Abstract
Purpose
This was a collaborative study from the Ophthalmology and Gastroenterology departments of a tertiary care hospital, designed to investigate the local ocular surface parameters in patients with celiac disease (CD).
Methods
Fifty-six eyes of 28 patients with CD and 58 eyes of 29 healthy adult subjects serving as controls were evaluated. The Schirmer test, tear-film break-up time, and conjunctival impression cytology were performed in addition to a complete ophthalmological examination. Impression cytology specimens of each group were graded and scored in the range 0–3.
Results
The 28 patients with CD consisted of 24 females (86%) and 4 males (14%). The mean age was 34.4±11.3 years (22–55 years). Tear-film break-up time and Schirmer test results were significantly lower in the study group than in the controls (P<0.05). Also, there was a significant difference between the groups for impression cytology grading scores (P=0.001).
Conclusions
The CD group showed a marked preponderance of females with an F/M ratio of six females per male, as reported in the literature. Tear-film functions and conjunctival surface epithelial morphology were significantly altered in patients with CD.
Introduction
Celiac disease (CD) is an immune-mediated enteropathy in genetically susceptible individuals that develops following the intake of gluten, a major protein found in wheat, barley, and rye.1 The prevalence of the disease is about 0.5–1% in the United States and developed countries.2 HLA-DQ2 and HLA-DQ8 (human leukocyte antigen) are found in 95% of the patients and are the most important genetic factors regarding susceptibility to the disorder.3 CD development is thought to be mediated by T cells and monocytes/macrophages.4, 5 Dry eye disease is present in about 5 million Americans aged 50 and over.6, 7 Women make up two-thirds of the patients.8 The ocular surface in these patients contains lymphoid cells such as dendritic cells, natural killer, and B and T cells that mediate the suppression or exacerbation of ocular surface inflammation.9 The coexistence of T helper1 (Th1) and T helper17 (Th17) in the conjunctiva of dry eye patients has been shown.10 Th1 cells are pro-inflammatory and play a role in the pathogenesis of certain autoimmune diseases.11 Dry eye syndrome has been reported in disorders such as Sjogren’s syndrome, rheumatoid arthritis, scleroderma, polymyositis, lymphoma, amyloidosis, hemochromatosis, sarcoidosis, and systemic lupus erythematous (SLE).12
CD increases the susceptibility to other autoimmune diseases as it is an immune-mediated disorder. The coexistence of CD and autoimmune disease has been shown in many studies.13, 14 The common point is thought to be related to the genetic background.15 Ocular surface and lacrimal gland inflammation can develop due to the active T lymphocytes and secreted cytokines in systemic autoimmune diseases.
The relationship between CD and the dry eye syndrome were investigated in this study.
Subjects and methods
All of the study procedures were conducted in accordance with the Declaration of Helsinki, and informed consent was obtained from all of the participants after approval from the Institutional Review Board. This study was approved by the Ethics Committee of Ankara Numune Training and Research Hospital. All patients were Turkish Caucasians. The subjects with celiac disease diagnosed in accordance with the ESPGHAN16 criteria that had no vitamin deficiency and were using a gluten free diet were included in the study. The study was conducted at Ulucanlar Eye Hospital between November 2014 and June 2015. A total of 28 celiac patients and 29 healthy cases were prospectively evaluated. This study is registered as Australian New Zealand Clinical Trials Registry, number ACTRN371738.
The study exclusion criteria were the use of topical or systemic cyclosporine or ocular steroids within the last 6 months, history of a punctal plug, use of contact lenses, other topical drug treatments, active ocular infection, blepharitis, meibomian gland dysfunction, herpetic keratitis, history of ocular surgery or trauma within the last 6 months, and presence of other ocular surface disease. None of the patients with CD had a history of Stevens–Johnson syndrome, SLE, inflammatory bowel disease, scleroderma or a chemical, thermal, or radiation injury, or any other systemic disorder, and none had undergone any ocular surgery that would create an ocular surface problem. The patients with CD have no signs and symptoms of dry eye.
All of the patients underwent visual acuity measurement with a Snellen chart, intraocular pressure measurement with an applanation tonometer, Schirmer test, tear break-up time (TBUT) evaluation, and conjunctival impression cytology. Biomicroscopy and dilated fundus examination were performed. Visual acuities determined with the Snellen chart were converted into logMAR for statistical evaluations.
