ABSTRACT
Cajal is commonly regarded as the father of modern neuroscience in recognition of his fundamental work on the structure of the nervous system. But Cajal also made seminal contributions to the knowledge of nuclear structure in the early 1900s, including the discovery of the “accessory body” later renamed “Cajal body” (CB). This important nuclear structure has emerged as a center for the assembly of ribonucleoproteins (RNPs) required for splicing, ribosome biogenesis and telomere maintenance. The modern era of CB research started in the 1990s with the discovery of coilin, now known as a scaffold protein of CBs, and specific probes for small nuclear RNAs (snRNAs). In this review, we summarize what we have learned in the recent decades concerning CBs in post-mitotic neurons, thereby ruling out dynamic changes in CB functions during the cell cycle. We show that CBs are particularly prominent in neurons, where they frequently associate with the nucleolus. Neuronal CBs are transcription-dependent nuclear organelles. Indeed, their number dynamically accommodates to support the high neuronal demand for splicing and ribosome biogenesis required for sustaining metabolic and bioelectrical activity. Mature neurons have canonical CBs enriched in coilin, survival motor neuron protein and snRNPs. Disruption and loss of neuronal CBs associate with severe neuronal dysfunctions in several neurological disorders such as motor neuron diseases. In particular, CB depletion in motor neurons seems to reflect a perturbation of transcription and splicing in spinal muscular atrophy, the most common genetic cause of infant mortality.
KEYWORDS: Amyotrophic lateral sclerosis, Cajal body, coilin, neurodegeneration, neurons, nucleolus, pre-mRNA splicing, snRNP, spinal muscular atrophy, survival motor neuron
Abbreviations
- ADH
antidiuretic hormone
- AIDA-1
AID associated protein 1d
- ALS
amyotrophic lateral sclerosis
- CB
Cajal body
- DRPLA
dentorubral-palidolusyan atrophy
- pcd
Purkinje Cell Degeneration
- NMDA
N-methyl-D-aspartate
- SMA
spinal muscular atrophy
- SMN
survival motor neuron
- snRNPs
small nuclear ribonucleoproteins
- snoRNPs
small nucleolar ribonucleoproteins
- SUMO1
small ubiquitin-like modifier 1
- UBF
upstream binding factor
Historical background
In 1906, Santiago Ramón y Cajal (1852–1934) and Camillo Golgi (1843–1926) shared the Nobel Prize in Physiology or Medicine in recognition of their seminal work on the structure of the nervous system. Curiously, their scientific conceptions concerning the organization of the nervous system were fundamentally opposed to each other, creating the “storm center of histological controversy.“1 In 1873, Golgi discovered a new chromoargentic staining method, “la reazione nera” (black reaction), which allowed him to demonstrate dendrites and axons. From his observations, Golgi embraced the most prevalent view among the scientific community, Gerlach's “reticular theory.” It stated that neuronal processes physically fuse with each other as a diffuse protoplasmic network. On the other hand, in 1888, Cajal obtained fascinating results with a modification of the Golgi stain that proved that dendrites and axons definitely ended freely, and formulated the “neuron theory,” which interpreted neurons as morphological and functional units. Three years later, Cajal proposed the “law of dynamic polarity,” which established that neurons are polarized cells: dendrites and neuronal bodies are involved in the reception of the nerve impulse, whereas the axon is the response element that propagates the nerve impulse. On the basis of the neuron theory and the law of dynamic polarity, Cajal provided us with the basic fundamentals for neuronal circuitry, proposing the conduction pathways of the nerve impulse in the nervous centers. Therefore, Cajal is recognized as the father of modern neuroscience.2,3
While the Golgi stain was a fundamental tool in characterizing neuronal populations and their connections in nervous centers, little progress had been achieved in the characterization of neuronal intracellular structures by the end of the 19th century. Dissatisfied with the available staining techniques, in 1903 Cajal developed a simple and reliable procedure for demonstrating diverse cellular structures, the “reduced silver staining method.“4 This is essentially a 2-step method. After fixation, tissue samples are impregnated with an aqueous silver nitrate solution to bind silver ions (Ag+) to the target tissue molecules, mainly proteins and ribonucleoproteins (RNPs). In a second step, a reducing agent (hydroquinone or pyrogallic acid) is used to reduce the silver ions to metallic silver (Ag) particles, which are insoluble and visible. The initial deposition of metallic silver promotes further deposition in an autocatalytic process which is necessary for microscopic detection. The new silver staining procedure enabled Cajal to characterize intracellular structures in great detail, essentially nuclear and cytoskeletal (neurofibrils) components, based on their distinct silver affinities. Interestingly, this technical work also included the discovery of a new important nuclear structure, the “accessory body” later renamed the Cajal Body (CB), as discussed later.4 Currently, the CB is considered to be a nuclear center of snRNA processing, snRNP assembly and snRNP surveillance.5-8
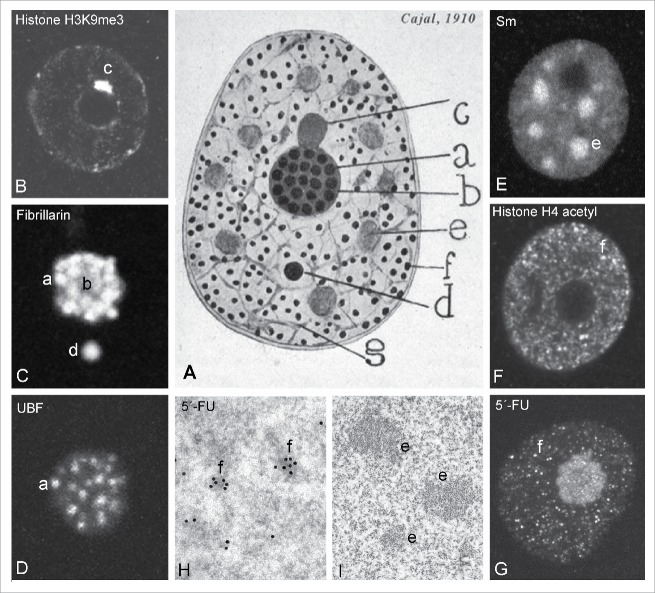
Having at hand the reduced silver staining method, in 1910 Cajal published an extensive monograph on the nuclear organization of the pyramidal neurons of the cerebral cortex, which represents a masterpiece of nuclear structure at light microscopy level.3,9 In the early 1900s, knowledge of the neuronal nucleus was essentially based mainly on the use of acid and basic aniline dyes which revealed the basophilic framework of “Flemming's chromatin.”9 Other nuclear structures revealed with aniline dyes at that time were the “nucleolus” —the site of rRNA synthesis, rRNA processing and preribosomal particle assembly10,11— and the “Levi basophilic grume,” a nucleolus-attached clump of centromeric heterochromatin.12 However, Cajal preferred to use the silver staining technique because of its reproducibility and ability to demonstrate a variety of new nuclear structures. Cajal provided a precise structural and cytochemical characterization of what he called “argyrophilic nucleolar spherules,” “accessory body,” “hyaline grumes,” “neutrophilic granules” and “nuclear membrane” (Fig. 1).3,9 Fig. 1A shows Cajal's original drawing of these nuclear structures and recent parallel images of the neuronal nucleus processed with modern specific molecular markers of nuclear compartments.
Figure 1.
Cajal's original drawing of the neuronal nucleus. (A) The scheme shows the nuclear structures identified in pyramidal neurons from the cerebral cortex (“Original drawing at the Cajal Institute, C.S.I.C., Madrid”). Panels B-I illustrate the equivalent nuclear components identified at the present time in mammalian neurons. (B) Nucleolus-attached heterochromatin (“Levi basophilic grume, c”). (C) nucleolus and CB (“argyrophilic nucleolar spherules,” a;” “ground substance, b” “accessory body, d”). (D) Fibrillar centers of the nucleolus (“argyrophilic nucleolar spherules, a”). (E) Nuclear speckles (“hyaline grumes, e”). (F) Nuclear microfoci of active chromatin immunolabeled for the acetylated histone H4 (“neutrophil grains, f”). (G) Nuclear foci of 5′-fluorouridine (5′-FU) incorporation into nascent RNA (“neutrophil grains, f”). (H) Ultrastructural characterization of nuclear foci of 5′-FU incorporation (I) Fine structure of interchromatin granule clusters in a neuron (“hyaline grumes e”). Note that Cajal's drawing shows the nucleus enclosed by 2 parallel lines. (C and E from Lafarga et al., Chromosoma 2009; G from Casafont et al., Acta Neuropathol 2011, reproduced with permission from © Springer).3,99
Cajal described the neuronal nucleolus as composed of small closely packed “argyrophilic nucleolar spherules” (from 0.25 to 0.30 µm in diameter), which clearly correspond to the “fibrillar centers” of the nucleolus. They are nucleolar sites that concentrate components of the RNA polymerase I transcription machinery, such as the upstream binding factor (UBF) and RNA polymerase I, involved in rRNA gene transcription (Fig. 1A, C–D and Fig. 2).10,11 Interestingly, Cajal noted that the number of “argyrophilic nucleolar spherules” correlated with neuronal size and that they tended to disappear in some neurological disorders, suggesting an important role of these nucleolar spherules in neuronal biology.
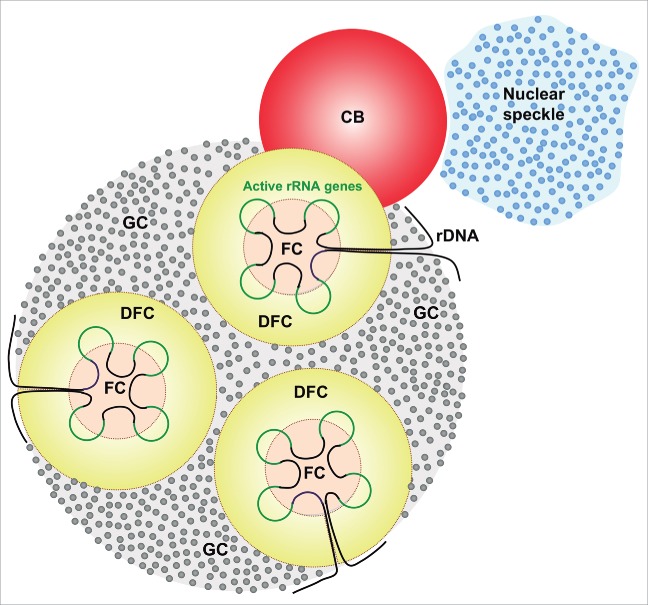
Figure 2.
