Abstract
Overload-exercise (OE) causes myocardial injury through inducing autophagy and apoptosis. In this study we examined whether an autophagy inhibitor 3-methyladenine (3-MA) could alleviate OE-induced cardiac injury. Rats were injected with 3-MA (15 mg/kg, iv) or saline before subjected to various intensities of OE, including no swim (control), 2 h swim (mild-intensity exercise, MIE), 2 h swim with 2.5% body weight overload (moderate OE; MOE), 5% overload (intensive OE; IOE) or 2.5% overload until exhausted (exhaustive OE; EOE). After OE, the hearts were harvested for morphological and biochemiacal analysis. The cardiac morphology, autophagosomes and apoptosis were examined with H&E staining, transmission electron microscopy and TUNEL analysis, respectively. Autophagy-related proteins to (LC3-II/I and Beclin-1) and apoptosis-related proteins (Bcl-2/Bax) were assessed using Western blotting. Our results showed that compared with the control, MIE did not change the morphological structures of the heart tissues that exhibited intact myocardial fibers and neatly arranged cardiomyocytes. However, IOE resulted in irregular arrangement of cardiomyocytes and significantly increased width of cardiomyocytes, whereas EOE caused more swollen and even disrupted cardiomyocytes. In parallel with the increased OE intensity (MOE, IOE, EOE), cardiomyocyte autophagy and apoptosis became more and more prominent, evidenced by the increasing number of autophagosomes and expression levels of LC3-II/I and Beclin-1 as well as the increasing apoptotic cells and decreasing Bcl-2/Bax ratio. 3-MA administration significantly attenuated OE-induced morphological changes of cardiomyocytes as well as all the autophagy- and apoptosis-related abnormalities in MOE, IOE and EOE rats. Thus, the autophagy inhibitor 3-MA could alleviate OE-induced heart injury in rats.
Keywords: overload exercise, autophagy, apoptosis, cardiac injury, 3-methyladenine
Introduction
Physical activity and exercise is associated with a reduced risk of cardiovascular disease in epidemiologic studies1. Appropriate exercise has been demonstrated to improve cardiovascular structure and function as well as maintain glucose and lipid homeostasis2,3,4. However, high-intensity exercise can result in increased autophagy in skeletal muscle1,5. We have previously shown that long-term overload-exercise (OE) in rats led to heart dysfunction by increasing cardiomyocyte apoptosis6,7. Later, we found that short-term OE in rats also induced cardiac injury through the induction of autophagy and apoptosis6,7. However, whether the inhibition of autophagy can alleviate OE-induced cardiac injury remains unknown.
Autophagy is a conserved intracellular degradation process that delivers cytoplasmic constituents to the lysosome to provide a protective mechanism for maintaining the function of organ and tissue. Increasing evidence has demonstrated that autophagy contributes to various diseases, such as cancer and heart diseases8,9. Massive and persistent activation of autophagy may contribute to excessive cell proliferation and pathological alterations10. Pharmacological inhibition of autophagy after viral insults has protective effects on acute lung injury11,12. Autophagy occurs at a low rate in the heart under physiological conditions13,14. However, it has been shown to be upregulated in cardiovascular diseases, such as ischemia-reperfusion and heart failure9,15,16,17. Thus, it is interesting and important to explore whether inhibition of autophagy could be beneficial in OE-induced cardiac injury.
The interactions between autophagy and apoptosis have been investigated with different results regarding autophagy in preventing or inducing apoptosis18,19. Elevated autophagy was reported to promote apoptosis in a hypoxic condition15. The B-cell lymphoma 2 (Bcl-2) family of proteins includes anti-apoptotic member Bcl-2 and pro-apoptotic member Bax. Previous studies have demonstrated that Bcl-2 can inhibit apoptosis and autophagy through binding to Bax and beclin-1, which is a key component involved in the initiation of autophagosomes formation20,21,22,23. However, whether autophagy inhibition can reduce different intensities of OE-induced cardiomyocyte apoptosis remains unknown.
In the present study, we investigated the effect of autophagy inhibition on different intensities of OE-induced cardiomyocyte injury.
Materials and methods
Animals
A total of 60 healthy male Sprague-Dawley (SD) rats (age: 2-months old; weight: 200±20 g) were purchased from Hubei Research Center of Experimental Animals (Hubei, China) and used for the study. All animals were maintained on a 12-h light/dark cycle and provided access to food and water ad libitum.
All experimental protocols were approved by the Animal Care and Use Committee of Wuhan Sports University, which is in compliance with the National Institutes of Health Guidelines. All experiments were dedicatedly designed and performed according to the ethical guidelines for the use of experimental pain-conscious animals24.
