positive cases.
In fact, in our preliminary experience, the NE calculation allowed a reduction of false positive cases at CT by excluding reactive enlarged lymph nodes with low NE and therefore increasing the specificity valued of this tool to 81% that is significantly higher as compared to previous series.
Our study has some important limitations mainly represented by the relative small number of the enrolled patients. All images were analysed in consensus, therefore, an inter-observer agreement was not calculated for the proposed new CT software. N staging evaluation was performed regardless of the size criterion. Another important limitation is represented by the choice of considering imaging positivity in at least 1 lymph node as overall positivity for lymph node involvement without a lymph node by lymph node comparison between imaging and surgical data. The proposed software was not tested for lymph nodes smaller than 6 mm as not found in the examined sample.
Finally the study has been reported in line with the PROCESS criteria [33].
9. Conclusions
CT Net enhancement represents an accurate tool for N staging of gastric cancer and could be considered as the CT correspondent quantitative parameter of F-FDG PET/CT SUV. It could be applied in the clinical practice for differentiating reactive lymph nodes from metastatic ones improving accuracy and specificity of CT.
Ethical approval
None.
Sources of funding
None.
Author contribution
1 guarantor of integrity of the entire study: Stabile Ianora A Antonio.
2 study concepts and design: Stabile Ianora A Antonio, Telegrafo Michele, Moschetta Marco.
3 literature research: Lorusso Valentina, Niccoli A Artor, Moschetta Marco.
4 clinical studies: Stabile Ianora A Antonio, Telegrafo Michele, Lucarelli N Maria, Lorusso Valentina, Scardapane Arnaldo, Niccoli A Artor, Moschetta Marco.
5 experimental studies/data analysis: Stabile Ianora A Antonio, Telegrafo Michele, Lucarelli N Maria, Lorusso Valentina, Scardapane Arnaldo, Niccoli A Artor, Moschetta Marco.
6 statistical analysis: Telegrafo Michele, Scardapane Arnaldo, Niccoli A Artor, Moschetta Marco.
7 manuscript preparation: Stabile Ianora A Antonio, Telegrafo Michele, Scardapane Arnaldo, Moschetta Marco.
8 manuscript editing: Stabile Ianora A Antonio, Telegrafo Michele, Lucarelli N Maria, Lorusso Valentina, Scardapane Arnaldo, Niccoli A Artor, Moschetta Marco.
Conflicts of interest
None.
Guarantor
Marco Moschetta.
Research registration unique identifying number (UIN)
Researchregistry 1858.
References
- 1.Kim J.H., Eun H.W., Choi J.H., Hong S.S., Kang W., Auh Y.H. Diagnostic performance of virtual gastroscopy using MDCT in early gastric cancer compared with 2D axial CT: focusing on interobserver variation. Am. J. Roentgenol. 2007;189:299–305. doi: 10.2214/AJR.07.2201. PMid:17646454. [DOI] [PubMed] [Google Scholar]
- 2.Habermann C.R., Weiss F., Riecken R., Honarpisheh H., Bohnacker S., Staedtler C., Dieckmann C., Schoder V., Adam G. Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology. 2004;230:465–471. doi: 10.1148/radiol.2302020828. PMid:14752188. [DOI] [PubMed] [Google Scholar]
- 3.Moschetta M., Stabile Ianora A.A., Anglani A., Marzullo A., Scardapane A., Angelelli G. Preoperative T staging of gastric carcinoma obtained by MDCT vessel probe reconstructions and correlations with histological findings. Eur. Radiol. 2010;20(1):138–145. doi: 10.1007/s00330-009-1482-7. PMid:19504100. [DOI] [PubMed] [Google Scholar]