Schematic diagram showing the association of CBs with the nucleolus and nuclear speckles or interchromatin granule clusters (IGC). The nucleolus shows its main components: fibrillar centers (FC), dense fibrillar component (DFC), containing active rRNA genes, and the granular component (GC), the site of preribosomal particle assembly.
In addition to the discovery of the “accessory body,” which will be addressed in a separate section, Cajal was the first to characterize rounded nuclear areas (6–11 in pyramidal neurons) of homogeneous texture that he termed “hyaline grumes,” today known as “nuclear speckles” or “interchromatin granule clusters” (Fig. 1A, E, I and Fig. 2). They are DNA-poor nuclear domains enriched in snRNPs and other non-snRNP protein splicing factors.13 Nuclear speckles play an important role in coordinating the supply and recycling of pre-mRNA splicing factors to transcription sites.14
According to Cajal, the “neutrophil granules” appeared as numerous sharply defined nuclear spots located throughout the nucleus excluding the nucleolus and “hyaline grumes” (Fig. 1A). We propose that they are remodeling foci of active chromatin immunolabeled for detecting both the acetylated histone H4 and the active phosphorylated form (Ser2) of RNA polymerase II (Fig. 1F).3,15 Moreover, the nuclear pattern of “neutrophil granules” is similar to the nuclear foci observed in neurons after a short pulse of 5′-fluorouiridine incorporation into nascent RNA, which also are excluded from “hyaline grumes/nuclear speckles” (Fig. 1G, H). In this vein, the preferential silver staining of “neutrophil granules” may reflect the local accumulation of nascent RNA and phosphoproteins with high affinity for silver.
Finally, it is noteworthy that Cajal, in his articles and seminal book Histology of the Nervous System of Man and Vertebrates,16 always depicted the neuronal nucleus enclosed in 2 parallel lines which resemble the appearance of the double nuclear membrane when viewed by electron microscopy (Fig. 1A). It is possible that the dilation of the perinuclear space produced by some fixative agents allowed Cajal to reveal the existence of a double membrane, even though this falls below of the resolution power of the light microscope.
The accessory body of cajal (“cajal body”)
Using the reduced silver nitrate method, Cajal discovered, in many neuronal types from several mammalian species, a round and sharply defined argyrophilic nuclear structure, approximately 0.5 µm in diameter, which he called “the accessory body” of the nucleolus (cuerpo accesorio, in Spanish) (Figs. 1A, C, 2, 3A-C).4,9 He found the accessory body in most neuronal types and also pointed out variations in number (1 to 3) and size depending on the neuronal type. Cajal obtained the best staining of the accessory body by fixation of nervous tissue samples with pyridine or formalin-ammoniacal alcohol, which reduced the silver affinity of the nucleolus and other nuclear structures, thus highlighting visualization of the accessory body (Fig. 3C). Although the function of the accessory body was unknown at that time, Cajal concluded that this nuclear body is a distinct entity which is clearly distinguished by its morphology, size and silver staining properties from other nuclear components such as the “Levi basophilic grume,” “argyrophilic nucleolar spherules,” micronucleoli and “neutrophil granules.“9
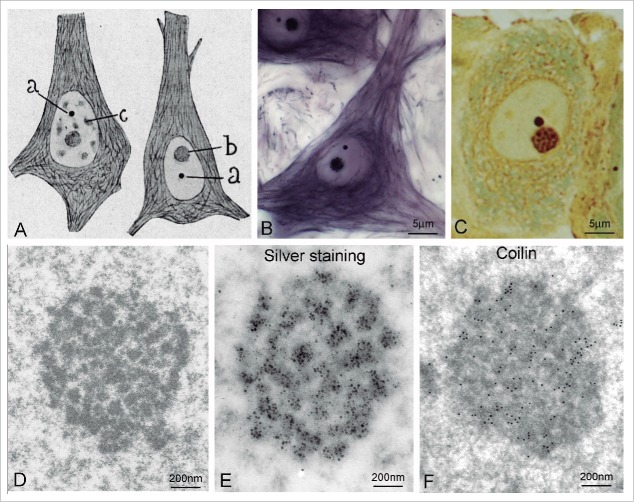
Figure 3.
Cajal body (“Cajal's cuerpo accesorio”) (A) Original drawing by Cajal showing 2 pyramidal neurons from the human cerebral cortex. The accessory body (a), the nucleolus (b) and nuclear speckles (c) are identified in the nucleus. The cytoplasm contains neurofibrils (“Original drawing at the Cajal Institute, C.S.I.C., Madrid”). (B) Neuron stained with Cajal's reduced silver nitrate method. The nucleolus, accessory body/CB and neurofibrils appear stained (courtesy of Dr. J.M. López-Cepero). (C) Semithin section (1 µm thick) of a sensory ganglion neuron stained with Cajal's method showing sharply defined argyrophilic nucleolar spherules and a nucleolus-attached accessory body/CB. (D) Electron micrograph of a neuronal accessory body/CB. (E) Fine structure of a silver-stained accessory body/CB from a Purkinje neuron. Silver precipitates specifically decorate the coiled threads of the CB. (F) Immunogold electron microscopy localization of coilin on the coiled threads of an accessory body/CB from a neuron. (B and E from Lafarga et al., Chromosoma (2009) reproduced with permission from © Springer; C from Lafarga el al. J Neurosci Meth (1986) reproduced with permission from © Elsevier).3,100
Following its characterization by Cajal, the accessory body attracted little attention until the 1950s, when Barr's laboratory, at the University of Western Ontario, focused its interest on the organization of the sex chromatin (“the inactive X chromosome”) in female neuronal nuclei. Barr's team confirmed Cajal's observations in feline neurons and, with the cytochemical method of Feulgen, they demonstrated that the accessory body, unlike the sex chromatin, is relatively DNA poor.17-19
It was not until 1969 that the first observation of the accessory body was made in non-neuronal tissue. By electron microscopy, Monneron and Bernhard (1969) reported a nuclear structure composed of dense coiled threads in hepatocytes and gave it the name of “coiled body” (Fig. 3D).20 They demonstrated the presence of RNA in the coiled body using the regressive EDTA staining for ribonucleoproteins. Curiously, in the same year, Hardin and co-workers (1969), who knew Cajal's work on the neuronal nucleus, reported a similar structure by electron microscopy in sensory ganglion neurons, but retained the name of “accessory body.“21 Using a modification of Cajal's silver nitrate method for electron microscopy, our laboratory definitively established that Cajal's accessory body and Monneron and Bernhard's coiled body are the same nuclear structure (Fig. 3D, E).22,23 However, it was not until the EMBO Workshop on “Functional Organization of the Cell Nucleus” held in Prague in 1999 that Gall proposed linking Cajal's name to the nuclear structure that he discovered in 1903.5,24 The same year saw the publication of the first 2 articles that introduced “Cajal body” (CB) nomenclature.25,26
The modern era of CB research started in the 1990s with the publication of 2 articles27,28 from Tan's team at the Scripps Research Institute in La Jolla. The authors identified human autoimmune sera that specifically labeled coiled bodies in cultured cells. The protein responsible for the immunostaining was purified and a cDNA clone encoding a coiled body-specific protein was isolated. The protein was named p80-coilin and since then it has become a molecular marker of CBs (Figs. 3F, 4A). Soon afterwards, an ortholog of human coilin was discovered in Xenopus laevis and localized to sphere organelles, which are homologous with somatic cell CBs, within the germinal vesicles of oocytes.5,29 In the 1990s, the introduction of new antibodies for nuclear proteins and specific probes for snRNAs allowed the identification of other essential CB components, such as snRNPs, snoRNPs, fibrillarin, Nopp140, SMN and several Gemin proteins.6,7,30 As expected, the presence of these key components has been confirmed in neurons with specific antibodies to CB proteins and the tri-methylguanosine cap on snRNAs, and human autoimmune sera against the Sm proteins of spliceosomal snRNPs (Fig. 4C-E).26,31-33
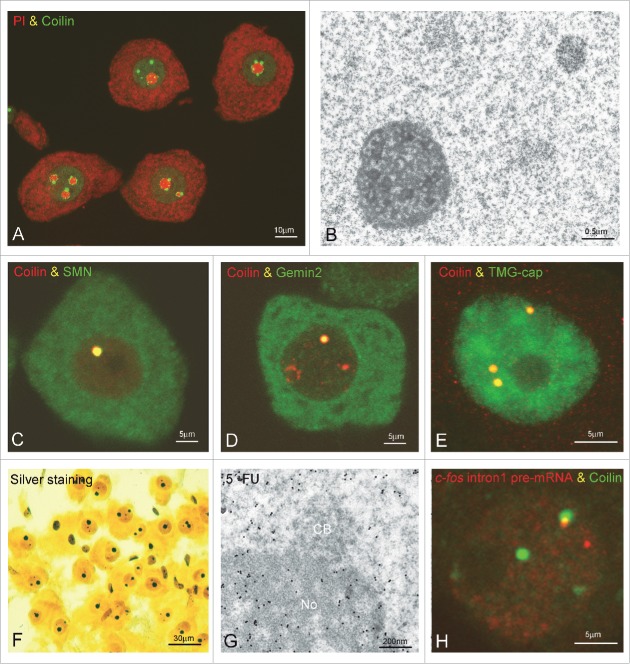
Figure 4.
