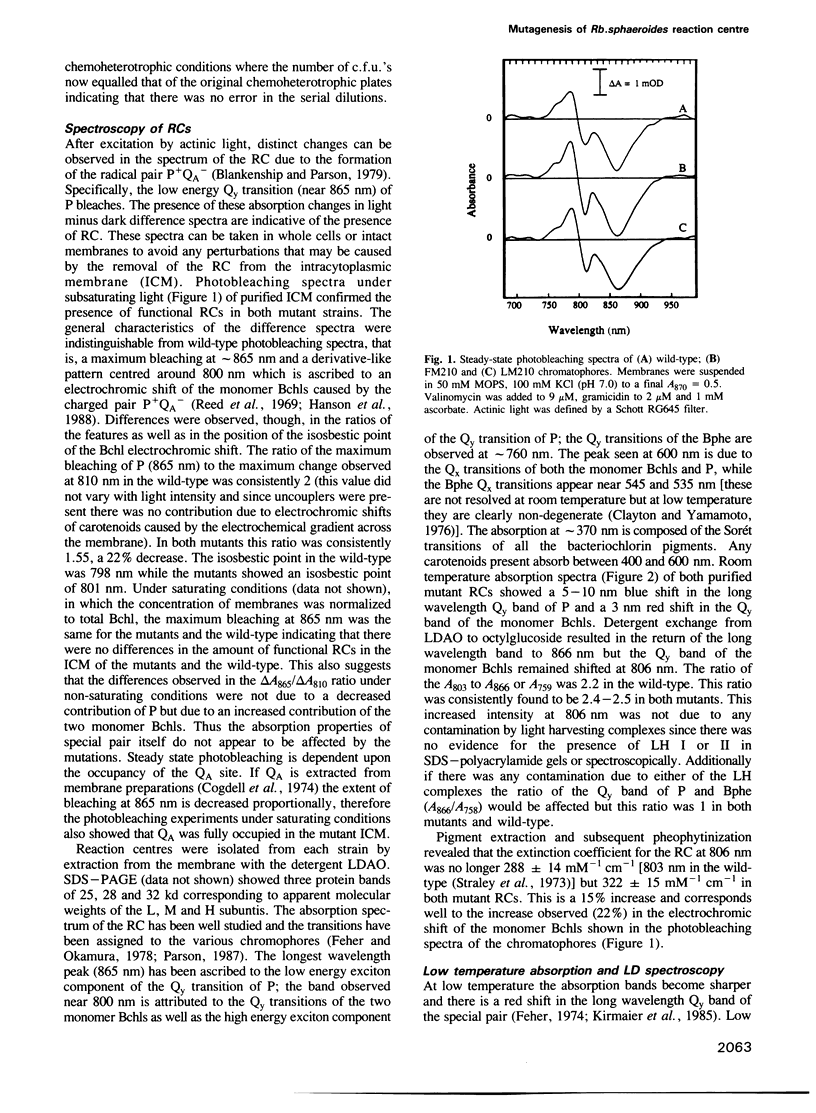
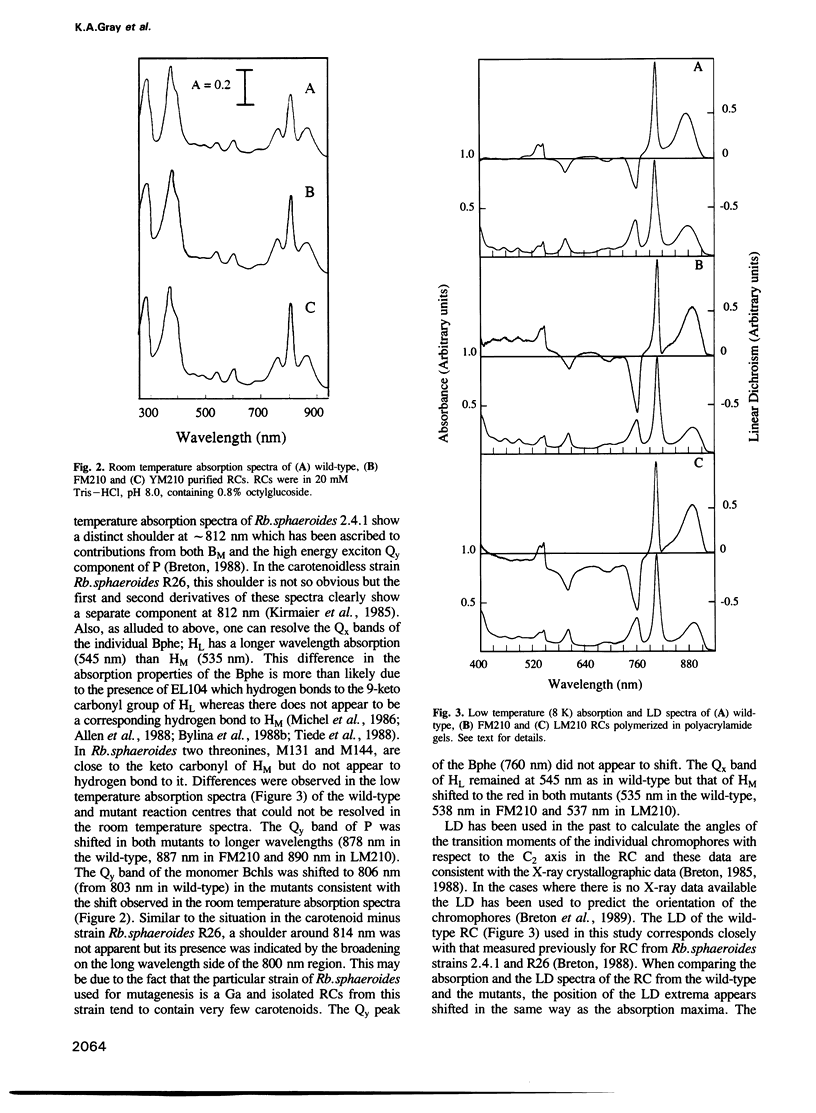
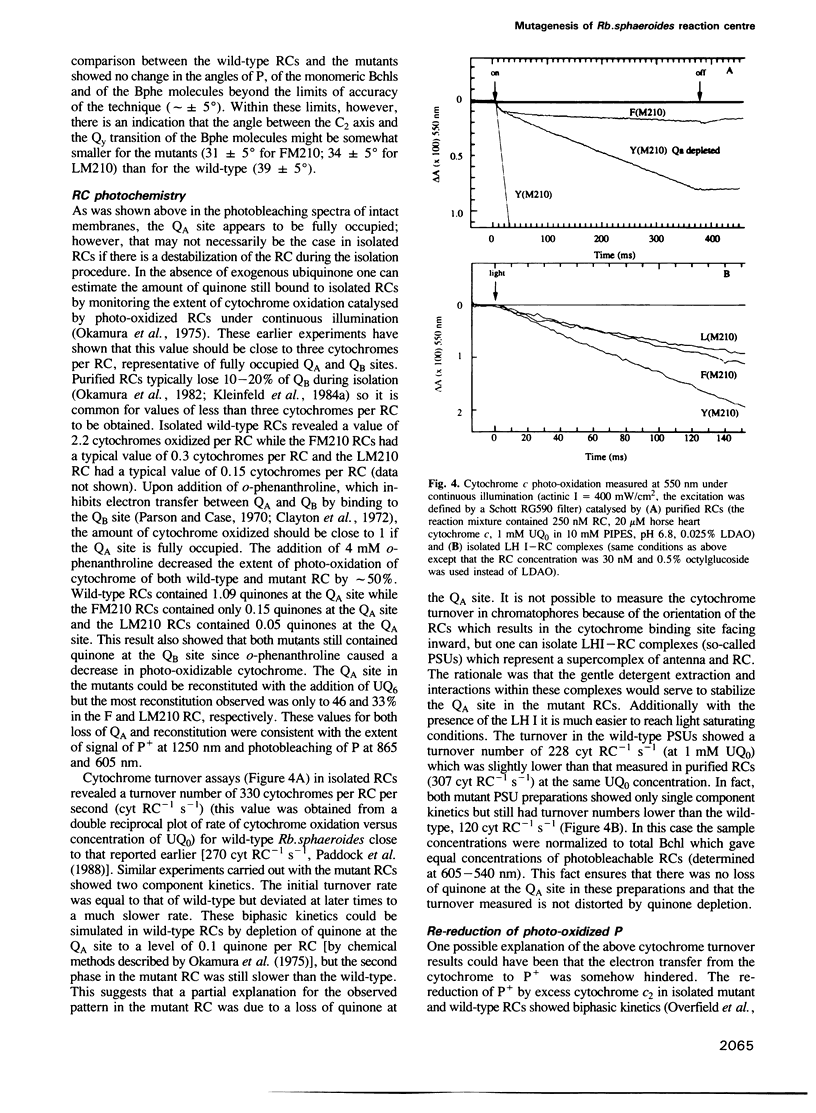
Abstract
A description of the properties of site-directed mutants of the reaction centre (RC) of Rhodobacter sphaeroides is presented. The residue tyrosine M210 (YM210) has been changed to phenylalanine (FM210) and leucine (LM210). Both mutants grew photoheterotrophically under conditions of high light but only the FM210 mutant grew under low light. Photobleaching spectra of chromatophores isolated from these mutants showed that the amount of functional RC was comparable to wild-type and that the spectral features were essentially unchanged. Shifts were observed in the absorption spectra in regions attributable to all the chromophores. An increase in intensity and a 3 nm red shift (from 803 to 806 nm) was observed in the Qy band of the monomer bacteriochlorophylls. A new extinction coefficient for the RC was determined at 806 nm (332 +/- 15 mM-1 cm-1). Linear dichroism (LD) spectra showed that there was no significant large scale change in the angles of the individual pigments relative to the C2 axis of symmetry. Cytochrome turnover assays were performed on isolated RC and light harvesting I complex (LH I)-RC (photosynthetic units, PSU) preparations. A turnover number of 120 cyt RC-1 s-1 was calculated for both the mutants while wild-type had a turnover number of 228 cyt RC-1 s-1. The cytochrome c2-mediated re-reduction kinetics of P+ were comparable to those observed in the wild-type. The half-time of charge recombination within the RC increased in the mutants to the wild-type (100 ms in the wild type, and 150 and 200 ms in FM210 and LM210, respectively).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the protein subunits. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6162–6166. doi: 10.1073/pnas.84.17.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer J. R., Tierney G. V., Crofts A. R. Secondary electron transfer in chromatophores of Rhodopseudomonas capsulata A1a pho. Binary out-of-phase oscillations in ubisemiauinone formation and cytochrome b50 reduction with consective light flashes. FEBS Lett. 1979 May 1;101(1):201–206. doi: 10.1016/0014-5793(79)81326-5. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Tiede D., Tang J., Smith U., Norris J., Schiffer M. Structure of Rhodopseudomonas sphaeroides R-26 reaction center. FEBS Lett. 1986 Sep 1;205(1):82–86. doi: 10.1016/0014-5793(86)80870-5. [DOI] [PubMed] [Google Scholar]