Experimental protocol of OE in rats
The swimming exercise protocol was described in our previous study with a slight modification6. All rats were pre-trained to swim in the absence of a load for one week. For formal OE procedures, all rats were randomly divided into two groups (n=30/group): the saline-treatment group was intravenously injected with 500 μL sterile saline by using 261/2 gauge needle via tail vein; the 3-MA-treatment group was intravenously injected with 3-MA at a dose of 15 mg/kg of body weight in a volume of 500 μL by using a 261/2 gauge needle via the tail vein. Then, rats in each group were randomly divided into five subgroups (n=6/subgroup) and subjected to one single bout of swimming training: saline+Con & 3-MA+Con: control group (no swim); saline+MIE & 3-MA+MIE: no overload, swim for 2 h (mild-intensity exercise; MIE); saline+MOE & 3-MA+MOE: with a load of 2.5% of the body weight on the tail, swim for 2 h (moderate-overload exercise; MOE); saline+IOE & 3-MA+IOE: with a load of 5% of the body weight on the tail, swim for 2 h (intensive-overload exercise; IOE); saline+EOE & 3-MA+EOE: with a load of 2.5% of the body weight on the tail, swim till the rats became exhausted (unable to float up within 10 s; exhausting-overload exercise; EOE). The load was composed of paper clips that were bound with a rope and tied to the tail base.
Histological analysis
After OE, three hearts from each group were harvested, and the left ventricles were isolated and treated as we described previously6,19. In brief, all left ventricles were fixed with 10% neutral formalin in phosphate buffer overnight, soaked with water, dehydrated through an alcohol series in a tissue processing machine (Tissue-tek, USA), and embedded in paraffin. The longitudinal sections (5-μm thickness) of heart tissue were prepared by using a microtome (Leica RM 2255, Germany) and stained with hematoxylin-eosin (H&E, Sigma, Louis, MO, USA) solution according to the manufacturer's instructions. All sections were examined using a light microscope (Leica, Germany) at 400×, and the width of cardiomyocytes was quantified as previously reported25. Briefly, the data of cardiomyocyte width were collected from ten representative cardiomyocytes per field, five random fields in each slide. The data from six slides were averaged for one rat. Distortion and disruption of myocardial fibers were grossly evaluated by visual observation.
Transmission electron microscopy (TEM)
For transmission electron microscopy (TEM) analysis, the harvested hearts from different groups were fixed with 2% glutaraldehyde and post-fixed with 1% osmium (all were purchased from Electron Microscopy Science, Hatfield, PA), then embedded with Epon 812 (Sigma, St Louis, MO, USA) and baked at 60 °C according to our previously described protocol6,19. Ultrathin sections (60–80 nm) were prepared with MT7000, mounted on 300-mesh copper grids, and stained with uranyl acetate and lead citrate. All samples were examined with a transmission electron microscope (H-7000FA, Japan) at an accelerating voltage of 70 kV. The quantification of autophagosomes was analyzed as previously described10. The autophagosomes on each slide were counted under five random fields, and the numbers of six slides were averaged for one rat.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling assay
In situ apoptosis was performed by using the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay kit (Roche Diagnostics, Indianapolis, IN, USA) according to our previous report6,19. Briefly, heart slices (5 μm-thickness) were adhered to gelatin-coated slides, dewaxed, rehydrated in gradient concentration ethanol, and washed with PBS. Then, the slides were incubated with Proteinase K solution in PBS, blocked and incubated with TUNEL reaction mixture (45 μL Equilibration buffer, 1 μL biotin-11-dUTP and 4 μL TdT, freshly prepared) for 60 min at 37 °C. Labeled nuclei were visualized with peroxidase-conjugated anti-digoxygenin antibody with diaminobenzidine as the chromogen. All heart tissue samples were examined under a light microscope (Leica, German). TUNEL-positive cells had brown or yellowish-brown granules in the nucleus, with the characteristic morphology of apoptosis. Apoptotic cells were counted at ten arbitrarily selected microscopic fields. The apoptotic rate of cardiomyocytes was calculated with the following formula: apoptotic rate (%)=(number of TUNEL-positive cells/total number of cells)×100.
Western blot analysis
Proteins from the hearts harvested from each group were extracted with tissue lysis buffer (Thermo scientific, Grand Island, NY, USA) supplemented with complete mini protease inhibitor tablet (Roche Diagnostics, Indianapolis, IN, USA). Protein lysates were electrophoresed on an SDS-PAGE gel and transferred to PVDF membranes. The membranes were blocked with 5% non-fat milk for 1 h and incubated with primary antibodies against Beclin-1 (1:500, Abcam, Cambridge, MA, USA), LC-3B (1:1000, Abcam, Cambridge, MA, USA), Bcl-2 (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA), Bax (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and β-actin (1:10 000; Cell Signaling Technologies, Danvers, MA, USA) at 4 °C overnight. After washing, the membranes were incubated with horseradish peroxidase-conjugated IgG (Jackson Immuno Research Lab, West Grove, PA, USA) for 1 h at RT. The blots were developed with enhanced chemiluminescence developing solutions and quantified with ImageJ software.
Statistical analysis
Experimental data were expressed as the mean±SD. Multiple comparisons were performed by one- or two-way ANOVA followed by post hoc test. SAS 6.12 software was used. For all tests, a value of P<0.05 was considered statistically significant.