- 4.Chen C.Y., Hsu J.S., Wu D.C., Kang W.Y., Hsieh J.S., Jaw T.S., Wu M.T., Liu G.C. Gastric cancer: preoperative local staging with 3D multi-detector row CT—correlation with surgical and histopathologic results. Radiology. 2007;242:472–482. doi: 10.1148/radiol.2422051557. PMid:17255419. [DOI] [PubMed] [Google Scholar]
- 5.Ahn H.S., Lee H.J., Yoo M.W., Kim S.G., Im J.P., Kim S.H., Kim W.H., Lee K.U., Yang H.K. Diagnostic accuracy of T and N stages with endoscopy, stomach protocol CT, and endoscopic ultrasonography in early gastric cancer. J. Surg. Oncol. 2009;99:20–27. doi: 10.1002/jso.21170. PMid:18937292. [DOI] [PubMed] [Google Scholar]
- 6.Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J. Clin. Oncol.. 2007; 25:2107–2116. doi: 10.1200/JCO.2006.09.5224 PMid:17513817. [DOI] [PubMed]
- 7.Moschetta M., Scardapane A., Telegrafo M., Lorusso V., Angelelli G., Stabile Ianora A.A. Differential diagnosis between benign and malignant ulcers:320-row CT virtual gastroscopy. Abdom. Imaging. 2012;37:1066–1073. doi: 10.1007/s00261-012-9849-7. PMid:22289996. [DOI] [PubMed] [Google Scholar]
- 8.Angelelli G., Moschetta M., Cosmo T., Binetti F., Scardapane A., Stabile Ianora A.A. CT diagnosis of the nature of bowel obstruction: morphological evaluation of the transition point. Radiol. Med. 2012;117:749–758. doi: 10.1007/s11547-011-0770-x. PMid:22228127. [DOI] [PubMed] [Google Scholar]
- 9.Angelelli G., Moschetta M., Sabato L., Morella M., Scardapane A., Stabile Ianora A.A. Value of “protruding lips” sign in malignant bowel obstructions. Eur. J. Radiol. 2011;80:681–685. doi: 10.1016/j.ejrad.2010.09.034. PMid:21030174. [DOI] [PubMed] [Google Scholar]
- 10.Angelelli G., Moschetta M., Binetti F., Cosmo T., Stabile Ianora A.A. Prognostic value of MDCT in malignant large-bowel obstructions. Radiol. Med. 2010;115:747–757. doi: 10.1007/s11547-010-0527-y. PMid:20177982. [DOI] [PubMed] [Google Scholar]
- 11.Seevaratnam R., Cardoso R., McGregor C., Lourenco L., Mahar A., Sutradhar R., Law C., Paszat L., Coburn N. How useful is preoperative imaging for tumor, node, metastasis (TNM) staging ofgastric cancer? A meta-analysis. Gastric Cancer. 2012;15(Suppl 1):S3–S18. doi: 10.1007/s10120-011-0069-6. PMid:21837458. [DOI] [PubMed] [Google Scholar]
- 12.Altini C., Niccoli Asabella A., Di Palo A., Fanelli M., Ferrari C., Moschetta M., Rubini G. 18F-FDG PET/CT role in staging of gastric carcinomas: comparison with conventional contrast enhancement computed tomography. Med. Baltim. 2015;94(20):e864. doi: 10.1097/MD.0000000000000864. PMid:25997066 PMCid:PMC4602890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NCCN clinical practice guidelines in oncology (NCCN guidelines) J. Natl. Compr. Canc. Netw. Gastric Cancer. 2013;11:531–546. http://www.jnccn.org/content/11/5/531.full [Google Scholar]
- 14.Waddell T., Verheij M., Allum W., Cunningham D., Cervantes A., Arnold D. Gastric cancer: ESMOESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013;24:57–63. doi: 10.1093/annonc/mdt344. PMid:24078663. [DOI] [PubMed] [Google Scholar]
- 15.Sobin L.H., Gospodarowicz M.K., Wittekind C. seventh ed. Wiley-Blackwell; New York: 2009. TNM Classification of Malignant Tumours. [Google Scholar]
- 16.Moschetta M., Ianora A.A., Marzullo A., Scardapane A., Angelelli G. Vessel probe CT protocol in the study of esophageal carcinoma: can it improve preoperative T staging? Eur. J. Surg. Oncol. 2010;36:663–669. doi: 10.1016/j.ejso.2010.05.011. PMid:20627648. [DOI] [PubMed] [Google Scholar]
- 17.Moschetta M., Scardapane A., Lorusso V., Rella L., Telegrafo M., Serio G., Angelelli G., Ianora A.A. Role of multidetector computed tomography in evaluating incidentally detected breast lesions. Tumori. 2015;101:455–460. doi: 10.5301/tj.5000291. PMid:25908028. [DOI] [PubMed] [Google Scholar]