The Schirmer’s test was performed using a standardized kit containing a strip of filter paper 5 mm × 30 mm and placed on to the lower lid margin in a temporal position. A value of <5 mm was accepted as abnormal. The stability of the tear-film layer was evaluated by determining the TBUT. After a drop of 2% sodium fluorescein was placed on the corneal surface, the patient was asked to blink so that the corneal surface was covered. The patient was asked not to blink afterwards. The interval between the last blink and the first randomly distributed dry spot was used as the TBUT. The mean of three measurements was recorded. A value of <10 s was accepted as abnormal.17
Impression cytology of the conjunctiva of the topically anesthetized eye was performed. Nelson graded conjunctival impression cytology specimens (grades 0–3) based on the appearance of the epithelial cells and the numbers of goblet cells.18 This grading score was used in present study. Small disks of cellulose acetate filter paper (MFS; Advantec MFS, Pleasanton, CA, USA; pore size 0.2 μm) were cut into pieces ~4 mm × 5 mm in size, placed on the superior nasal bulbar conjunctiva 5 mm from the limbus, gently pressed for 5 s, and then removed. The specimens were placed in a fixative solution and stained with Papanicolau’s modification of Gill’s technique.19 The specimens were examined with light microscopy by a pathologist who was masked to the history of each specimen. The examination employed the Nelson’s method, and the appearance of the conjunctival epithelial cells and goblet cells (if present) was recorded.18 Two observers, similarly masked, examined all the slides. All specimens were graded according to the following four levels. Grade 0: the epithelial cells are small and round with eosinophilic-staining cytoplasm. The nuclei are large with a nucleocytoplasmic ratio of 1 : 2. The goblet cells are abundant, plump, and oval with strongly periodic acid Schiff (PAS)-positive cytoplasm. Grade I: the epithelial cells are slightly larger than those in grade 0 and more polygonal, with eosinophilic-staining cytoplasm. The nuclei are smaller with a nucleocytoplasmic ratio of 1 : 3. The goblet cells are fewer in number; however, they still maintain their plump, oval shape with strongly PAS-positive cytoplasm. Grade II: the epithelial cells are larger than those in grade I and polygonal, occasionally multinucleated, with eosinophilic-staining cytoplasm. They have a nucleocytoplasmic ratio of 1 : 4–1 : 5. The goblet cells are markedly fewer in number, and are smaller, less strongly PAS-positive, and poorly defined. Grade III: the epithelial cells are larger than those in grade II and polygonal with basophilic-staining cytoplasm. The nuclei are small, pycnotic, or in many cells, completely absent. The goblet cells are completely absent. These alterations are also called as metaplasia of conjunctiva.
We used the unpaired two-tailed Student’s t-test and the Mann–Whitney U-test to evaluate the between-group differences. The level of significance was set at P<0.05. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) for Windows Version 17.0 (SPSS Inc., Chicago, IL, USA). For a study power of 80%, we determined 25 subjects will be enough to test all parameters.
Results
A total of 28 adult celiac patients and 29 healthy cases were included in the study. The demographic data of the study subjects are summarized in Table 1. There was no difference regarding age (P=0.810) and gender (P=0.957) between the groups. The duration since diagnosis was 5.5±3.1 years in the celiac group. The best-corrected visual acuity was 20/20 in both groups. The mean intraocular pressure was 14.1±2.2 mm Hg in the celiac group and 14.8±2.4 mm Hg in the control group. No pathology was observed on biomicroscopy and dilated fundus examinations in either group.
Table 1. Demographic data of the patients in the two groups.