- Cogdell R. J., Brune D. C., Clayton R. K. Effects of extraction and replacement of ubiquinone upon the photochemical activity of reaction centers and chromatophores from Rhodopseudomonas spheriodes. FEBS Lett. 1974 Sep 1;45(1):344–347. doi: 10.1016/0014-5793(74)80877-x. [DOI] [PubMed] [Google Scholar]
- Debus R. J., Barry B. A., Babcock G. T., McIntosh L. Site-directed mutagenesis identifies a tyrosine radical involved in the photosynthetic oxygen-evolving system. Proc Natl Acad Sci U S A. 1988 Jan;85(2):427–430. doi: 10.1073/pnas.85.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus R. J., Barry B. A., Sithole I., Babcock G. T., McIntosh L. Directed mutagenesis indicates that the donor to P+680 in photosystem II is tyrosine-161 of the D1 polypeptide. Biochemistry. 1988 Dec 27;27(26):9071–9074. doi: 10.1021/bi00426a001. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Michel H. Nobel lecture. The photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis. EMBO J. 1989 Aug;8(8):2149–2170. doi: 10.1002/j.1460-2075.1989.tb08338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault D. Quantum mechanical tunnelling in biological systems. Q Rev Biophys. 1980 Nov;13(4):387–564. doi: 10.1017/s003358350000175x. [DOI] [PubMed] [Google Scholar]
- Farchaus J. W., Gruenberg H., Oesterhelt D. Complementation of a reaction center-deficient Rhodobacter sphaeroides pufLMX deletion strain in trans with pufBALM does not restore the photosynthesis-positive phenotype. J Bacteriol. 1990 Feb;172(2):977–985. doi: 10.1128/jb.172.2.977-985.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farchaus J. W., Oesterhelt D. A Rhodobacter sphaeroides puf L, M and X deletion mutant and its complementation in trans with a 5.3 kb puf operon shuttle fragment. EMBO J. 1989 Jan;8(1):47–54. doi: 10.1002/j.1460-2075.1989.tb03347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld D., Okamura M. Y., Feher G. Electron transfer in reaction centers of Rhodopseudomonas sphaeroides. I. Determination of the charge recombination pathway of D+QAQ(-)B and free energy and kinetic relations between Q(-)AQB and QAQ(-)B. Biochim Biophys Acta. 1984 Jul 27;766(1):126–140. doi: 10.1016/0005-2728(84)90224-x. [DOI] [PubMed] [Google Scholar]