Cajal bodies in neurons. (A) Dissociated neuronal bodies from a sensory ganglion co-stained for nucleic acids with propidium iodide (PI) and for coilin clearly revealed the distribution of CBs free in the nucleoplasm and associated with the nucleolus. (B) Electron micrograph of a typical neuronal nucleus illustrating a typical nucleolus with numerous fibrillar centers and a CB free in the nucleoplasm. (C-E) Confocal images of double immunostained for coilin in combination with SMN (C), Gemin2 (D) and TMG-cap of spliceosomal snRNPs (E) in mammalian neurons demonstrate the colocalization of these molecular constituents in the CB. (F) Dissociated neurons from the supraoptic nucleus silver stained with Cajal's procedure showing the specific staining of nucleoli and CBs. (G) Immunogold electron microscopy detection of 5′-FU incorporation sites in the nucleolus (No) and euchromatin domains after a 30-min pulse of of 5′-FU. Gold particles are absent from the inner side of the CB. (H) In situ hybridization for the c-fos intron I pre-mRNA (red) in combination with coilin immunolabeling (green) revealed the spatial association of a CB with a gene loci of c-fos following a osmotic stress in supraoptic neurons.
Organization of cajal bodies in mammalian neurons
In this section, we focus on nuclear architecture and its relationship to the CB organization in post-mitotic differentiated neurons. We also discuss the composition and molecular heterogeneity of CBs, as well as the functional importance of the relationship between CB number and neuronal size. Finally, we address the dynamic behavior of neuronal CBs in response to changes in transcriptional activity, as well as the structural and functional link between CBs and nucleoli.
The architecture of the neuronal nucleus in most mammalian neurons is characterized by the predominance of relaxed euchromatin. This open chromatin configuration facilitates the access of transcription factors to active genes and is clearly related to the high transcriptional activity of neurons required to maintain their metabolic and bioelectrical activities.34-36 In this chromatin landscape, 2 prominent structures stand out: the nucleolus and the CB (Fig. 4A, B). Both compartments are nuclear epicenters of the RNA world and their dysfunction could have a profound impact on protein synthesis and on information processing and storage by neurons, as reflected in the recently discovered signaling pathway between neuronal synapses and the nucleolus and CBs.37,38
Mammalian neurons provide an excellent model to study CBs because their condition of post-mitotic (G0) cells with a constant diploid amount of DNA rules out variations in CB organization and function associated with cell-cycle stage and ploidy. This is an important issue because some CBs may have specific S-phase functions. These may include the biogenesis of U7 snRNPs, required for histone mRNA 3′end processing, and the assembly of telomerase, an important mechanism for telomere maintenance.39,40 Moreover, CBs disassemble during mitosis when transcription is off and reassemble in early G1 when transcription resumes.41,42 Therefore, the behavior of neuronal CBs may be strictly correlated to the global transcriptional and splicing activity required to sustain neuronal size and metabolic and bioelectrical activity.
Neuronal CBs concentrate coilin, fibrillarin, SMN and components of the splicing machinery (Figs. 4C-E, 6A, D),26,31,32,43,44 indicating that they are canonical CBs potentially engaged in the assembly and maturation of spliceosomal snRNPs. Since the 1960s, electron microscopy studies have found that neuronal CBs have well-defined coiled electron-dense strands embedded in an amorphous matrix (Figs. 3D-F).21,23,35,45 While key molecular components of CBs, such as silver staining proteins, coilin, fibrillarin, SMN and snRNPs, are specifically localized on the dense coiled threads (Fig. 3E, F), no molecular constituents have been characterized in the amorphous matrix.28,31,43,45
Figure 6.
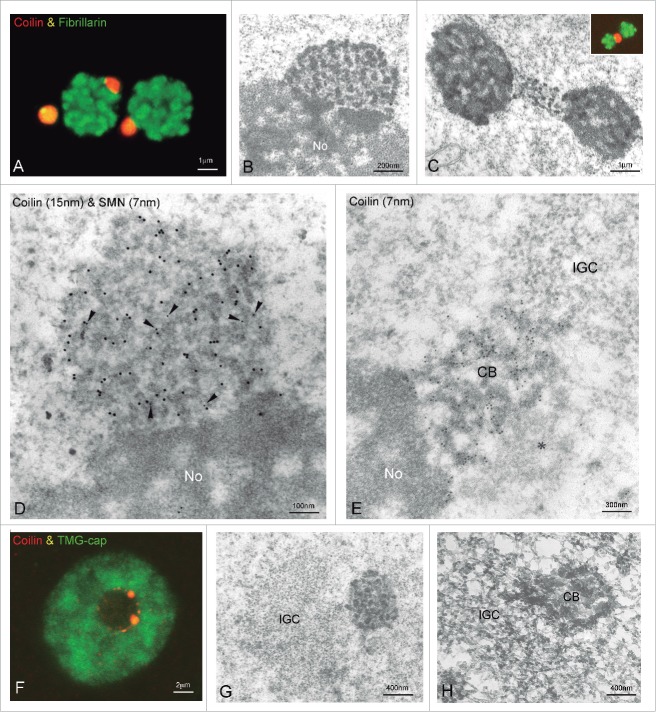
Association of the CB with the neuronal nucleolus. (A) Double immunostaining for coilin and fibrillarin illustrates the close association of CBs with nucleoli in a sensory ganglion neuron. (B) Electron micrograph of a nucleolus (No) attached CB. Note the association of the CB with the dense fibrillar component of the nucleolus. (C) Electron micrograph of 2 nucleoli physically linked with a CB. (inset) Similar confocal picture co-stained for fibrillarin and coilin. (D) Double immunogold electron microscopy labeling for coilin and SMN (arrowheads) of a nucleolus-attached CB. (E) Electron micrograph of a CB immunogold labeled for coilin connecting the nucleolus (No) with an interchromatin granule cluster (IGC). Note the presence of an amorphous material associated with the CB (asterisk). (F) Confocal picture of a neuronal nucleus illustrating the distribution of nuclear speckles, immunostained for the TMG-cap, and their association with CBs immunolabeled for coilin. (G) Electron micrograph showing the association of an interchromatin granule cluster (IGC) with a CB. (H) High voltage electron micrograph from a resinless preparation of neuronal nucleus illustrates the spatial association of an interchromatin granule cluster (IGC) with a CB. (E from Lafarga et al., J. Neurocytol 1998, reproduced with permission from © Springer).59
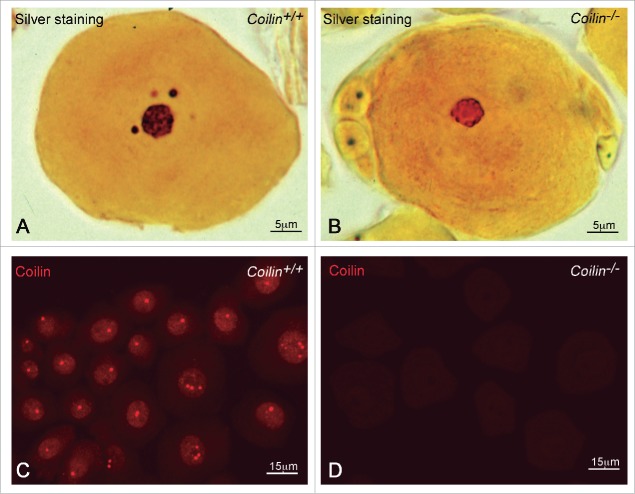
It is noteworthy that coilin is essential for the correct formation and/or maintenance of neuronal CBs, as illustrated by loss of silver-stained nuclear bodies in coilin knockout mice (Fig. 5).33 Similarly, coilin depletion in zebrafish embryos leads to CB dispersion and deficient snRNP biogenesis.42 Thus, coilin plays an essential role in promoting macromolecular assembly of snRNPs in CBs.46 However, an important puzzle concerning CBs has emerged from observations in Drosophila melanogaster that small CB-specific RNAs (scaRNAs), which guide the modification of spliceosomal snRNAs (methylation and pseudouridylation), function in coilin-null flies that lack CBs. This indicates that concentration of snRNAs and scaRNA in CBs is not essential in flies.47
Figure 5.
Loss of CBs in coilin knockout neurons. (A, B) Sensory ganglion neurons from wild-type and coilin knockout mice were silver stained with Cajal's procedure. Note the staining of the nucleolus and CBs in the wild-type neuron and the absence of CBs in the knockout cell. (C, D) Coilin immunostaining in wild-type and knockout sensory ganglion neurons shows typical CBs in the wild-type neurons and the lack of coilin staining in the knockout cells.
It is also important to note the existence of a molecular heterogeneity in some neuronal CBs. Coilin-positive CBs free of SMN-complex proteins and snRNPs occasionally appear under physiological conditions (Fig. 4D). They are presumably immature CBs not involved in the maturation of snRNPs. Indeed, in cultured non-neuronal cell lines, upon nuclear import, snRNPs concentrate in CBs that contain coilin and SMN but not in bodies that contain coilin but lack SMN.48 During neuronal recovery after osmotic stress, which induces early disruption of CBs followed by their reformation, a subset of CBs transiently accumulates active SUMO1, suggesting that the sumoylation of some CB proteins is involved in the reformation of these nuclear bodies.49 Moreover, it has been reported in some cell lines that a subset of CBs contains the Ubc9 conjugating enzyme and the SUMO isopeptidase USPL1.49,50 These findings, together with the recent identification of SMN as a new SUMO1 substrate, support the hypothesis that sumoylation/desumoylation events may participate in the molecular dynamics of CBs.51
In the 1990s, a partner for CBs was reported and called Gem, for Gemini of CBs, in a HeLa cell line (PV).52 Gems were characterized by immunofluorescence as coilin-free nuclear bodies that concentrate SMN and Gemin2 and Gemin3.53,54 Later studies showed the presence of Gemins2,3,4,6,7 which are shared with CBs.7 Gemin proteins are integral components of the SMN complex involved in snRNP biogenesis.7 According to the original description, Gems are frequently associated with CBs, but do not colocalize with them and sometimes appear as separate structures free in the nucleoplasm.52 However, in several cell lines and differentiated cells, especially neurons, SMN and Gemin 2 commonly colocalize with coilin and snRNPs in the CBs (Fig. 4D).26,31,32,55 Although Gems have been reported in fetal motor neurons,56 our group has not found Gems in mature mammalian neurons. The function of Gems remains unknown. Their absence in mature neurons suggests that under physiological conditions differentiated cells accommodate nuclear concentrations of SMN complex proteins (SMN and Gemins) to the functional requirements for CB assembly and maintenance.