| Celiac group n=48 | Control group n=33 | P-value | |
|---|---|---|---|
| Age (years) | |||
| Mean | 34.4±11.3 | 31.7±7.1 | 0.810 |
| Range | 22–55 | 23–52 | |
| Gender | |||
| Female | 24 | 25 | 0.957 |
| Male | 4 | 4 | |
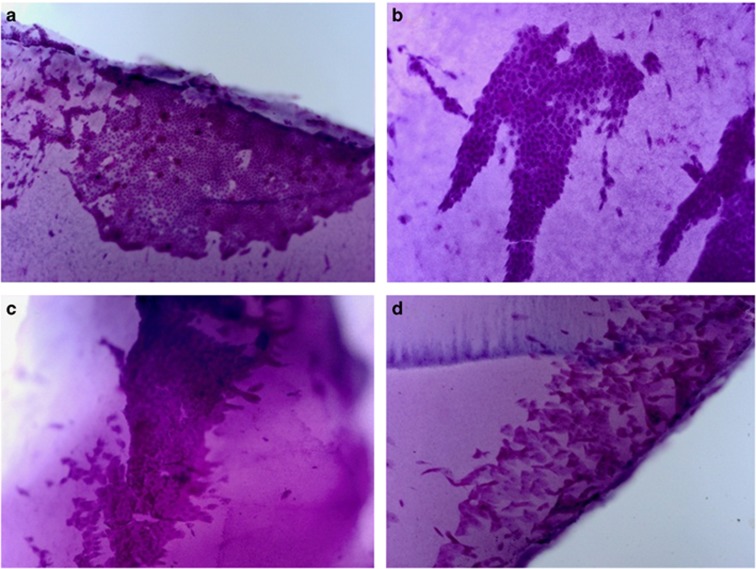
Most of the control group subjects had grade 0 or 1 impression cytology results. Normal goblet cells were clearly distinguishable due to their intense pink color and the normal cells were flat with a prominent nucleus in this group. The nucleocytoplasmic ratio was low (Figure 1a). There were fewer but well-formed goblet cells (grade I) (Figure 1b). In the CD cases, most cases were grade II and III, and goblet cells were significantly lower in number or not present (grade II; Figure 1c), and epithelial cells were larger with small nuclei (grade III; Figure 1d).
Figure 1.
Impression cytology of conjunctival surface of cases. (a) Impression cytology of the conjunctival surface of a control case showing conjunctival epithelial cells (grade 0). These cells are flat with a prominent nucleus and the nuclear cytoplasmic ratio is low; (b) impression cytology of the conjunctival surface of a patient with celiac disease showing the epithelial cells are slightly larger and goblet cells are fewer in number (grade I); (c) impression cytology of the conjunctival surface of a patient with celiac disease showing the epithelial cells are larger and goblet cells are markedly fewer in number (grade II); and (d) impression cytology of the conjunctival surface of a patient with celiac disease showing dysplastic squamous cells with increased nucleus: cytoplasmic ratio and hyperchromatic nuclei (grade III; 100 ×, periodic acid Schiff staining).
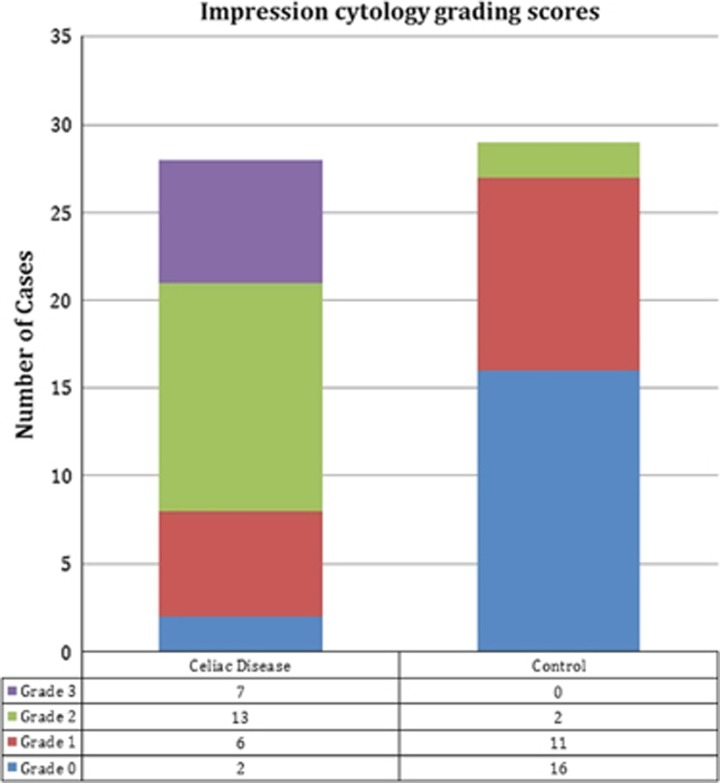
The Schirmer test I (without topical anesthesia) was performed in both eyes simultaneously. The mean Schirmer test values were 8.2±2.3 and 9.9±1.7 mm in CD patients and controls, respectively. The mean break-up time values were 10.1±3.5 and 12.3±2 s in the CD and control groups, respectively. The Schirmer test scores and break-up time values were statistically significantly different between the groups (P<0.05). Impression cytology grading scores for all groups are summarized in Figure 2. CD patients had significantly higher mean impression cytology grades (P=0.001) than controls.