Results
OE-induced heart injury model in rats
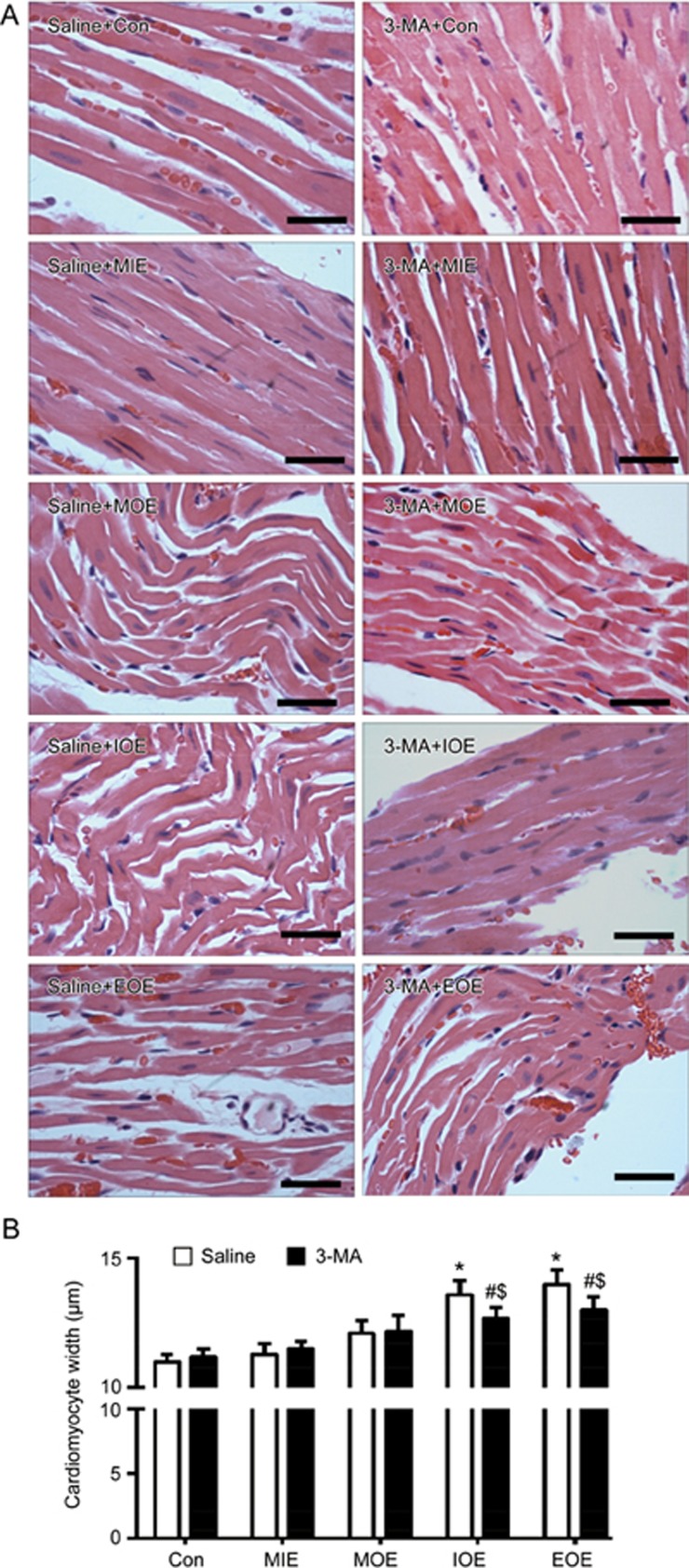
The myocardial structure was assessed by H&E staining. As shown in Figure 1, in the saline+Con and saline+MIE groups, the cardiomyocytes of the left ventricles maintained normal morphological structures, such as neatly arranged cardiomyocytes and intact myocardial fibers with clearly visible stripes. In the saline+MOE group, the myocardium displayed fiber distortion. In the saline+IOE group, we found that cardiomyocytes became swollen and distorted. Moreover, in the saline+EOE group, the cardiomyocytes became more swollen and some fibers were disrupted. The data demonstrate the success of the rat model of OE-induced heart injury.
Figure 1.
3-MA reduced OE-induced heart injury in rats. (A) Representative histologic images showing the morphology of cardiomyocytes in the left ventricle of the heart in various OE groups treated with/without 3-MA. (B) Quantitative data of the cardiomyocyte width. *P<0.05 vs saline+Con, saline+MIE or saline+MOE. #P<0.05 vs 3-MA+Con or 3-MA+MIE. $P<0.05 vs saline+IOE or saline+EOE. The data are expressed as the mean±SD. n=3/group. Magnification: ×400, Scale bar: 40 μm. 3-MA: 3-methyladenine; MIE: mild-intensity exercise; MOE: moderate-overload exercise; IOE: intensive-overload exercise; EOE: exhausting-overload exercise.
3-MA reduced cardiac morphology changes in OE rats
When compared to saline+MIE group, 3-MA+MIE did not affect the morphology of cardiomyocytes, such as fiber arrangement and the width of cardiomyocytes. In the 3-MA+MOE group, 3-MA treatment decreased the distortion of myocardium fibers when compared to those in saline-treated rats. In the 3-MA+IOE group, 3-MA treatment remarkably reduced the distortion of fibers. Moreover, in the EOE group, 3-MA treatment partially reduced the fiber distortion and significantly decreased the width of cardiomyocytes (P<0.05, vs saline+EOE; Figure 1).