- 18.Ly Q.P., Sasson A.R. Modern surgical considerations for gastric cancer. J. Natl. Compr. Cancer Netw. 2008;6:885–894. doi: 10.6004/jnccn.2008.0067. PMid:18926098. [DOI] [PubMed] [Google Scholar]
- 19.Kovoor P.A., Hwang J. Treatment of resectable gastric cancer: current standards of care. Expert Rev. Anticancer Ther. 2009;9:135–142. doi: 10.1586/14737140.9.1.135. PMid:19105713. [DOI] [PubMed] [Google Scholar]
- 20.Hartgrink H.H., Jansen E.P., van Grieken N.C., van de Velde C.J. Gastric cancer. Lancet. 2009;374:477–490. doi: 10.1016/S0140-6736(09)60617-6. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catalano V., Labianca R., Beretta G.D., Gatta G., de Braud F., Van Cutsem E. Gastric cancer. Crit. Rev. Oncol. Hematol. 2009;71:127–164. doi: 10.1016/j.critrevonc.2009.01.004. PMid:19230702. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz R.E. Factors influencing change of preoperative treatment intent in a gastrointestinal cancer practice. World J. Surg. Oncol. 2007;5:32. doi: 10.1186/1477-7819-5-32. PMid:17355626 PMCid:PMC1838912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network N . National Comprehensive Cancer Network, Inc.; 2010. NCCN Clinical Practice Guidelines in Oncology—gastric Cancer—V.2.2010; p. 7. [DOI] [PubMed] [Google Scholar]
- 24.Lim J.H., Ko Y.T., Lee D.H. Transabdominal US staging of gastric cancer. Abdom. Imaging. 1994;19:527–531. doi: 10.1007/BF00198255. PMid:7820025. [DOI] [PubMed] [Google Scholar]
- 25.Suk K.T., Lim D.W., Kim M.Y., Park D.H., Kim K.H., Kim J.M., Kim J.W., Kim H.S., Kwon S.O., Baik S.K., Park S.J. Thickening of the gastric wall on transabdominal sonography: a sign of gastric cancer. J. Clin. Ultrasound. 2008;36:462–466. doi: 10.1002/jcu.20450. PMid:18335513. [DOI] [PubMed] [Google Scholar]
- 26.Solbiati L., Tonolini M., Cova L., Goldberg S.N. The role of contrast-enhanced ultrasound in the detection of focal liver lesions. Eur. Radiol. 2001;11(Suppl 3):E15–E26. doi: 10.1007/pl00014125. PMid:11793049. [DOI] [PubMed] [Google Scholar]
- 27.Chen C.Y., Wu D.C., Kang W.Y., Hsu J.S. Staging of gastric cancer with 16-channel MDCT. Abdom. Imaging. 2006;31:514–520. doi: 10.1007/s00261-005-0218-7. PMid:16465577. [DOI] [PubMed] [Google Scholar]
- 28.Kim A.Y., Kim H.J., Ha H.K. Gastric cancer by multidetector row CT: preoperative staging. Abdom. Imaging. 2005;30:465–472. doi: 10.1007/s00261-004-0273-5. PMid:15785907. [DOI] [PubMed] [Google Scholar]
- 29.Tunaci M. Carcinoma of stomach and duodenum: radiologic diagnosis and staging. Eur. J. Radiol. 2002;42(3):181–192. doi: 10.1016/s0720-048x(02)00035-9. [DOI] [PubMed] [Google Scholar]
- 30.Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J. Clin. Oncol.. 2007;25:2107–2116. doi: 10.1200/JCO.2006.09.5224 PMid:17513817. [DOI] [PubMed]
- 31.Koh D.M., Collins D.J. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am. J. Roentgenol. 2007;188:1622–1635. doi: 10.2214/AJR.06.1403. PMid:17515386. [DOI] [PubMed] [Google Scholar]
- 32.Dassen A.E., Lips D.J., Hoekstra C.J., Pruijt J.F., Bosscha K. FDGPET has no definite role in preoperative imaging in gastric cancer. Eur. J. Surg. Oncol. 2009;35:449–455. doi: 10.1016/j.ejso.2008.11.010. PMid:19147324. [DOI] [PubMed] [Google Scholar]
- 33.Agha R.A., Fowler A.J., Rajmohan S., Barai I., Orgill D.P. Preferred reporting of case series in surgery; the PROCESS guidelines. Int. J. Surg. 2016;36(Pt A):319–323. doi: 10.1016/j.ijsu.2016.10.025. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]