Figure 2.
Impression cytology grading scores for all groups.
Discussion
CD is an immune-mediated enteropathy that emerges in genetically susceptible individuals with gluten intake.1 HLA-DQ2 and HLA-DQ8 molecules are the most important genetic factors creating susceptibility to the disorder.3 CD has been shown to develop as a result of a gliadin-related T-cell-mediated hypersensitivity reaction, and the characteristic lesions are due to cytokine secretion.5 Th1-type cytokines (interferon gamma—(IFN gamma)), macrophage-derived cytokines (tumor necrosis factor (TNF) alpha, and interleukins (IL)-6 and IL-8), and Th2-type cytokines (IL-4 and IL-5) play a role in CD immunopathogenesis.20 IL-2, IFN gamma, TNF beta, IL-4, and IL-10 have been found to increase in peripheral blood samples of celiac patients compared to a control group in the study of Lahat et al.21 The coexistence of CD and autoimmune disease has been shown in many studies,22, 23 and the most commonly associated disorder is Sjogren’s syndrome (3.3%).24
Dry eye is a multifactorial disorder of tears and ocular surface accompanied by increased osmolarity of the tear-film layer that can result in ocular surface damage and inflammation, discomfort, and decreased visual quality. The IL-1, IL-6, TNF alpha, and IL-17 inflammatory cytokines have been shown to increase in the cornea and conjunctiva epithelium as an indicator of the inflammation occurring at the ocular surface.25 IFN gamma has been shown to disrupt the signalization of IL-13, one of the main cytokines of TH2 cells, and to be responsible for a decrease in goblet cells in dry eye patients.26 We examined the relationship between dry eye syndrome and CD where Th1-mediated cytokines play an active role. The impression cytology results revealed that ocular surface inflammation develops in celiac patients.
The relationship of the two disorders with an increase of autoreactive T cells plays a role in the etiopathogenesis has been investigated. Gürdal et al27 showed that ocular surface damage and inflammation also developed in patients without thyroid ophthalmopathy in the study they conducted in Graves patients. TBUT and Schirmer scores were shown to be significantly decreased in RA patients without Sjogren’s syndrome compared to the control group in a study.28 Some studies have reported that ocular inflammation increases in SLE, where similar mechanisms play a role in the etiopathogenesis. Resch et al29 reported that ocular surface Langerhans cell density increased in SLE patients, leading to abnormal Schirmer test and tear-film break-up time values. We found inflammation of the ocular surface to increase in celiac patients where a similar mechanism is known to play a role in the etiopathogenesis.
There is no gold standard diagnostic method for dry eye disease. Symptom-based assessment is therefore very important in the diagnosis. The Schirmer test and tear-film break-up time are not sufficient in the evaluation of conjunctival morphology and cytology. Kumar et al30 reported that conjunctival impression cytology was more valuable in dry eye diagnosis than the other tests. Since impression cytology of the conjunctiva is a useful investigational tool for analyzing the changes in the conjunctival surface, we intended to assess the status of the conjunctiva by this technique in cases with CD. Ocular surface epithelia have an active role in stabilizing the tear-film.31 Goblet cell density reflects the overall health of the ocular surface, and a decrease in the density of goblet cells is an early sign of squamous metaplasia. Circulating factors that maintain the normal epithelial differentiation were lacking, and intense inflammation might introduce different factors to facilitate the epithelial alterations.31 It is known that any inflammation of ocular surface can lead to squamous metaplasia in epithelial cells, loss of glycocalyx, and goblet cells. As a result, the tear-film becomes unstable secondary to a reduction in the mucin layer of the tear-film and hence conjunctival mucosa gradually develops into a non-secreting keratinized epithelium.31 Impression cytology analysis of CD patients had significant squamous metaplasia and goblet cell loss as compared to the control patients. These findings support the existence of an ocular surface disease in patients with CD. Although Schirmer and TBUT scores of the celiac patients were significantly lower than the control group, they were higher than the values we determined as threshold values for dry eye diagnosis. This is possibly due to decreased severity of the disease with gluten-free diet treatment. The impression cytology findings of our patients were consistent with their Schirmer and TBUT results.