- Komiya H., Yeates T. O., Rees D. C., Allen J. P., Feher G. Structure of the reaction center from Rhodobacter sphaeroides R-26 and 2.4.1: symmetry relations and sequence comparisons between different species. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9012–9016. doi: 10.1073/pnas.85.23.9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel H., Epp O., Deisenhofer J. Pigment-protein interactions in the photosynthetic reaction centre from Rhodopseudomonas viridis. EMBO J. 1986 Oct;5(10):2445–2451. doi: 10.1002/j.1460-2075.1986.tb04520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierendorf R. C., Pfeffer D. Direct sequencing of denatured plasmid DNA. Methods Enzymol. 1987;152:556–562. doi: 10.1016/0076-6879(87)52061-4. [DOI] [PubMed] [Google Scholar]
- Okamura M. Y., Isaacson R. A., Feher G. Primary acceptor in bacterial photosynthesis: obligatory role of ubiquinone in photoactive reaction centers of Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3491–3495. doi: 10.1073/pnas.72.9.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Shmuckler B. E., Zargarov A. A., Kutuzov M. A., Telezhinskaya I. N., Levina N. B., Zolotarev A. S. Photosynthetic reaction centre of Chloroflexus aurantiacus. Primary structure of M-subunit. FEBS Lett. 1988 May 23;232(2):364–368. doi: 10.1016/0014-5793(88)80770-1. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Zolotarev A. S., Shmukler B. E., Zargarov A. A., Kutuzov M. A., Telezhinskaya I. N., Levina N. B. Photosynthetic reaction centre of Chloroflexus aurantiacus. I. Primary structure of L-subunit. FEBS Lett. 1988 Apr 11;231(1):237–242. doi: 10.1016/0014-5793(88)80739-7. [DOI] [PubMed] [Google Scholar]
- Overfield R. E., Wraight C. A., Devault D. Microsecond photooxidation kinetics of cytochrome c2 from Rhodopseudomonas sphaeroides: in vivo and solution studies. FEBS Lett. 1979 Sep 1;105(1):137–142. doi: 10.1016/0014-5793(79)80903-5. [DOI] [PubMed] [Google Scholar]