The number, size and spatial organization of CBs and nucleoli have been carefully analyzed using preparations of dissociated neuronal bodies immunostained for coilin and counterstained with propidium iodide (Fig. 4A).31,32,57,58 The size of neuronal CBs ranges from approximately 0.5 to 1.5 µm in diameter. Whereas nucleoli exhibit size scaling in sensory ganglion neurons, a linear relationship between CB size and neuronal size has not been demonstrated in this neuronal population.31,32 Several studies have shown that CB number correlates with neuronal size and global transcriptional activity.31,57,59,60 For example, sensory ganglion neurons include 3 cell-size categories related to their different transcriptional and bioelectrical activities: small, medium and large neurons. In rats, the mean CB number increases from 1.1 per cell in small neurons, to 1.8 and 2.9 in medium and large neurons, respectively, indicating a close relationship between cell body size, a parameter directly related to global transcriptional and translational activity of the neuron,61 and CB formation.31 This relationship is consistent with mathematical prediction models suggesting that nuclei with higher CBs support greater rates of snRNP assembly, due to the increased likelihood of molecular interactions between CB components.42,62 Intriguingly, a remarkably higher number of CBs occurs in human ganglion neurons, with a mean value of 2.8 and 13.4 in small and large-size neurons, respectively, as compared with rat neurons.32 Although human ganglion neurons are moderately larger than their counterparts in rats, other unknown factors, including differences between species in genome organization, could be determinants of the great abundance of CBs in human neurons.
The first experimental evidence that CBs are transcription-dependent organelles in neurons came from our study in osmotically-stimulated supraoptic neurons, neurosecretory cells that produce the antidiuretic hormone (ADH). Using Cajal's silver staining procedure (Fig. 4F), we observed that the metabolic activation of ADH synthesis and secretion by dehydration in animals induced a 3-fold increase in the mean number of CBs per neuron, which rapidly returned to control values once the osmotic stimulation ceased by rehydration.57 In contrast, osmotic cellular stress, which causes a severe inhibition of global transcription in supraoptic neurons, led to a dramatic decrease in CB number within the first 2 hours after stress induction.59 Collectively, these observations support the notion that the formation of CBs depends on ongoing transcription.
In electron microscopy studies, in situ transcription assays in neurons have demonstrated that the incorporation sites of 5′-fluorouridine into nascent RNA occur at the nucleolus and euchromatin domains, and may also appear closely associated with CBs.36 However, this incorporation does not occur within the CB (Fig. 4G).36,58 Moreover, using in situ hybridization with a probe for the c-fos intron-1 pre-mRNA in osmotically stimulated supraoptic neurons, our group has found spatial associations of CBs with the nascent transcripts accumulated at c-fos gene loci (Fig. 4H). These observations are consistent with the emerging evidence of a dynamic association between CBs and genomic loci, indicating that CBs may be nucleated at particular active transcription sites.63-65 In this vein, Dundr's team has recently demonstrated that CBs are linked to genome conformation, orchestrating genome-wide clustering of highly expressed spliceosomal sn/snoRNA and histone genes.66
Neuronal CBs frequently associate with 2 nuclear compartments, the nucleolus and nuclear speckles (Figs. 2, 3C, 4A, 6).21,23,32,43,57 For example, approximately 30% of CBs appear attached to the nucleolus in human sensory ganglia32; we have even found CBs physically connecting 2 adjacent nucleoli (Fig. 6C). In cultured hippocampal neurons, the association with the nucleolus increased at the final stage of neuronal differentiation with the formation of a perinucleolar “rosette” of CBs.67 The authors suggest that rosette formation correlates with full neuronal polarization and represents a novel marker for the final stage of differentiation.
Physically, CBs specifically associate with the dense fibrillar component of the nucleolus (Fig. 6B, D),21,23,31,43 the site of synthesis and early processing of pre-rRNAs.10 Interestingly, CBs and the dense fibrillar component share several macromolecules, such as fibrillarin, Nopp140, NAP57 and snoRNPs, which provide a molecular link for a nucleolus-CB interaction.68 A dynamic interaction between these 2 nuclear structures is also supported by the observation of CBs moving to and from nucleoli in living HeLa cells.69 CB-nucleolar association may facilitate the delivery of snoRNPs or other factors involved in pre-rRNA processing to the nucleolus, a particularly important mechanism in neurons that have high requirements for ribosome biogenesis. In fact, a recent study from Neugebauer's group shows that all classes of snoRNAs concentrate in CBs and the authors speculate that CBs are the sites of specific steps in snoRNP assembly.70 The functional coupling between CBs and nucleoli is also supported by the observation in primary cultures that neuronal stimulation by activation of NMDA (“N-methyl-D-aspartate”) receptors significantly increases nucleolar number, CB-nucleolar association and protein synthesis compared to control neurons.37 This neuronal response is mediated by a new protein of the postsynaptic region identified as AIDA-1d (“AID associated protein 1d”), which harbors nuclear localization sequences. Following neuronal stimulation, AIDA-1d is translocated to the nucleus, where it is targeted to CBs and the nucleolus, coinciding with CB-nucleolar association. Because AIDA-1 interacts with coilin,71 it may link CBs with nucleoli by binding to an unknown factor in the nucleolus.37 It seems likely that this synaptic signaling to CBs and the nucleolus regulates ribosome biogenesis and perhaps RNA dynamics.37,38
Nuclear speckles or their ultrastructural counterpart, interchromatin granule clusters,13 are very often located in close proximity to CBs in neurons (Figs. 2, 4E, 6F, G). The spatial association of interchromatin granule clusters with CBs is particularly visible in thick resinless preparations of neuronal nuclei processed with critical point drying and examined with high voltage electron microscopy (Fig. 6H). Moreover, as is illustrated in Fig. 6E, the coilin-positive coiled threads of a nucleolus-attached CB can establish direct contact with interchromatin granule clusters. Collectively, the frequent spatial associations of CBs with both the nucleolus and nuclear speckles observed in neurons provides the structural basis for efficient transfer of snoRNPs and snRNPs from CBs to their respective target compartments of the neuronal nucleus.
Cajal bodies in neuropathological disorders
There is little current literature on the contribution of CBs to the molecular pathophysiology of neurodegenerative disorders. In the following section, we focus on the behavior of CBs in CAG repeat disorders, ataxias and 2 motor neuron diseases, amyotrophic lateral sclererosis (ALS) and spinal muscular atrophy (SMA).
Expansion of a CAG repeat encoding a polyglutamine tract causes at least 8 neurodegenerative disorders characterized by the formation of intranuclear neuronal inclusions containing the mutant protein.72 In 2 of these human diseases, dentorubral-palidolusyan atrophy (DRPLA) and Machado-Joseph disease, Yamada and coworkers have shown that CBs frequently appear physically associated with nuclear inclusions.73 The authors confirmed this association in the DRPLA transgenic mouse brain and suggested that CBs are trapped at the nuclear inclusion surface, which may prevent their mobility69 and functions associated with snRNP biogenesis.6,30,74 More recently, Hebert's group has demonstrated that the expression in Hela cells of the mutant ataxin 3, which is mutated in Machado-Joseph disease, not only reproduces the attachment of CBs to nuclear inclusions found in patient neurons, but also disrupts the splicing of an artificial reporter.75 The authors conclude that the spatial association of CBs with ataxin 3 nuclear inclusions correlates with a dysfunction of pre-mRNA splicing, possibly as a consequence of altered CB activity in snRNP biogenesis.
A useful model for analyzing the dysfunction of the nucleolus and CB that occur in neurodegeneration is the pcd (“Purkinje cell degeneration”) mouse. The phenotype of this mouse is caused by mutation in the Nna 1 gene—encodes a cytosolic carboxypeptidase— which produces the degeneration and death of Purkinje cells/neurons during postnatal life, resulting in a severe cerebellar ataxia (gross lack of coordination of movement).76 A cellular hallmark of the disease is the progressive accumulation of DNA damage associated with a defective DNA repair, resulting in a severe inhibition of transcription of both ribosomal and protein-coding genes.60 Gene silencing in Purkinje cells clearly correlates with progressive disruption of both nucleoli and CBs.60 Interestingly, in addition to a severe loss of CBs, coilin was redistributed as a thin shell around the nucleolus (Fig. 7A, B, F), and even located inside the nucleolus (Fig. 7C). Moreover, immunoelectron microscopy detection of coilin revealed the loosening and fragmentation of the electron-dense coiled threads of CBs, reflecting a possible process of disassembly of these bodies (Fig. 7D, E).60 Given the importance of CBs for the assembly of snRNPs and snoRNPs, required for pre-mRNA and pre-rRNA processing, repectively,5,7,77 the CB depletion in Purkinje cells of the pcd mouse may reflect a reduced demand of RNA processing under conditions of DNA damage-induced transcriptional repression.60
Figure 7.