There are few studies on ocular involvement in CD. A study on 38 children reported no difference between CD patients and healthy children in terms of visual acuity, cataract, and uveitis.32 The risk of uveitis was reported to increase in celiac patients in another study.33 Some case presentations demonstrated improvement with gluten-free treatment.34, 35 Scleritis, bilateral cataract, and xerophthalmic fundus were among the ocular signs seen with CD in case presentations.36, 37, 38 We had not observed any uveitis, scleritis, and cataract in our cases throughout the clinical follow-up period. As far as we know, there is no study where ocular surface inflammation in CD was investigated.
One limitation of the present study was that immunologic markers that indicate the effectiveness of gluten-free diet treatment in celiac patients could not be evaluated. We did not evaluate the relationship between the severity of the CD and ocular inflammation. We also did not evaluate dry eye symptoms with OSDI scoring. In addition, this is a small cohort of patients with CD, so the conclusion provided here is not statistically powerful enough.
In conclusion, our study indicates that impression cytology grades are altered in patients with CD. These findings suggest a possible association between impression cytology grading scores and CD. It also seems reasonable to evaluate and follow up CD cases with functional and cytological ocular surface investigations for the early detection of corneal and conjunctival disorders in these patients. As we found some associations between CD and ocular surface disorders, further investigations should be conducted to explain the mechanism of squamous metaplasia formation in these patients.
Acknowledgments
Written permission has been received from all persons.
Footnotes
The authors declare no conflict of interest.
References
- Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH et al. The Oslo definitions for coeliac disease and related terms. Gut 2013; 62: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Tapia A, Ludvigsson JF, Brantner TL, Murray JA, Everhart JE. The prevalence of celiac disease in the United States. Am J Gastroenterol 2012; 107: 1538–1544. [DOI] [PubMed] [Google Scholar]
- Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol 2002; 2: 647–655. [DOI] [PubMed] [Google Scholar]
- Scott H, Nilsen E, Sollid LM, Lundin KE, Rugtveit J, Molberg O et al. Immunopathology of gluten-sensitive enteropathy. Springer Semin Immunopathol 1997; 18: 535–553. [DOI] [PubMed] [Google Scholar]
- MacDonald T, Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med 1988; 167: 1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop. Ocul Surf 2007; 5: 75–92. [DOI] [PubMed] [Google Scholar]
- The epidemiology of dry eye disease: report of the epidemiology subcommittee of the international dry eye workshop. Ocul Surf 2007; 5: 93–107. [DOI] [PubMed] [Google Scholar]
- Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol 2003; 136: 318–326. [DOI] [PubMed] [Google Scholar]
- Zhang X, Volpe EA, Gandhi NB, Schaumburg CS, Siemasko KF, Pangelinan SB. NK Cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoS One 2012; 7: e36822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan SK, El AJ, Ecoiffier T, Goyal S, Zhang Q, Saban DR et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol 2009; 182: 1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J Autoimmun 2008; 31: 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djalilian AR, Hamrah P, Pflugfelder SC. Dry eye. In: Krachmer JH, Mannis MJ, Holland EJ (eds). Cornea. 2nd ed.Elsevier/Mosby; Philadelphia; 2005: 521–540.