3-MA decreased autophagosome formation and down-regulated the ratio of LC-3 II/I in the hearts of in OE rats
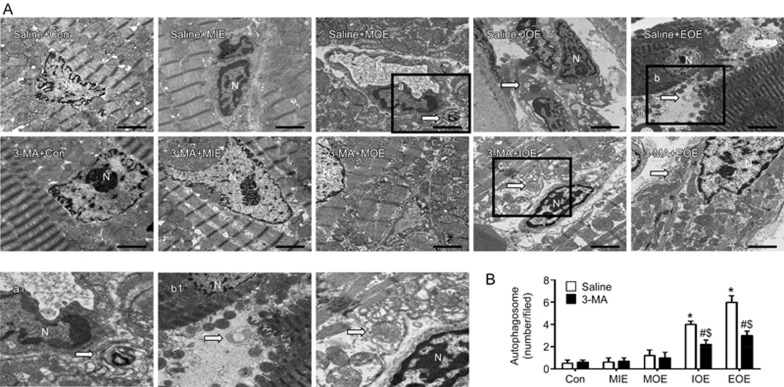
Autophagosome formation is an important morphological feature of autophagy. As shown in Figure 2, a rare autophagosome was found in the myocardium of control or MIE or MOE rats. More autophagosomes were observed in the myocardium of saline-treated IOE and EOE rats (P<0.05, vs saline+Con or saline+MIE). 3-MA treatment remarkably reduced autophagosome formation in the myocardium of IOE and EOE rats (P<0.05, vs saline+IOE or saline+EOE).
Figure 2.
3-MA reduced OE-induced autophagosome formation in the left ventricle. (A) Representative TEM plots showing the formation of autophagosomes (white arrow) in the left ventricle of the heart in various OE groups treated with/without 3-MA. a1 is the enlarged view of box a; b1 is the enlarged view of box b. Scale bar: 2 μm. (B) Quantification of autophagosomes. *P<0.05 vs saline+Con, saline+MIE or saline+MOE. #P<0.05 vs 3-MA+Con, 3-MA+MIE or 3-MA+MOE. $P<0.05 vs saline+IOE or saline+EOE. The data are expressed as the mean±SD. n=3/group. N: nucleus. 3-MA: 3-methyladenine; MIE: mild-intensity exercise; MOE: moderate-overload exercise; IOE: intensive-overload exercise; EOE: exhausting-overload exercise.
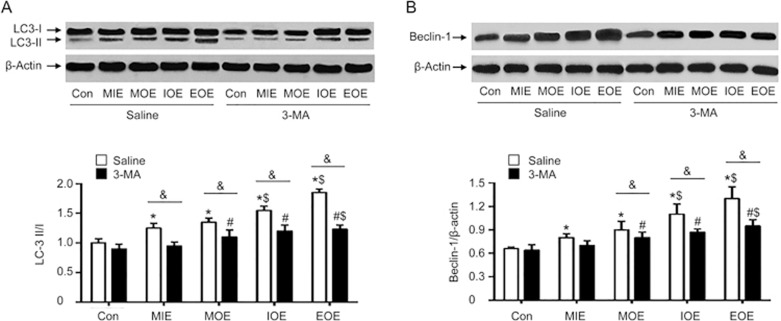
To further assess the autophagy activity, we measured the levels of autophagy-related proteins, LC3-II/I and Beclin-1. Our western blot results (Figure 3) revealed that OE elevated the ratio of LC-3 II/I in saline-treated rats (P<0.05 vs saline+Con). Further, this increase was paralleled by the increasing of the intensity of OE (EOE>IOE>MOE). 3-MA treatment did not significantly change the ratio of LC-3 II/I in MIE rats (P<0.05 vs 3-MA+Con), whereas 3-MA significantly decreased MOE-, IOE- and EOE-induced elevated ratios of LC-3 II/I, compared to those of saline-treated rats (P<0.05, vs saline+MOE, or saline+IOE, or saline+EOE). Similarly, we found that the expression of Beclin-1, another autophagy-related protein, was upregulated in the saline-treated MIE, MOE, IOE and EOE rats (P<0.05, vs saline+Con), paralleled by the intensity of OE (EOE>IOE>MOE). 3-MA decreased MOE-induced upregulation of beclin-1(P<0.05, vs saline+MOE). IOE- or EOE- induced elevated expression of Beclin-1 was also largely reduced by 3-MA (P<0.05, vs saline+IOE or saline+EOE). Taken together, these data are in agreement with the findings in autophagosome formation as well as in histological changes.
Figure 3.
3-MA prevented OE-induced elevated ratio of LC-3 II/I and increased expression of beclin-1 in the left ventricle. (A) Representative Western blot bands and summarized data of LC3-I and LC3-II. (B) Representative bands and summarized data of beclin-1. *P<0.05 vs saline+Con. #P<0.05 vs 3-MA+Con. $P<0.05, vs saline+MIE or 3-MA+MIE. &P<0.05 vs saline+MOE, saline+IOE or saline+EOE. The data are expressed as the mean±SD. n=3/group. 3-methyladenine; MIE: mild-intensity exercise; MOE: moderate-overload exercise; IOE: intensive-overload exercise; EOE: exhausting-overload exercise.
