Abstract
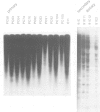
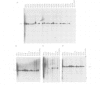
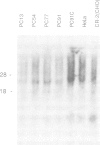
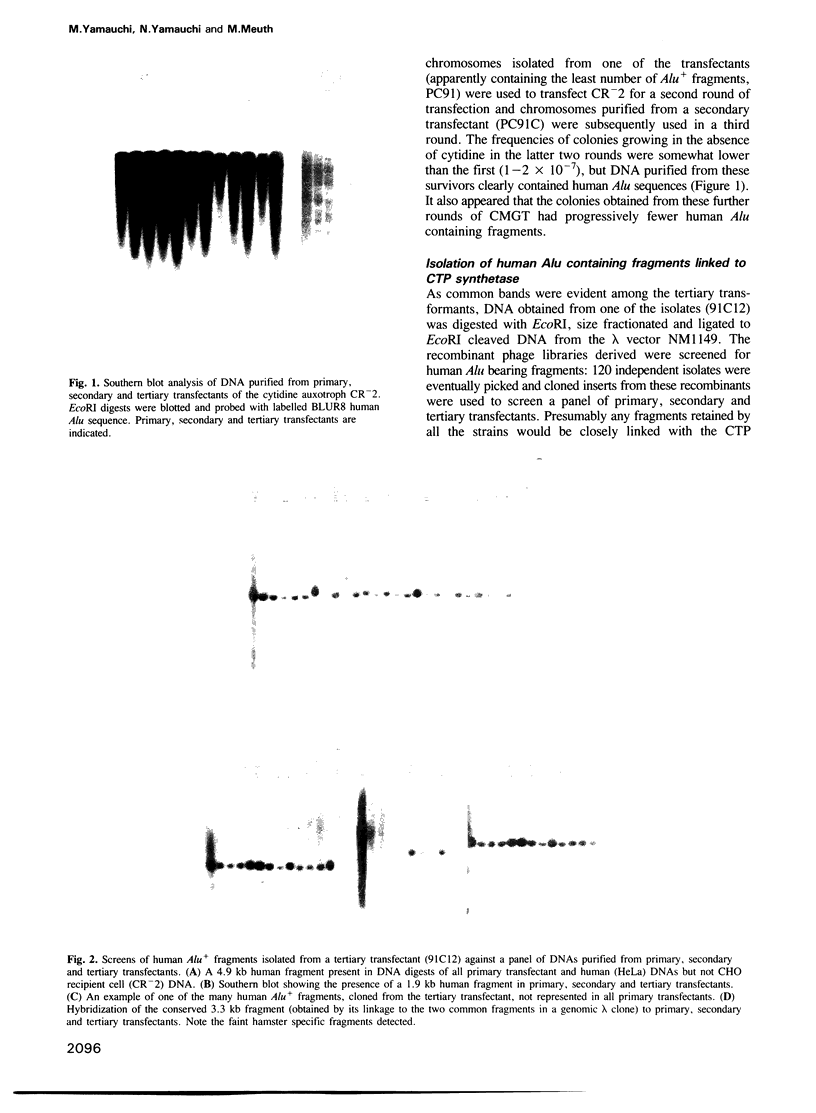
Successive rounds of chromosome-mediated gene transfer were used to complement a hamster cytidine auxotroph deficient in CTP synthetase activity and eventually to clone human genomic and cDNA fragments coding for the structural gene. Our approach was to isolate human Alu+ fragments from a tertiary transfectant and to utilize these fragments to screen a panel of primary transfectants. In this manner two DNA fragments, both mapping within the structural gene, were identified and used to clone a partial length cDNA. The remaining portion of the open reading frame was obtained through the RACE polymerase chain reaction technique. The open reading frame encodes 591 amino acids having a striking degree of similarity to the Escherichia coli structural gene (48% identical amino acids with 76% overall similarity including conservative substitutions) with the glutamine amide transfer domain being particularly conserved. As regulatory mutations of CTP synthetase confer both multi-drug resistance to agents widely used in cancer chemotherapy and a mutator phenotype, the cloning of the structural gene will be important in assessing the relevance of such phenotypes to the development of cellular drug resistance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronow B., Watts T., Lassetter J., Washtien W., Ullman B. Biochemical phenotype of 5-fluorouracil-resistant murine T-lymphoblasts with genetically altered CTP synthetase activity. J Biol Chem. 1984 Jul 25;259(14):9035–9043. [PubMed] [Google Scholar]
- Caras I. W., Levinson B. B., Fabry M., Williams S. R., Martin D. W., Jr Cloned mouse ribonucleotide reductase subunit M1 cDNA reveals amino acid sequence homology with Escherichia coli and herpesvirus ribonucleotide reductases. J Biol Chem. 1985 Jun 10;260(11):7015–7022. [PubMed] [Google Scholar]
- Chu E. H., McLaren J. D., Li I. C., Lamb B. Pleiotropic mutants of Chinese hamster cells with altered cytidine 5'-triphosphate synthetase. Biochem Genet. 1984 Aug;22(7-8):701–715. doi: 10.1007/BF00485854. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman E. R. Altered CTP synthetase activity confers resistance to 5-bromodeoxyuridine toxicity and mutagenesis. Mutat Res. 1986 Jun;161(1):19–27. doi: 10.1016/0027-5107(86)90096-5. [DOI] [PubMed] [Google Scholar]
- Kelsall A., Meuth M. Direct selection of Chinese hamster ovary strains deficient in CTP synthetase activity. Somat Cell Mol Genet. 1988 Mar;14(2):149–154. doi: 10.1007/BF01534400. [DOI] [PubMed] [Google Scholar]
- Kizaki H., Ohsaka F., Sakurada T. CTP synthetase from Ehrlich ascites tumor cells. Subunit stoichiometry and regulation of activity. Biochim Biophys Acta. 1985 May 20;829(1):34–43. doi: 10.1016/0167-4838(85)90065-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lewis W. H., Srinivasan P. R., Stokoe N., Siminovitch L. Parameters governing the transfer of the genes for thymidine kinase and dihydrofolate reductase into mouse cells using metaphase chromosomes or DNA. Somatic Cell Genet. 1980 May;6(3):333–347. doi: 10.1007/BF01542787. [DOI] [PubMed] [Google Scholar]
- McPartland R. P., Weinfeld H. Cooperative effects of CTP on calf liver CTP synthetase. J Biol Chem. 1979 Nov 25;254(22):11394–11398. [PubMed] [Google Scholar]
- Meuth M., Gonçalves O., Thom P. A selection system specific for the Thy mutator phenotype. Somatic Cell Genet. 1982 Jul;8(4):423–432. doi: 10.1007/BF01538705. [DOI] [PubMed] [Google Scholar]