- Bizzaro N, Villalta D, Tonutti E, Tampoia M, Bassetti D, Tozzoli R et al. Association of celiac disease with connective tissue diseases and autoimmune diseases of the digestive tract. Autoimmun Rev 2003; 2: 358–363. [DOI] [PubMed] [Google Scholar]
- Viljamaa M, Kaukinen K, Huhtala H, Kyrönpalo S, Rasmussen M, Collin P et al. Coeliac disease, autoimmune diseases and gluten exposure. Scand J Gastroenterol 2005; 40: 437–443. [DOI] [PubMed] [Google Scholar]
- Freitag T, Schulze-Koops H, Niedobitek G, Niedobitek G, Melino G, Schuppan D. The role of the immune response against tissue transglutaminase in the pathogenesis of coeliac disease. Autoimmun Rev 2004; 3: 13–20. [DOI] [PubMed] [Google Scholar]
- Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Pediatric Gastroenterology and Nutrition. Arch Dis Child 1990; 65: 909–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallarackal GU, Ansari EA, Amos N, Martin JC, Lane C, Camilleri JP. A comparative study to assess the clinical use of fluorescein meniscus time (FMT) with tear break up time (TBUT) and Schirmer's tests (ST) in the diagnosis of dry eyes. Eye 2002; 16: 594–600. [DOI] [PubMed] [Google Scholar]
- Nelson JD. Impression cytology. Cornea 1988; 7: 71–81. [PubMed] [Google Scholar]
- Gill GW, Frost JK, Miller KA. A new formula for a half-oxidized hematoxylin solution that neither over stains nor requires differentiation. Acta cytol 1974; 18: 300–311. [PubMed] [Google Scholar]
- Kontakou M, Przemioslo RT, Sturgess RP, Limb GA, Ellis HJ, Day P et al. Cytokine mRNA expression in the mucosa of treated celiac patients after wheat peptide challenge. Gut 1995; 37: 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat N, Shapiro S, Karban A, Gerstein R, Kinarty A, Lerner A et al. Cytokine profile in coeliac disease. Scand J Immunol 1999; 49: 441–446. [DOI] [PubMed] [Google Scholar]
- Lancaster-Smith MJ, Perrin J, Swarbrick ET, Wright JT. Coeliac disease and autoimmunity. Postgrad Med J 1974; 50: 45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BT, Holmes GK, Cooke WT. Coeliac disease and immunological disorders. Br Med J 1978; 1: 537–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin P, Reunala T, Pukkala E, Laippala P, Keyriläinen O, Pasternack A. Coeliac disease—associated disorders and survival. Gut 1994; 35: 1215–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC. Anti-inflammatory therapy of dry eye. Am J Ophthalmol 2004; 137: 337–342. [DOI] [PubMed] [Google Scholar]
- Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol 2009; 147: 198–205. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürdal C, Saraç O, Genç I, Kırımlıoğlu H, Takmaz T, Can I. Ocular surface and dry eye in Graves' disease. Curr Eye Res 2011; 36: 8–13. [DOI] [PubMed] [Google Scholar]
- Fujita M, Igarashi T, Kurai T, Sakane M, Yoshino S, Takahashi H. Correlation between dry eye and rheumatoid arthritis activity. Am J Ophthalmol 2005; 140: 808–813. [DOI] [PubMed] [Google Scholar]
- Resch MD, Marsovszky L, Németh J, Bocskai M, Kovács L, Balog A. Dry eye and corneal langerhans cells in systemic lupus erythematous. J Ophthalmol 2015; 2015: 543835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Bhargava R, Kumar M, Ranjan S, Kumar M, Verma P. The correlation of routine tear function tests and conjunctival impression cytology in dry eye syndrome. Korean J Ophthalmol 2014; 28: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng SC, Hirst LW, Maumenee AE, Kenyon KR, Sun TT, Green WR. Possible mechanisms for the loss of goblet cells in mucin-deficient disorders. Ophthalmology 1984; 91: 545–552. [DOI] [PubMed] [Google Scholar]
- Urganci N, Kalyoncu D. Eye disorders in children with celiac disease. Eur J Ophthalmol 2016; 26: 85–87. [DOI] [PubMed] [Google Scholar]
- Mollazadegan K, Kugelberg M, Tallstedt L, Ludvigsson JF. Increased risk of uveitis in coeliac disease: a nationwide cohort study. Br J Ophthalmol 2012; 96: 857–861. [DOI] [PubMed] [Google Scholar]
- Krifa F, Knani L, Sakly W, Ghedira I, Essoussi AS, Boukadida J et al. Uveitis responding on gluten free diet in a girl with celiac disease and diabetes mellitus type 1. Gastroenterol Clin Biol 2010; 34: 319–320. [DOI] [PubMed] [Google Scholar]
- Klack K, Pereira RM, de Carvalho JF. Uveitis in celiac disease with an excellent response to gluten-free diet: third case described. Rheumatol Int 2011; 31: 399–402. [DOI] [PubMed] [Google Scholar]
- Keller J, Torres-Torres R, Sainz de la Maza M. Anterior scleritis and celiac disease: a proposed association. Ocul Immunol Inflamm 2013; 21: 410–412. [DOI] [PubMed] [Google Scholar]
- Raina UK, Goel N, Sud R, Thakar M, Ghosh B. Bilateral total cataract as the presenting feature of celiac disease. Int Ophthalmol 2011; 31: 47–50. [DOI] [PubMed] [Google Scholar]
- Witherspoon SR, Callanan D. Celiac disease presenting as a xerophthalmic fundus. Retina 2008; 28: 525–526. [DOI] [PubMed] [Google Scholar]