3-MA decreased cell apoptosis in the left ventricle of OE rats
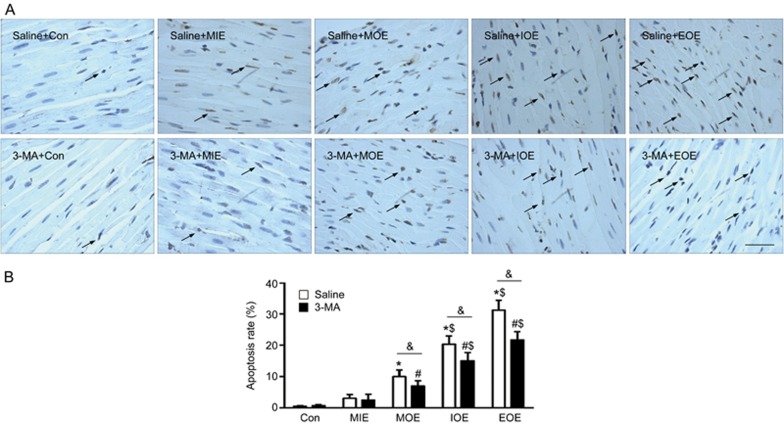
As shown in Figure 4, the cell apoptosis in the left ventricle was analyzed by TUNEL staining. In the saline-treated rats, there was a significant increase of cell apoptosis in those completed MOE, IOE and EOE compared to that of the control and MIE rats (P<0.05, vs saline+Con or saline+MIE). More cells underwent apoptosis as the intensity of OE (EOE>IOE>MOE) increased. 3-MA did not display an anti-apoptotic effect in MIE rats (P>0.05, vs saline+MIE). However, it significantly decreased MOE-induced apoptosis and largely reduced IOE-, and EOE- induced apoptosis (P<0.05, vs saline+ MOE, or saline+IOE, or saline+EOE.
Figure 4.
3-MA decreased OE-induced cell apoptosis in rats. (A) Representative images showing the TUNEL positive cells which were labeled in brown (arrows). Scale bar: 100 μm. (B) Summarized data. *P<0.05 vs saline+Con or saline+MIE; #P<0.05 vs 3-MA+Con or 3-MA+MIE. $P<0.05 vs saline+MOE or 3-MA+MOE. &P<0.05 vs saline+MOE, saline+IOE or saline+EOE. The data are expressed as the mean±SD. n=3/group. 3-MA: 3-methyladenine; MIE: mild-intensity exercise; MOE: moderate-overload exercise; IOE: intensive-overload exercise; EOE: exhausting-overload exercise.
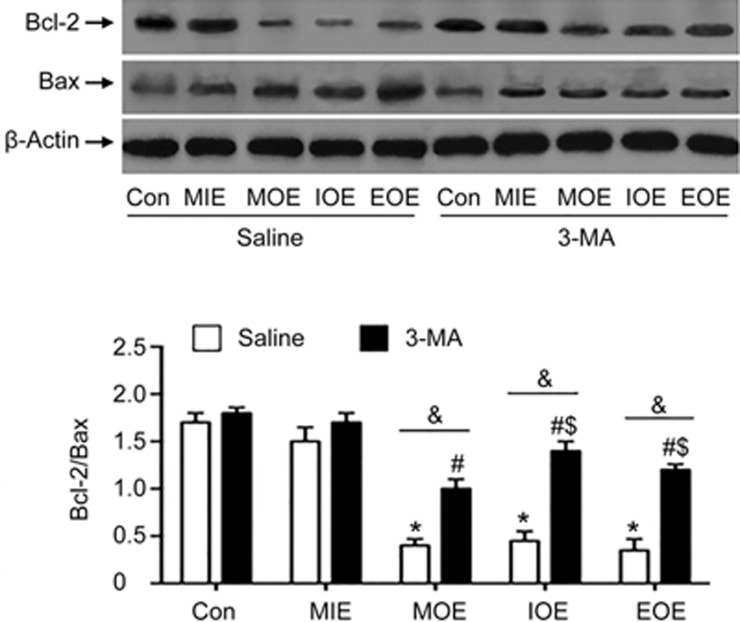
3-MA prevented the reduction of Bcl-2/Bax in the left ventricle of OE rats
To further explore the mechanism of 3-MA, we measured the expression of Bcl-2 and Bax in the proteins extracted from the left ventricle. As shown in Figure 5, MIE did not significantly change the levels of Bcl-2 and Bax, or the ratio of Bcl-2/Bax, in both saline and 3-MA treated rats (P>0.05, vs saline+Con or 3-MA+Con). MOE, IOE and EOE significantly decreased the ratio of Bcl-2/Bax in saline-treated rats (P<0.05, vs saline+Con). 3-MA treatment significantly increased the ratio of Bcl-2/Bax, which was associated with down-regulated expression of Bax and elevated expression of Bcl-2 (P<0.05, vs saline+MOE, or saline+IOE, or saline+EOE). These data help to interpret the findings on cell apoptosis in MOE, IOE and EOE rats.