- Reed D. W., Zankel K. L., Clayton R. K. THE EFFECT OF REDOX POTENTIAL ON P870 FLUORESCENCE IN REACTION CENTERS FROM Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1969 May;63(1):42–46. doi: 10.1073/pnas.63.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. C., Komiya H., Yeates T. O., Allen J. P., Feher G. The bacterial photosynthetic reaction center as a model for membrane proteins. Annu Rev Biochem. 1989;58:607–633. doi: 10.1146/annurev.bi.58.070189.003135. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Jun;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- Shiozawa J. A., Lottspeich F., Oesterhelt D., Feick R. The primary structure of the Chloroflexus aurantiacus reaction-center polypeptides. Eur J Biochem. 1989 Mar 1;180(1):75–84. doi: 10.1111/j.1432-1033.1989.tb14617.x. [DOI] [PubMed] [Google Scholar]
- Sinning I., Michel H., Mathis P., Rutherford A. W. Characterization of four herbicide-resistant mutants of Rhodopseudomonas viridis by genetic analysis, electron paramagnetic resonance, and optical spectroscopy. Biochemistry. 1989 Jun 27;28(13):5544–5553. doi: 10.1021/bi00439a031. [DOI] [PubMed] [Google Scholar]
- Stanssens P., Opsomer C., McKeown Y. M., Kramer W., Zabeau M., Fritz H. J. Efficient oligonucleotide-directed construction of mutations in expression vectors by the gapped duplex DNA method using alternating selectable markers. Nucleic Acids Res. 1989 Jun 26;17(12):4441–4454. doi: 10.1093/nar/17.12.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Parson W. W., Mauzerall D. C., Clayton R. K. Pigment content and molar extinction coefficients of photochemical reaction centers from Rhodopseudomonas spheroides. Biochim Biophys Acta. 1973 Jun 28;305(3):597–609. doi: 10.1016/0005-2728(73)90079-0. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Feher G. Primary structure of the reaction center from Rhodopseudomonas sphaeroides. Proteins. 1986 Dec;1(4):312–325. doi: 10.1002/prot.340010405. [DOI] [PubMed] [Google Scholar]
- Yeates T. O., Komiya H., Chirino A., Rees D. C., Allen J. P., Feher G. Structure of the reaction center from Rhodobacter sphaeroides R-26 and 2.4.1: protein-cofactor (bacteriochlorophyll, bacteriopheophytin, and carotenoid) interactions. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7993–7997. doi: 10.1073/pnas.85.21.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]