- Meuth M., Gonçalves O., Trudel M. The genetic consequences of the Thy- mutation to CHO cells. Basic Life Sci. 1985;31:297–312. doi: 10.1007/978-1-4613-2449-2_18. [DOI] [PubMed] [Google Scholar]
- Meuth M., L'Heureux-Huard N., Trudel M. Characterization of a mutator gene in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6505–6509. doi: 10.1073/pnas.76.12.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth M. Role of deoxynucleoside triphosphate pools in the cytotoxic and mutagenic effects of DNA alkylating agents. Somatic Cell Genet. 1981 Jan;7(1):89–102. doi: 10.1007/BF01544750. [DOI] [PubMed] [Google Scholar]
- Meuth M. The molecular basis of mutations induced by deoxyribonucleoside triphosphate pool imbalances in mammalian cells. Exp Cell Res. 1989 Apr;181(2):305–316. doi: 10.1016/0014-4827(89)90090-6. [DOI] [PubMed] [Google Scholar]
- Miller C. L., Ruddle F. H. Co-transfer of human X-linked markers into murine somatic cells via isolated metaphase chromosomes. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3346–3350. doi: 10.1073/pnas.75.7.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbantoglu J., Hartley D., Phear G., Tear G., Meuth M. Spontaneous deletion formation at the aprt locus of hamster cells: the presence of short sequence homologies and dyad symmetries at deletion termini. EMBO J. 1986 Jun;5(6):1199–1204. doi: 10.1002/j.1460-2075.1986.tb04347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen L. C., Aasland R., Wittwer C. U., Krokan H. E., Helland D. E. Molecular cloning of human uracil-DNA glycosylase, a highly conserved DNA repair enzyme. EMBO J. 1989 Oct;8(10):3121–3125. doi: 10.1002/j.1460-2075.1989.tb08464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteous D. J., Morten J. E., Cranston G., Fletcher J. M., Mitchell A., van Heyningen V., Fantes J. A., Boyd P. A., Hastie N. D. Molecular and physical arrangements of human DNA in HRAS1-selected, chromosome-mediated transfectants. Mol Cell Biol. 1986 Jun;6(6):2223–2232. doi: 10.1128/mcb.6.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard C. A., Goodfellow P. N. Investigation of chromosome-mediated gene transfer using the HPRT region of the human X chromosome as a model. Genes Dev. 1987 Apr;1(2):172–178. doi: 10.1101/gad.1.2.172. [DOI] [PubMed] [Google Scholar]
- Takeishi K., Kaneda S., Ayusawa D., Shimizu K., Gotoh O., Seno T. Nucleotide sequence of a functional cDNA for human thymidylate synthase. Nucleic Acids Res. 1985 Mar 25;13(6):2035–2043. doi: 10.1093/nar/13.6.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander L., Berg P. Isolation and characterization of expressible cDNA clones encoding the M1 and M2 subunits of mouse ribonucleotide reductase. Mol Cell Biol. 1986 Oct;6(10):3433–3442. doi: 10.1128/mcb.6.10.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. E., Lamb B. J., Chu E. H. Purification of cytidine-triphosphate synthetase from rat liver, and demonstration of monomer, dimer and tetramer. Biochim Biophys Acta. 1988 Apr 14;953(3):334–344. doi: 10.1016/0167-4838(88)90042-8. [DOI] [PubMed] [Google Scholar]
- Weng M., Makaroff C. A., Zalkin H. Nucleotide sequence of Escherichia coli pyrG encoding CTP synthetase. J Biol Chem. 1986 Apr 25;261(12):5568–5574. [PubMed] [Google Scholar]
- Yamauchi M., Ayusawa D., Shimizu K., Seno T., Matsuhashi M. Two types of mouse FM3A cell mutants deficient in 5-aminoimidazole-4-carboxamide ribonucleotide transformylase and their transformants isolated by human chromosome-mediated gene transfer. Somat Cell Mol Genet. 1989 Jan;15(1):39–48. doi: 10.1007/BF01534668. [DOI] [PubMed] [Google Scholar]