Figure 5.
3-MA prevented OE-induced reduction of Bcl-2/Bax in the left ventricle. Top panels: Representative Western blot bands. The histogram shows the summarized data of Bcl-2/Bax. *P<0.05 vs saline+Con or saline+MIE. #P<0.05 vs 3-MA+Con or 3-MA+MIE. $P<0.05 vs 3-MA+MOE. &P<0.05 vs saline+MOE, saline+IOE or saline+EOE. The data are expressed as the mean±SD. n=3/group. 3-MA: 3-methyladenine; MIE: mild-intensity exercise; MOE: moderate-overload exercise; IOE: intensive-overload exercise; EOE: exhausting-overload exercise.
Discussion
In this study, we showed a rat model of short-term OE-induced heart injury characterized by histological changes and alterations in gene expression. Moreover, we revealed that 3-MA, an autophagy inhibitor, effectively attenuated the heart injury in this OE model through modulating autophagy and apoptosis.
It is well known that regular physical exercise can help maintain heart health26. Swimming exercise training can induce a physiological left ventricular hypertrophy for the hemodynamic overload of the heart27. Nevertheless, vigorous exercise causes pathological cardiac hypertrophy, triggers sudden cardiac cell death, and even acute myocardial infarctio28,29. The changes of cardiac morphology have been shown to be associated with poor prognosis in patients with heart failure and correlate with adverse cardiac remodeling30. We have previously demonstrated that short-term OE can induce cardiac injury, as evidenced by the disorganization of myocardial fibers and increased collagen volume fraction in the left ventricle. Such injury mainly was through OE-induced activation of autophagy and apoptosis19. Interestingly, in that study, we found that transcutaneous electrical acupoint stimulation, a novel noninvasive and low-risk alternative to electroacupuncture, could counteract the short-term OE-induced cardiac injury19. In this study, we found that OE induced an irregular arrangement of cardiomyocytes, an increase of the width of cardiomyocytes, and an increase of cardiomyocyte autophagy and apoptosis, which paralleled the intensity of OE (EOE>IOE>MOE). This made it possible to investigate the effects of different intensities of OE on the heart.
Autophagy has been characterized as an essential cellular process in the heart. In general, cells exhibit a low rate of autophagy at basal levels, however, it can increase in response to stress conditions31. Though autophagy is documented as a cell survival mechanism, excess autophagy might be detrimental and cause cell death13. Autophagy has been reported in myocardial ischemia/reperfusion, cardiac oxidative stress and heart failure32,33. To investigate whether inhibition of autophagy can affect OE-induced acute detrimental effects in the heart, we pre-treated rats with autophagy inhibitor 3-MA. Our EM data showed that 3-MA largely alleviated MOE-, IOE- and EOE- induced autophagosome formation in the myocardium, indicating that OE-induced excess autophagy can be inhibited by 3-MA. In addition, our results showed that 3-MA treatment remarkably decreased the MOE-induced irregular arrangement of cardiomyocytes and partially reduced IOE- or EOE-induced swelling of cardiomyocytes and myocardium fiber distortion, suggesting the therapeutic potential of 3-MA on OE-induced cardiac injury. In our study, we did not observe any acute deaths of animals. However, whether 3-MA could induce later detrimental effects or even a considerable rate of mortality awaits further investigation. In addition, we will further investigate whether other autophagy inhibitors such as wortmannin34 have anti-autophagic effects on OE-induced acute cardiac injury rat model.
To elucidate the relationship of autophagy and OE-induced cardiac injury, we determined the autophagy-related gene expression in the myocardium in rats. It is well known that the conversion of LC-3 I to LC-3 II is an autophagic marker35,36. The ratio of LC-3 II/I has traditionally been used to evaluate the extent of autophagy. In our study, 3-MA treatment decreased the ratio of LC-3 II/I in MOE, IOE and EOE rats, suggesting that 3-MA can alleviate excessive autophagy and prevent myocardium damage. We also measured beclin-1, which is another key protein shown to be involved in the regulation of autophagy13. Consistently, our data showed that 3-MA treatment reduced the OE-induced upregulation of beclin-1 in the heart. Taken together, 3-MA can decrease the ratio of LC-3 II/I and the level of beclin-1, as well as alleviate OE-induced histological injury of the heart, although we did not perform a heart function assessment. These findings demonstrate the anti-autophagy effect of 3-MA in the heart of rats. Meanwhile, our result is supported by a previous study showing that 3-MA has an anti-autophagy effect in the isolated rat atria subjected to ischemia/reperfusion injury37. However, we did not determine whether 3-MA could promote autophagy flux, which can be blocked by using autophagy-lysosome fusion inhibitors or lysosomal hydrolases inhibitors34.