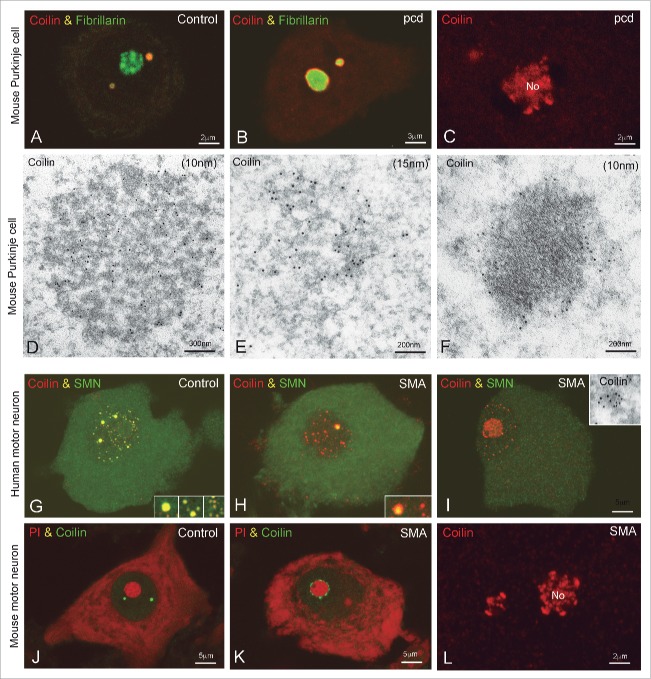
Reorganization of Cajal bodies in Purkinje cells of the pcd mice and motor neurons from SMA. (A-C) Confocal microscopy images from squash preparations of Purkinje cells from wild type (A) and pcd mice (B, C) co-stained for fibrillarin and coilin (A, B) or single immunostained for coilin (C). (A) Fibrillarin and coilin colocalize in a CB from a control Purkinje cell. (B, C) At advanced stages of Purkinje cell degeneration, with nucleolar segregation and fragmentation, coilin appears redistributed as a thin ring surrounding the segregated masses of fibrillarin or inside the nucleolus (No). (D) In wild type Purkinje cells, CBs show the typical morphology of coiled threads immunogold labeled for coilin. (E) CB with an irregular morphology and loosely arranged threads in a degenerating Purkinje cell. (F) Gold particles of coilin immunoreactivity are also observed surrounding an electrondense mass presumably corresponding to a segregated portion of the dense fibrillar component of the nucleolus. (G-I) Double immunostained for coilin and SMN on dissociated motor neurons from control and SMA samples. (G) In a control neuron coilin concentrates in several large CBs and numerous mini-CBs where it colocalizes with SMN (inset). (H) This motor neuron from an SMA patient shows a large CB immunolabeled for coilin and SMN and numerous coilin-positive and SMN-negative mini-CBs (inset). (I) SMA motor neuron with an eccentric nucleus, intranucleolar accumulation of coilin and numerous coilin microfoci free of SMN. (inset) Detail of a coilin microfocus with immunogold electron microscopy. (J-L) Motor neurons from a wild type mouse (J) and an SMA mouse model (K and L). (J, K) Co-staining for coilin and propidium iodide (PtdIns) illustrates the typical organization of CBs in the control neuron (J) and the redistribution of coilin as perinucleolar caps in the SMA motor neuron (K). (L) Intranucleolar localization of coilin (No) in an SMA motor neuron. (A; B, D-F, from Baltanas et al., Brain Pathol 2011, reproduced with permission from © John Wiley and Sons; G-I from Tapia et al. Histochem Cell Biol 2012, reproduced with permission from © Springer.60,96
An important point is the contribution of CB dysfunction to the molecular pathophysiology of 2 motor neuron diseases: SMA and ALS. The former is the most common genetic cause of infant mortality. SMA pathological hallmarks are lower motor neuron degeneration and muscular atrophy with paralysis. This disease is caused by deletion or mutation of the survival motor neuron 1 (SMN1) gene, resulting in reduced levels of SMN with diminished snRNP activity and the disruption of CBs (Fig. 7G-I).51,78-81
ALS is the most common adult-onset neurodegenerative disease affecting upper and lower motor neurons. Mutations in the RNA binding proteins FUS and TDP-43, involved in transcription and mRNA splicing and transport, are responsible for a subset of ALS cases.82 Interestingly, the number of Gems —identified with a single immunostaining for SMN— decreased in fibroblasts and mouse motor neurons depleted of FUS or TDP-43, suggesting a possible role of these bodies in ALS pathophysiology.83-85 In the spinal cord of ALS mouse models, uridylate-rich (U) snRNAs belonging to minor spliceosome, which carries out U12-dependent splicing, are markedly reduced, as occurs in SMA.78,86 Moreover, reduced SMN protein levels have been found in murine models of ALS.44,87 Therefore, SMA and ALS are related motor neuron diseases that share a dysfunction of mRNA metabolism. In this vein, FUS has been reported to interact with TDP-43 and to associate with the SMN complex, a process mediated by U1-snRNP and by direct interaction between FUS and the SMN Tudor domain.84,88 ALS-causative mutations in FUS deregulate SMN functions with a loss of Gems and reduced splicing activity.84,88 Collectively, these findings suggest the existence of converging pathogenic mechanisms in SMA and ALS, including the dysfunction of SMN and the depletion of Gems or CBs.
Accurate determination of the molecular composition of nuclear bodies is crucial for their identification and for understanding their functions and pathological implications. Most reports on nuclear alterations in SMA consider that the loss of Gems —SMN-positive and coilin- and snRNP-negative nuclear bodies52 —is a cellular hallmark of the disease.84,88,89 Similarly, Gem depletion has been reported in murine models of ALS.85,87,89,90 However, the identification of Gems in these SMA and ALS studies is not surprising given that the authors commonly use a single SMN immunolabeling for counting nuclear bodies,83-85,90,91 establishing by default that they are Gems although the presence of the CB marker coilin is highly likely.
In the light of the experience of our group in mammalian neuronal models under normal and pathological conditions, we propose that canonical CBs, rather than Gems, are disrupted in motor neurons in SMA and ALS. Several lines of evidence support CB dysfunction in motor neuron diseases: i) in postnatal and mature mammalian neurons SMN and coilin co-localize in typical canonical CBs (Figs. 4C, 6D, 7A), while Gems are not detected;26,31 ii) in cell lines and mammalian nervous tissue, the formation and integrity of CBs are dependent on ongoing transcription and snRNP biogenesis,7,57,59,92,93 2 nuclear functions altered in motor neuron diseases;81,86,94 iii) lack of CBs in cells derived from SMA patients correlates with decreased U4/U6-U5 tri-snRNP assembly, a maturation step of spliceosomal snRNPs that is 10-fold faster in CBs than in nucleoplasm,62,95 and with splicing alterations of particular minor introns;78,80 iv) coilin protein, which is lacking in Gems, scaffolds CBs and couples snRNP and snoRNP biogenesis, making CBs the center of small non-coding RNA processing;7,33,42 and v) in motor neurons of a 3-month-old SMA patient, we have observed that lowered SMN levels induce severe depletion of canonical CBs, whereas Gems were conspicuously absent in both SMA and age-matched control neurons (Fig. 7G-I).96
Taken together, the depletion of canonical CBs seems to be a hallmark feature of SMA motor neurons. Moreover, most of the remaining coilin-positive CBs detected in SMA motor neurons fail to recruit SMN and snRNPs96,97 and frequently appear as unstructured mini CBs (Fig. 7H, I, inset), suggesting that they are not involved in snRNP biogenesis. A shift of coilin from CBs to perinucleolar caps lacking SMN and snRNP, or even inside the nucleolus, is also observed in human and murine SMA motor neurons (Fig. 7J-L).96 This coilin redistribution probably reflects the impact of reduced SMN levels on transcription and splicing in motor neurons, resulting in a deficient nucleation of canonical CBs. This interpretation is consistent with experimental observations showing that CB disruption with transcriptional inhibitors (actinomycin D or DRB) causes coilin to relocalize and accumulate in perinucleolar caps.36,98
Concluding remarks
Neurons provide an excellent model to study CBs because their condition of post-mitotic cells (G0) rules out variations in CB function associated with cell-cycle progression. This is an important point because CBs play an essential role in the assembly of U7 snRNPs during the S phase of the cell cycle, and mitotic disassembly/reassembly. Therefore, the behavior of neuronal CBs may be related to the global transcriptional activity required to sustain neuronal size and bioelectrical activity. Several lines of evidence support the notion that neuronal CBs are transcription-dependent nuclear compartments whose number dynamically accommodates to the demand for snRNP and snoRNP assembly required for pre-mRNA splicing and ribosome biogenesis. In neuropathological disorders, particularly in motor neuron diseases, depletion of CBs, rather than Gems, is a hallmark feature associated with perturbations of transcription and splicing. This reflects the crucial role of CB in the assembly of spliceosomal snRNPs and also suggests the contribution of CB dysfunction to the splicing pathology associated with several neurodegenerative disorders. Although SMN deficiency is directly associated with CB dysfunction in SMA, and probably in other motor neuron diseases, further molecular studies are necessary to determine the molecular interactions that govern the assembly/disassembly cycle of CBs under normal and pathological conditions. This particularly includes changes in coilin interactions with proteins and RNAs, given that coilin is now known to scaffold the CB.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to thank Raquel García Ceballos for technical assistance. We thank Drs Jordi Caldero, Josep Esquerda, Javier Riancho and Prof Larry Gerace for comments on the manuscript.
Funding
This work was supported by the following grants: “Dirección General de Investigación” (BFU2014-54754-P) and “Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas” (CIBERNED; CB06/05/0037) Spain. Olga Tapia is a recipient of a grant from SMA Europe.
References
- 1.Frixione E. Cajal's second great battle for the neuron doctrine: the nature and function of neurofibrils. Brain Res Rev 2009; 59:393-409; PMID:19111572; https://doi.org/ 10.1016/j.brainresrev.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 2.DeFelipe J. Sesquicentenary of the birthday of Santiago Ramón y Cajal, the father of modern neuroscience. Trends Neurosci 2002; 25:481-4; PMID:12183210; https://doi.org/ 10.1016/S0166-2236(02)02214-2 [DOI] [PubMed] [Google Scholar]
- 3.Lafarga M, Casafont I, Bengoechea R, Tapia O, Berciano MT. Cajal's contribution to the knowledge of the neuronal cell nucleus. Chromosoma 2009; 118:437-443; PMID:19404660; https://doi.org/ 10.1007/s00412-009-0212-x [DOI] [PubMed] [Google Scholar]
- 4.Cajal S. Un sencillo método de coloración selectiva del retículo protoplasmático y sus efectos en diversos órganos nerviosos. Trab Lab Invest Biol 1903; 2:129-221. [Google Scholar]
- 5.Gall JG. Cajal bodies: the first 100 years. Annu Rev Cell Dev Biol 2000; 16:273-300; PMID:11031238; https://doi.org/ 10.1146/annurev.cellbio.16.1.273 [DOI] [PubMed] [Google Scholar]
- 6.Stanek D, Neugebauer KM. The Cajal body: a meeting place for spliceosomal snRNPs in the nuclear maze. Chromosoma 2006; 115:343-54; PMID:16575476; https://doi.org/ 10.1007/s00412-006-0056-6 [DOI] [PubMed] [Google Scholar]
- 7.Machyna M, Heyn P, Neugebauer KM. Cajal bodies: where form meets function. Wiley Interdiscip Rev RNA 2013; 4:17-34; PMID:23042601; https://doi.org/ 10.1002/wrna.1139 [DOI] [PubMed] [Google Scholar]
- 8.Novotný I, Malinová A, Stejskalová E, Matějů D, Klimešová K, Roithová A, Švéda M, Knejzlík Z, Staněk D. SART3-Dependent Accumulation of incomplete spliceosomal snRNPs in Cajal bodies. Cell Rep 2015; 10:429–40; PMID:25600876; https://doi.org/ 10.1016/j.celrep.2014.12.030 [DOI] [PubMed] [Google Scholar]
- 9.Cajal S. El núcleo de las células piramidales del cerebro humano y de algunos mamíferos. Trab Lab Invest Biol 1910; 8:27-62. [Google Scholar]
- 10.Boisvert F-M, van Koningsbruggen S, Navascués J, Lamond AI. The multifunctional nucleolus. Nat Rev Mol Cell Biol 2007; 8:574-85; PMID:17519961; https://doi.org/ 10.1038/nrm2184 [DOI] [PubMed] [Google Scholar]
- 11.Pederson T. The nucleolus. Cold Spring Harb Perspect Biol 2011; 3:a000638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manuelidis L. Indications of centromere movement during interphase and differentiation. Ann N Y Acad Sci 1985; 450:205-21; PMID:3860180; https://doi.org/ 10.1111/j.1749-6632.1985.tb21494.x [DOI] [PubMed] [Google Scholar]
- 13.Lamond AI, Spector DL. Nuclear speckles: a model for nuclear organelles. Nat Rev Mol Cell Biol 2003; 4:605-612; PMID:12923522; https://doi.org/ 10.1038/nrm1172 [DOI] [PubMed] [Google Scholar]
- 14.Spector DL, Lamond AI. Nuclear speckles. Cold Spring Harb Perspect Biol 2011; 3:a000646; PMID:20926517; https://doi.org/ 10.1101/cshperspect.a000646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casafont I, Palanca A, Lafarga V, Mata-Garrido J, Berciano MT, Lafarga M. Dynamic behavior of the RNA polymerase II and the ubiquitin proteasome system during the neuronal DNA damage response to ionizing radiation. Mol Neurobiol 2015; PMID:26660115; https://doi.org/ 10.1007/s12035-015-9565-8 [DOI] [PubMed] [Google Scholar]
- 16.Cajal S. Textura del sistema nervioso del hombre y de los vertebrados. Moya: Madrid, 1899. [Google Scholar]
- 17.Thompson BK, Haggar RA, Barr ML. The accessory body of Cajal in nerve cell nuclei of the cat. J Comp Neurol 1957; 108:253-67; PMID:13502477; https://doi.org/ 10.1002/cne.901080205 [DOI] [PubMed] [Google Scholar]
- 18.Haggar RA. Behavior of the accessory body of Cajal during axon reaction. J Comp Neurol 1957; 108:269-83; PMID:13502478; https://doi.org/ 10.1002/cne.901080206 [DOI] [PubMed] [Google Scholar]
- 19.Nayyar RP, Barr ML. Histochemical studies on the accessory body of cajal in neurones of the cat. J Comp Neurol 1968; 132:125-34; PMID:5732426; https://doi.org/ 10.1002/cne.901320107 [DOI] [PubMed] [Google Scholar]
- 20.Monneron A, Bernhard W. Fine structural organization of the interphase nucleus in some mammalian cells. J Ultrastruct Res 1969; 27:266-88; PMID:5813971; https://doi.org/ 10.1016/S0022-5320(69)80017-1 [DOI] [PubMed] [Google Scholar]
- 21.Hardin JH, Spicer SS, Greene WB. The paranucleolar structure, accessory body of Cajal, sex chromatin, and related structures in nuclei of rat trigeminal neurons: a cytochemical and ultrastructural study. Anat Rec 1969; 164:403-31; PMID:5797926; https://doi.org/ 10.1002/ar.1091640403 [DOI] [PubMed] [Google Scholar]
- 22.Lafarga M, Hervas J. Light and electron microscopic characterization of the ‘Accesory Body’ of Cajal in the neuronal nucleus. Elsevier Science Amsterdam, 1983. [DOI] [PubMed] [Google Scholar]
- 23.Lafarga M, Hervás JP, Santa-Cruz MC, Villegas J, Crespo D. The ‘accessory body’ of Cajal in the neuronal nucleus. A light and electron microscopic approach. Anat Embryol (Berl) 1983; 166:19-30; PMID:6301310; https://doi.org/ 10.1007/BF00317942 [DOI] [PubMed] [Google Scholar]
- 24.Gall JG. The centennial of the Cajal body. Nat Rev Mol Cell Biol 2003; 4:975-80; PMID:14685175; https://doi.org/ 10.1038/nrm1262 [DOI] [PubMed] [Google Scholar]
- 25.Gall JG, Bellini M, Wu Z, Murphy C. Assembly of the nuclear transcription and processing machinery: Cajal bodies (coiled bodies) and transcriptosomes. Mol Biol Cell 1999; 10:4385-402; PMID:10588665; https://doi.org/ 10.1091/mbc.10.12.4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carvalho T, Almeida F, Calapez A, Lafarga M, Berciano MT, Carmo-Fonseca M. The spinal muscular atrophy disease gene product, SMN: A link between snRNP biogenesis and the Cajal (coiled) body. J Cell Biol 1999; 147:715-28; PMID:10562276; https://doi.org/ 10.1083/jcb.147.4.715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrade LE, Chan EK, Raska I, Peebles CL, Roos G, Tan EM. Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80-coilin. J Exp Med 1991; 173:1407-19; PMID:2033369; https://doi.org/ 10.1084/jem.173.6.1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raska I, Andrade LE, Ochs RL, Chan EK, Chang CM, Roos G, Tan EM. Immunological and ultrastructural studies of the nuclear coiled body with autoimmune antibodies. Exp Cell Res 1991; 195:27-37; PMID:2055273; https://doi.org/ 10.1016/0014-4827(91)90496-H [DOI] [PubMed] [Google Scholar]
- 29.Tuma RS, Stolk JA, Roth MB. Identification and characterization of a sphere organelle protein. J Cell Biol 1993; 122:767-73; PMID:8349728; https://doi.org/ 10.1083/jcb.122.4.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cioce M, Lamond AI. Cajal bodies: a long history of discovery. Annu Rev Cell Dev Biol 2005; 21:105-31; PMID:16212489; https://doi.org/ 10.1146/annurev.cellbio.20.010403.103738 [DOI] [PubMed] [Google Scholar]
- 31.Pena E, Berciano MT, Fernandez R, Ojeda JL, Lafarga M. Neuronal body size correlates with the number of nucleoli and Cajal bodies, and with the organization of the splicing machinery in rat trigeminal ganglion neurons. J Comp Neurol 2001; 430:250-63; PMID:11135260; https://doi.org/ 10.1002/1096-9861(20010205)430:2%3c250::AID-CNE1029%3e3.0.CO;2-L [DOI] [PubMed] [Google Scholar]
- 32.Berciano MT, Novell M, Villagra NT, Casafont I, Bengoechea R, Val-Bernal JF, Lafarga M. Cajal body number and nucleolar size correlate with the cell body mass in human sensory ganglia neurons. J Struct Biol 2007; 158:410-20; PMID:17275332; https://doi.org/ 10.1016/j.jsb.2006.12.008 [DOI] [PubMed] [Google Scholar]
- 33.Tucker KE, Berciano MT, Jacobs EY, LePage DF, Shpargel KB, Rossire JJ, Chan EK, Lafarga M, Conlon RA, Matera AG. Residual Cajal bodies in coilin knockout mice fail to recruit Sm snRNPs and SMN, the spinal muscular atrophy gene product. J Cell Biol 2001; 154:293-307; PMID:11470819; https://doi.org/ 10.1083/jcb.200104083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilczynski GM. Significance of higher-order chromatin architecture for neuronal function and dysfunction. Neuropharmacology 2014; 80:28-33; PMID:24456745; https://doi.org/ 10.1016/j.neuropharm.2014.01.016 [DOI] [PubMed] [Google Scholar]
- 35.Peters A, Palay SL, Webster H deF. The fine structure of the nervous system: Neurons and their supporting cells. 3rd ed. Oxford Unviersity Press: New York, 1991. [Google Scholar]
- 36.Casafont I, Navascués J, Pena E, Lafarga M, Berciano MT. Nuclear organization and dynamics of transcription sites in rat sensory ganglia neurons detected by incorporation of 5′-fluorouridine into nascent RNA. Neuroscience 2006; 140:453-62; PMID:16563640; https://doi.org/ 10.1016/j.neuroscience.2006.02.030 [DOI] [PubMed] [Google Scholar]