Apoptotic cell death has been demonstrated to be a contributing factor to the progression of heart disease38. Whether autophagy functions as a pro-death or pro-survival program during the pathogenesis of heart disease has not been completely understood. A previous study showed that the apoptotic rate of cardiomyocytes was considerably increased in mice experiencing long-term moderate-intensity and exhausting exercise32. Yan et al reported that autophagy triggered by ischemia could be a homeostatic mechanism by which apoptosis is inhibited39. In this study, we found that 3-MA as an autophagy inhibitor decreased MOE-, IOE-, and EOE- induced cell apoptosis in the left ventricle. To further explore the pathway mechanism, we investigated the effect of 3-MA on the ratio of Bcl-2/Bax. We found that 3-MA significantly counteracted the reduction of Bcl-2/Bax induced by MOE, or IOE or EOE. These data are consistent with the TUNEL staining assay showing the decreased apoptotic rate of cardiomyocytes of the MOE, IOE and EOE rats. These findings are supported by an in vitro study showing that 3-MA can inhibit autophagy and apoptosis in palmitate-treated osteoblasts18, and an in vivo study demonstrating that intracerebroventricular injection of 3-MA elicited an inhibitory effect on traumatic brain injury40.
In conclusion, the inhibition of autophagy can attenuate short-term OE-induced cardiac injury in rats with the mechanism of autophagy/apoptosis inhibition. The findings provide insights on developing pharmacological strategies for preventing OE-induced cardiac injury.
Author contribution
Zhang-hua LI, Yan-fang CHEN, Qi-fa YE, and Yi YANG designed the research; Hua LIU, Hui LEI, and Yue SHI performed the research; Hua LIU, Hui LEI, Yue SHI, Ning CHEN, Zhang-hua LI, and Qi-fa YE contributed new reagents or analytic tools; Hua LIU, Hui LEI, Yue SHI, Ning CHEN, Zhang-hua LI, Yue SHI, and Yi YANG analyzed the data; Hua LIU, Hui LEI, Jin-ju WANG, Zhang-hua LI, Yan-fang CHEN, and Yi YANG wrote the paper.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (No 30700388), the Key Program of Natural Science Foundation of Hubei Province, China (No 2015CFA084), the Outstanding Young Scientific and Technological Innovation Team in the Colleges and Universities of Hubei Province, China (No T201523), and the Scientific Research Project supported by Wuhan Sports University (No 2014QZ07, 2016XH24).
References
- Berlin JA, Colditz GA. A meta-analysis of physical activity in the prevention of coronary heart disease. Am J Epidemiol 1990; 132: 612–28. [DOI] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age (Dordr) 2010; 32: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo FG, Goodpaster BH. The role of weight loss and exercise in correcting skeletal muscle mitochondrial abnormalities in obesity, diabetes and aging. Mol Cell Endocrinol 2013; 379: 30–4. [DOI] [PubMed] [Google Scholar]
- DA Silva NDJ, Fernandes T, Soci UP, Monteiro AW, Phillips MI, DE Oliveira EM. Swimming training in rats increases cardiac microRNA-126 expression and angiogenesis. Med Sci Sports Exerc 2012; 44: 1453–62. [DOI] [PubMed] [Google Scholar]
- Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med 1999; 341: 650–8. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li ZH, Liu H, Shi WD, Zhang J. Inhibitory effect of tetramethylpyrazine preconditioning on overload training-induced myocardial apoptosis in rats. Chin J Integr Med 2015; 21: 423–30. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li Z, Liu H, Shi W, Zhang J. Effects of heat preconditioning on overload training induced myocardial injury. J Sports Med Phys Fitness 2013; 53: 93–100. [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Defective autophagy leads to cancer. Cancer Cell 2003; 4: 422–4. [DOI] [PubMed] [Google Scholar]
- De Meyer GR, De Keulenaer GW, Martinet W. Role of autophagy in heart failure associated with aging. Heart Fail Rev 2010; 15: 423–30. [DOI] [PubMed] [Google Scholar]
- Xu D, Chen B, Gu J, Chen L, Belguise K, Wang X, et al. Inhibition of autophagy ameliorates pulmonary microvascular dilation and PMVECs excessive proliferation in rat experimental hepatopulmonary syndrome. Sci Rep 2016; 6: 30833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Li C, Shu Y, Ju X, Zou Z, Wang H, et al. Inhibition of autophagy ameliorates acute lung injury caused by avian influenza A H5N1 infection. Sci Signal 2012; 5: ra16. [DOI] [PubMed] [Google Scholar]
- Li GG, Guo ZZ, Ma XF, Cao N, Geng SN, Zheng YQ, et al. The M2 macrophages induce autophagic vascular disorder and promote mouse sensitivity to urethane-related lung carcinogenesis. Dev Comp Immunol 2016; 59: 89–98. [DOI] [PubMed] [Google Scholar]
- Nishida K, Kyoi S, Yamaguchi O, Sadoshima J, Otsu K. The role of autophagy in the heart. Cell Death Differ 2009; 16: 31–8. [DOI] [PubMed] [Google Scholar]
- Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 2007; 13: 619–24. [DOI] [PubMed] [Google Scholar]
- Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, et al. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol 2009; 29: 2570–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaapen MW, Davies MJ, De BM, Haven AJ, Martinet W, Kockx MM. Apoptotic versus autophagic cell death in heart failure. Cardiovasc Res 2001; 51: 304–12. [DOI] [PubMed] [Google Scholar]
- Gustafsson AB, Gottlieb RA. Autophagy in ischemic heart disease. Circ Res 2009; 104: 150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaratnam K, Vidal C, Boadle R, Thekkedam C, Duque G. Mechanisms of palmitate-induced cell death in human osteoblasts. Biol Open 2013; 2: 1382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yang X, Dong Y, Chen N, Xiao X, Liu H, et al. Transcutaneous electrical acupoint stimulation alleviates adverse cardiac remodeling induced by overload training in rats. J Appl Physiol 2016; 120: 1269–76. [DOI] [PubMed] [Google Scholar]
- Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 2011; 18: 571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalier L, Cartron PF, Juin P, Nedelkina S, Manon S, Bechinger B, et al. Apoptosis 2007; 12: 887–96. [DOI] [PubMed]
- Salminen A, Kaarniranta K, Kauppinen A. Beclin 1 interactome controls the crosstalk between apoptosis, autophagy and inflammasome activation: impact on the aging process. Ageing Res Rev 2013; 12: 520–34. [DOI] [PubMed] [Google Scholar]
- Ciechomska IA, Goemans GC, Skepper JN, Tolkovsky AM. Bcl-2 complexed with Beclin-1 maintains full anti-apoptotic function. Oncogene 2009; 28: 2128–41. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–10. [DOI] [PubMed] [Google Scholar]
- Olah A, Nemeth BT, Matyas C, Hidi L, Lux A, Ruppert M, et al. Physiological and pathological left ventricular hypertrophy of comparable degree is associated with characteristic differences of in vivo hemodynamics. Am J Physiol Heart Circ Physiol 2016; 310: H587–H597. [DOI] [PubMed] [Google Scholar]
- Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 2007; 115: 3086–94. [DOI] [PubMed] [Google Scholar]
- Ma Z, Qi J, Meng S, Wen B, Zhang J. Swimming exercise training-induced left ventricular hypertrophy involves microRNAs and synergistic regulation of the PI3K/AKT/mTOR signaling pathway. Eur J Appl Physiol 2013; 113: 2473–86. [DOI] [PubMed] [Google Scholar]
- Lavie CJ, Milani RV, Marks P, de Gruiter H. Exercise and the heart: risks, benefits, and recommendations for providing exercise prescriptions. Ochsner J 2001; 3: 207–13. [PMC free article] [PubMed] [Google Scholar]
- Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NA III, et al. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 2007; 115: 2358–68. [DOI] [PubMed] [Google Scholar]
- Karaahmet T, Tigen K, Dundar C, Pala S, Guler A, Kilicgedik A, et al. The effect of cardiac fibrosis on left ventricular remodeling, diastolic function, and N-terminal pro-B-type natriuretic peptide levels in patients with nonischemic dilated cardiomyopathy. Echocardiography 2010; 27: 954–60. [DOI] [PubMed] [Google Scholar]
- Mughal W, Dhingra R, Kirshenbaum LA. Striking a balance: autophagy, apoptosis, and necrosis in a normal and failing heart. Curr Hypertens Rep 2012; 14: 540–7. [DOI] [PubMed] [Google Scholar]
- Valentim L, Laurence KM, Townsend PA, Carroll CJ, Soond S, Scarabelli TM, et al. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol 2006; 40: 846–52. [DOI] [PubMed] [Google Scholar]
- Takemura G, Miyata S, Kawase Y, Okada H, Maruyama R, Fujiwara H. Autophagic degeneration and death of cardiomyocytes in heart failure. Autophagy 2006; 2: 212–4. [DOI] [PubMed] [Google Scholar]
- Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, et al. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 2010; 285: 10850–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, Ueno T, Kominami E. LC3 and autophagy. Methods Mol Biol 2008; 445: 77–88. [DOI] [PubMed] [Google Scholar]
- Cherra SJ III, Kulich SM, Uechi G, Balasubramani M, Mountzouris J, Day BW, et al. Regulation of the autophagy protein LC3 by phosphorylation. J Cell Biol 2010; 190: 533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann R, Velez DE, Rusiecki TM, Fernandez Pazos ML, Mestre Cordero VE, et al. Effects of 3-methyladenine on isolated left atria subjected to simulated ischaemia-reperfusion. Clin Exp Pharmacol Physiol 2015; 42: 41–51. [DOI] [PubMed] [Google Scholar]
- Prech M, Marszalek A, Schroder J, Filas V, Lesiak M, Jemielity M, et al. Apoptosis as a mechanism for the elimination of cardiomyocytes after acute myocardial infarction. Am J Cardiol 2010; 105: 1240–5. [DOI] [PubMed] [Google Scholar]
- Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, et al. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A 2005; 102: 13807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Lei J, Lin Y, Gao GY, Jiang JY. Autophagy inhibitor 3-MA weakens neuroprotective effects of posttraumatic brain injury moderate hypothermia. World Neurosurg 2016; 88: 433–46. [DOI] [PubMed] [Google Scholar]