- 37.Jordan BA, Fernholz BD, Khatri L, Ziff EB. Activity-dependent AIDA-1 nuclear signaling regulates nucleolar numbers and protein synthesis in neurons. Nat Neurosci 2007; 10:427-435; PMID:17334360; https://doi.org/ 10.1038/nn1867 [DOI] [PubMed] [Google Scholar]
- 38.Richter JD, Fallon JR. Synapses go nucle(ol)ar. Nat Neurosci 2007; 10:399-400; PMID:17387325; https://doi.org/ 10.1038/nn0407-399 [DOI] [PubMed] [Google Scholar]
- 39.Jády BE, Richard P, Bertrand E, Kiss T. Cell cycle-dependent recruitment of telomerase RNA and Cajal bodies to human telomeres. Mol Biol Cell 2006; 17:944-54; PMID:16319170; https://doi.org/16339074 10.1091/mbc.E05-09-0904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomlinson RL, Ziegler TD, Supakorndej T, Terns RM, Terns MP. Cell cycle-regulated trafficking of human telomerase to telomeres. Mol Biol Cell 2006; 17:955-65; PMID:16339074; https://doi.org/ 10.1091/mbc.E05-09-0903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carmo-Fonseca M, Ferreira J, Lamond AI. Assembly of snRNP-containing coiled bodies is regulated in interphase and mitosis-evidence that the coiled body is a kinetic nuclear structure. J Cell Biol 1993; 120:841-52; PMID:7679389; https://doi.org/ 10.1083/jcb.120.4.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strzelecka M, Trowitzsch S, Weber G, Lührmann R, Oates AC, Neugebauer KM. Coilin-dependent snRNP assembly is essential for zebrafish embryogenesis. TL - 17. Nat Struct Mol Biol 2010; 17 VN - r:403-409; PMID:20357773; https://doi.org/2088441 10.1038/nsmb.1783 [DOI] [PubMed] [Google Scholar]
- 43.Raska I, Ochs RL, Andrade LE, Chan EK, Burlingame R, Peebles C, Gruol D, Tan EM. Association between the nucleolus and the coiled body. J Struct Biol 1990; 104:120-7; PMID:2088441; https://doi.org/ 10.1016/1047-8477(90)90066-L [DOI] [PubMed] [Google Scholar]
- 44.Turner BJ, Bäumer D, Parkinson NJ, Scaber J, Ansorge O, Talbot K. TDP-43 expression in mouse models of amyotrophic lateral sclerosis and spinal muscular atrophy. BMC Neurosci 2008; 9:104; PMID:18957104; https://doi.org/ 10.1186/1471-2202-9-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lafarga M, Andrés MA, Fernández-Viadero C, Villegas J, Berciano MT. Number of nucleoli and coiled bodies and distribution of fibrillar centres in differentiating Purkinje neurons of chick and rat cerebellum. Anat Embryol (Berl) 1995; 191:359-67; PMID:7645762; https://doi.org/ 10.1007/BF00534689 [DOI] [PubMed] [Google Scholar]
- 46.Machyna M, Neugebauer KM, Staněk D. Coilin: The first 25 years. RNA Biol 2015; 12:590-6; PMID:25970135; https://doi.org/ 10.1080/15476286.2015.1034923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deryusheva S, Gall JG. Small Cajal body-specific RNAs of Drosophila function in the absence of Cajal bodies. Mol Biol Cell 2009; 20:5250-9; PMID:19846657; https://doi.org/ 10.1091/mbc.E09-09-0777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sleeman JE, Ajuh P, Lamond AI. snRNP protein expression enhances the formation of Cajal bodies containing p80-coilin and SMN. J Cell Sci 2001; 114:4407-4419; PMID:11792806 [DOI] [PubMed] [Google Scholar]
- 49.Navascues J, Bengoechea R, Tapia O, Casafont I, Berciano MT, Lafarga M. SUMO-1 transiently localizes to Cajal bodies in mammalian neurons. J Struct Biol 2008; 163:137-46; PMID:18571432; https://doi.org/ 10.1016/j.jsb.2008.04.013 [DOI] [PubMed] [Google Scholar]
- 50.Schulz S, Chachami G, Kozaczkiewicz L, Winter U, Stankovic-Valentin N, Haas P, Hofmann K, Urlaub H, Ovaa H, Wittbrodt J, et al.. Ubiquitin-specific protease-like 1 (USPL1) is a SUMO isopeptidase with essential, non-catalytic functions. EMBO Rep 2012; 13:930-8; PMID:22878415; https://doi.org/ 10.1038/embor.2012.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tapia O, Lafarga V, Bengoechea R, Palanca A, Lafarga M, Berciano MT. The SMN Tudor SIM-like domain is key to SmD1 and coilin interactions and to Cajal body biogenesis. J Cell Sci 2014; 127:939-46; PMID:24413165; https://doi.org/ 10.1242/jcs.138537 [DOI] [PubMed] [Google Scholar]
- 52.Liu Q, Dreyfuss G. A novel nuclear structure containing the survival of motor neurons protein. EMBO J 1996; 15:3555-65; PMID:8670859 [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Q, Fischer U, Wang F, Dreyfuss G. The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell 1997; 90:1013-21; PMID:9323129; https://doi.org/ 10.1016/S0092-8674(00)80367-0 [DOI] [PubMed] [Google Scholar]
- 54.Charroux B, Pellizzoni L, Perkinson RA, Shevchenko A, Mann M, Dreyfuss G. Gemin3: A novel DEAD box protein that interacts with SMN, the spinal muscular atrophy gene product, and is a component of gems. J Cell Biol 1999; 147:1181-94; PMID:10601333; https://doi.org/ 10.1083/jcb.147.6.1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matera AG, Frey MR. Coiled bodies and gems: Janus or gemini? Am J Hum Genet 1998; 63:317-21; PMID:9683623; https://doi.org/ 10.1086/301992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young PJ, Le TT, Dunckley M, Nguyen TM, Burghes AH, Morris GE. Nuclear gems and Cajal (coiled) bodies in fetal tissues: Nucleolar distribution of the spinal muscular atrophy protein, SMN. Exp Cell Res 2001; 265:252-61; PMID:11302690; https://doi.org/ 10.1006/excr.2001.5186 [DOI] [PubMed] [Google Scholar]
- 57.Lafarga M, Andres MA, Berciano MT, Maquiera E. Organization of nucleoli and nuclear bodies in osmotically stimulated supraoptic neurons of the rat. J Comp Neurol 1991; 308:329-39; PMID:1865004; https://doi.org/ 10.1002/cne.903080302 [DOI] [PubMed] [Google Scholar]
- 58.Palanca A, Casafont I, Berciano MT, Lafarga M. Reactive nucleolar and Cajal body responses to proteasome inhibition in sensory ganglion neurons. Biochim Biophys Acta 2014; 1842:848-59; PMID:24269586; https://doi.org/ 10.1016/j.bbadis.2013.11.016 [DOI] [PubMed] [Google Scholar]
- 59.Lafarga M, Berciano MT, Garcia-Segura LM, Andres MA, Carmo-Fonseca M. Acute osmotic/stress stimuli induce a transient decrease of transcriptional activity in the neurosecretory neurons of supraoptic nuclei. J Neurocytol 1998; 27:205-17; PMID:10640180; https://doi.org/ 10.1023/A:1006937032068 [DOI] [PubMed] [Google Scholar]
- 60.Baltanás FC, Casafont I, Weruaga E, Alonso JR, Berciano MT, Lafarga M. Nucleolar disruption and cajal body disassembly are nuclear hallmarks of DNA damage-induced neurodegeneration in Purkinje cells. Brain Pathol 2011; 21:374-388; PMID:21054627; https://doi.org/7523596 10.1111/j.1750-3639.2010.00461.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sato S, Burgess SB, McIlwain DL. Transcription and motoneuron size. J Neurochem 1994; 63:1609-15; PMID:7523596; https://doi.org/ 10.1046/j.1471-4159.1994.63051609.x [DOI] [PubMed] [Google Scholar]
- 62.Klingauf M, Stanek D, Neugebauer KM. Enhancement of U4/U6 small nuclear ribonucleoprotein particle association in Cajal bodies predicted by mathematical modeling. Mol Biol Cell 2006; 17:4972-81; PMID:16987958; https://doi.org/ 10.1091/mbc.E06-06-0513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matera AG, Izaguire-Sierra M, Praveen K, Rajendra TK. Nuclear bodies: Random aggregates of sticky proteins or crucibles of macromolecular assembly? Dev Cell 2009; 17:639-647; PMID:19922869; https://doi.org/ 10.1016/j.devcel.2009.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shevtsov SP, Dundr M. Nucleation of nuclear bodies by RNA. Nat Cell Biol 2011; 13:167-173; PMID:21240286; https://doi.org/ 10.1038/ncb2157 [DOI] [PubMed] [Google Scholar]
- 65.Mao YS, Zhang B, Spector DL. Biogenesis and function of nuclear bodies. Trends Genet 2011; 27:295-306; PMID:21680045; https://doi.org/ 10.1016/j.tig.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Q, Sawyer IA, Sung M-H, Sturgill D, Shevtsov SP, Pegoraro G, Hakim O, Baek S, Hager GL, Dundr M. Cajal bodies are linked to genome conformation. Nat Commun 2016; 7:10966; PMID:26997247; https://doi.org/ 10.1038/ncomms10966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santama N, Dotti CG, Lamond AI. Neuronal differentiation in the rat hippocampus involves a stage-specific reorganization of subnuclear structure both in vivo and in vitro. Eur J Neurosci 1996; 8:892-905; PMID:8743737; https://doi.org/ 10.1111/j.1460-9568.1996.tb01576.x [DOI] [PubMed] [Google Scholar]
- 68.Isaac C, Yang Y, Meier UT. Nopp 140 functions as a molecular link between the nucleolus and the coiled bodies. J Cell Biol 1998; 142:319-329; PMID:9679133; https://doi.org/ 10.1083/jcb.142.2.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Platani M, Goldberg I, Swedlow JR, Lamond AI. In vivo analysis of Cajal body movement, separation, and joining in live human cells. J Cell Biol 2000; 151:1561-74; PMID:11134083; https://doi.org/ 10.1083/jcb.151.7.1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Machyna M, Kehr S, Straube K, Kappei D, Buchholz F, Butter F, Ule J, Hertel J, Stadler PF, Neugebauer KM. The coilin interactome identifies hundreds of small noncoding RNAs that traffic through Cajal bodies. Mol Cell 2014; 56:389-99; PMID:25514182; https://doi.org/ 10.1016/j.molcel.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 71.Xu H, Hebert MD. A novel EB-1/AIDA-1 isoform, AIDA-1c, interacts with the Cajal body protein coilin. BMC Cell Biol 2005; 6:23; PMID:15862129; https://doi.org/ 10.1186/1471-2121-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ross CA. Intranuclear neuronal inclusions: a common pathogenic mechanism for glutamine-repeat neurodegenerative diseases? Neuron 1997; 19:1147-50; PMID:9427237; https://doi.org/ 10.1016/S0896-6273(00)80405-5 [DOI] [PubMed] [Google Scholar]
- 73.Yamada M, Sato T, Shimohata T, Hayashi S, Igarashi S, Tsuji S, Takahashi H. Interaction between neuronal intranuclear inclusions and promyelocytic leukemia protein nuclear and coiled bodies in CAG repeat diseases. Am J Pathol 2001; 159:1785-95; PMID:11696439; https://doi.org/ 10.1016/S0002-9440(10)63025-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hebert MD. Signals controlling Cajal body assembly and function. Int J Biochem Cell Biol 2013; 45:1314-7; PMID:23583661; https://doi.org/ 10.1016/j.biocel.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun J, Xu H, Negi S, Subramony SH, Hebert MD. Differential effects of polyglutamine proteins on nuclear organization and artificial reporter splicing. J Neurosci Res 2007; 85:2306-2317; PMID:17526020; https://doi.org/ 10.1002/jnr.21369 [DOI] [PubMed] [Google Scholar]
- 76.Fernandez-Gonzalez A, La Spada AR, Treadaway J, Higdon JC, Harris BS, Sidman RL, Morgan JI, Zuo J. Purkinje cell degeneration (pcd) phenotypes caused by mutations in the axotomy-induced gene, Nna1. Science 2002; 295:1904-6; PMID:11884758; https://doi.org/ 10.1126/science.1068912 [DOI] [PubMed] [Google Scholar]
- 77.Matera AG, Wang Z. A day in the life of the spliceosome. Nat Rev Mol Cell Biol 2014; 15:108-21; PMID:24452469; https://doi.org/ 10.1038/nrm3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Z, Lotti F, Dittmar K, Younis I, Wan L, Kasim M, Dreyfuss G. SMN deficiency causes tissue-specific perturbations in the repertoire of snRNAs and widespread defects in splicing. Cell 2008; 133:585-600; PMID:18485868; https://doi.org/ 10.1016/j.cell.2008.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burghes AHM, Beattie CE. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat Rev Neurosci 2009; 10:597-609; PMID:19584893; https://doi.org/ 10.1038/nrn2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boulisfane N, Choleza M, Rage F, Neel H, Soret J, Bordonné R. Impaired minor tri-snRNP assembly generates differential splicing defects of U12-type introns in lymphoblasts derived from a type I SMA patient. Hum Mol Genet 2011; 20:641-8; PMID:21098506; https://doi.org/ 10.1093/hmg/ddq508 [DOI] [PubMed] [Google Scholar]
- 81.Li DK, Tisdale S, Lotti F, Pellizzoni L. SMN control of RNP assembly: From post-transcriptional gene regulation to motor neuron disease. Semin Cell Dev Biol 2014; 32:22-29; PMID:24769255; https://doi.org/ 10.1016/j.semcdb.2014.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ratti A, Buratti E. Physiological functions and pathobiology of TDP-43 and FUS/TLS proteins. J Neurochem 2016; 138:95–111; PMID:27015757; https://doi.org/ 10.1111/jnc.13625 [DOI] [PubMed] [Google Scholar]
- 83.Shan X, Chiang P-M, Price DL, Wong PC. Altered distributions of Gemini of coiled bodies and mitochondria in motor neurons of TDP-43 transgenic mice. Proc Natl Acad Sci 2010; 107:16325-16330; PMID:20736350; https://doi.org/ 10.1073/pnas.1003459107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamazaki T, Chen S, Yu Y, Yan B, Haertlein TC, Carrasco MA, Tapia JC, Zhai B, Das R, Lalancette-Hebert M, et al.. FUS-SMN Protein Interactions Link the Motor Neuron Diseases ALS and SMA. Cell Rep 2012; 2:799-806; PMID:23022481; https://doi.org/ 10.1016/j.celrep.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishihara T, Ariizumi Y, Shiga A, Kato T, Tan C-F, Sato T, Miki Y, Yokoo M, Fujino T, Koyama A, et al.. Decreased number of Gemini of coiled bodies and U12 snRNA level in amyotrophic lateral sclerosis. Hum Mol Genet 2013; 22:4136-47; PMID:23740936; https://doi.org/ 10.1093/hmg/ddt262 [DOI] [PubMed] [Google Scholar]
- 86.Onodera O, Ishihara T, Shiga A, Ariizumi Y, Yokoseki A, Nishizawa M. Minor splicing pathway is not minor any more: Implications for the pathogenesis of motor neuron diseases. Neuropathology 2014; 34:99-107; PMID:24112438; https://doi.org/ 10.1111/neup.12070 [DOI] [PubMed] [Google Scholar]
- 87.Kariya S, Re DB, Jacquier A, Nelson K, Przedborski S, Monani UR. Mutant superoxide dismutase 1 (SOD1), a cause of amyotrophic lateral sclerosis, disrupts the recruitment of SMN, the spinal muscular atrophy protein to nuclear Cajal bodies. Hum Mol Genet 2012; 21:3421-34; PMID:22581780; https://doi.org/ 10.1093/hmg/dds174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun S, Ling S, Qiu J, Albuquerque CP, Zhou Y, Tokunaga S, Li H, Qiu H, Bui A, Yeo GW, Huang EJ, et al.. ALS-causative mutations in FUS/TLS confer gain and loss of function by altered association with SMN and U1-snRNP. Nat Commun 2015; 6:6171; PMID:25625564; https://doi.org/ 10.1038/ncomms7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cauchi RJ. Gem depletion: Amyotrophic lateral sclerosis and spinal muscular atrophy crossover. CNS Neurosci Ther 2014; 20:574-581; PMID:24645792; https://doi.org/ 10.1111/cns.12242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gertz B, Wong M, Martin LJ. Nuclear localization of human SOD1 and mutant SOD1-specific disruption of survival motor neuron protein complex in transgenic amyotrophic lateral sclerosis mice. J Neuropathol Exp Neurol 2012; 71:162-77; PMID:22249462; https://doi.org/ 10.1097/NEN.0b013e318244b635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Coovert DD, Le TT, McAndrew PE, Strasswimmer J, Crawford TO, Mendell JR, Coulson SE, Androphy EJ, Prior TW, Burghes AH. The survival motor neuron protein in spinal muscular atrophy. Hum Mol Genet 1997; 6:1205-1214; PMID:9259265; https://doi.org/ 10.1093/hmg/6.8.1205 [DOI] [PubMed] [Google Scholar]
- 92.Carmo-Fonseca M, Pepperkok R, Carvalho MT, Lamond AI. Transcription-dependent colocalization of the U1, U2, U4/U6, and U5 snRNPs in coiled bodies. J Cell Biol 1992; 117:1-14; PMID:1532583; https://doi.org/ 10.1083/jcb.117.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lemm I, Girard C, Kuhn AN, Watkins NJ, Schneider M, Bordonné R, Lührmann R. Ongoing U snRNP biogenesis is required for the integrity of Cajal bodies. Mol Biol Cell 2006; 17:3221-31; PMID:16687569; https://doi.org/ 10.1091/mbc.E06-03-0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dundr M. Nuclear bodies: Multifunctional companions of the genome. Curr Opin Cell Biol 2012; 24:415-422; PMID:22541757; https://doi.org/ 10.1016/j.ceb.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Novotný I, Blažíková M, Staněk D, Herman P, Malinsky J. In vivo kinetics of U4/U6·U5 tri-snRNP formation in Cajal bodies. Mol Biol Cell 2011; 22:513-523; PMID:21177826; https://doi.org/ 10.1091/mbc.E10-07-0560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tapia O, Bengoechea R, Palanca A, Arteaga R, Val-Bernal JF, Tizzano EF, Berciano MT, Lafarga M. Reorganization of Cajal bodies and nucleolar targeting of coilin in motor neurons of type I spinal muscular atrophy. Histochem Cell Biol 2012; 137:657-67; PMID:22302308; https://doi.org/ 10.1007/s00418-012-0921-8 [DOI] [PubMed] [Google Scholar]
- 97.Lefebvre S, Burlet P, Liu Q, Bertrandy S, Clermont O, Munnich A, Dreyfuss G, Melki J. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet 1997; 16:265-269; PMID:9207792; https://doi.org/ 10.1038/ng0797-265 [DOI] [PubMed] [Google Scholar]
- 98.Cioce M, Boulon S, Matera AG, Lamond AI. UV-induced fragmentation of Cajal bodies. J Cell Biol 2006; 175:401-13; PMID:17088425; https://doi.org/ 10.1083/jcb.200604099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Casafont I, Palanca A, Lafarga V, Berciano MT, Lafarga M. Effect of ionizing radiation in sensory ganglion neurons: organization and dynamics of nuclear compartments of DNA damage/repair and their relationship with transcription and cell cycle. Acta Neuropathol 2011; 122:481-93; PMID:21915754; https://doi.org/ 10.1007/s00401-011-0869-0 [DOI] [PubMed] [Google Scholar]
- 100.Lafarga M, Gonzalez C, Berciano MT. An improved cytological silver staining method for the demonstration of neuronal nuclear bodies. J Neurosci Methods 1986; 18:317-24; PMID:2432363; https://doi.org/ 10.1016/0165-0270(86)90019-1 [DOI] [PubMed] [Google Scholar]