Abstract
Purpose
To report long-term outcomes of deep sclerectomy (DS) in eyes with raised intraocular pressure (IOP) and glaucoma secondary to uveitis.
Patients and methods
Retrospective consecutive case series of 43 eyes of 43 patients with uveitic glaucoma. Mitomycin C (MMC) 0.2–0.4 mg/ml was applied sub-conjunctivally prior to scleral flap dissection for 2–3 min in 35 eyes (81%). Combined phacoemulsification and DS was done in 4 cases (9%).
Results
Mean follow-up was 68.5±33.5 months. In total, 23 eyes (53.5%) had previous intraocular surgery. Pre-operative IOP was 33.6±12.0 mm Hg. Mean IOP at one, three and five years after surgery was 15.5±5.0 mm Hg, 16.9±6.7 mm Hg and 16.4±5.2 mm Hg, respectively.
The probability of IOP <22 and <19 mm Hg was 69 and 62% at 3 years and 60 and 51% at 5 years, respectively. This included eyes that had undergone needle revision and/or laser goniopuncture within that period but had not needed glaucoma medication or further glaucoma procedures. The overall number of glaucoma medications decreased from 3.0±1.2 to 0.8±1.2 by last follow-up (P<0.001). Serious complications included hypotony with macular folds in two eyes and occlusion of the trabeculo-Descemet's membrane (TDM) by iris in two eyes. Recurrence of uveitis was observed in 16 eyes. Seven eyes (16.3%) had subsequent procedures including trabeculectomy with MMC in one eye, DS with MMC in two eyes and Baerveldt tube implantation in five eyes.
Conclusions
DS is a safe and effective procedure to lower IOP in uveitic glaucoma. However, as with other glaucoma procedures, a significant proportion of patients will require another IOP-lowering procedure in the long-term.
Introduction
Glaucoma is a frequent and serious complication of uveitis with the reported prevalence ranging from 10 to 23% depending on the glaucoma definition used.1, 2, 3, 4 Intraocular pressure (IOP) may not be controlled in a significant proportion of uveitic patients despite maximal topical therapy and surgery may be indicated despite the absence of glaucomatous optic neuropathy in eyes with significantly high IOP. Multiple causes of poor IOP control may be present, including intolerance to or side effects from topical medications and/or oral acetazolamide, the use of concomitant steroid therapy with subsequent hypertensive response, and poor compliance due to multiple drop regimes.
Trabeculectomy for glaucoma associated with uveitis (UG) carries one of the highest failure rates for glaucoma filtration surgery.5, 6 Despite this it remains a common first line surgical procedure in these cases, particularly when augmented with Mitomycin C (MMC) or 5-Fluorouracil (5-FU).6, 7, 8, 9, 10, 11, 12 This may reflect the widespread choice of trabeculectomy as the preferred primary procedure by many glaucoma surgeons, which still leaves the option of subsequent glaucoma drainage implantation (GDI) if necessary. There are increasing numbers of reports in the literature regarding the use and efficacy of GDI for UG with some authors suggesting this as a primary procedure in all cases with a high failure risk.13, 14, 15, 16, 17, 18, 19, 20, 21
‘Non-penetrating’ glaucoma procedures like deep sclerectomy (DS) and viscocanalostomy may be advantageous in eyes with intraocular inflammation or previous surgery. A study has reported less post-operative inflammation after DS compared to trabeculectomy while the application of intra-operative MMC also seems to improve IOP outcomes in DS.22, 23, 24 Satisfactory medium to long-term results have been reported for DS with MMC in primary open angle, pseudo-exfoliative, and normal-tension glaucoma.23, 24, 25 In UG, encouraging preliminary results have been reported for both DS and viscocanalostomy.26, 27, 28, 29, 30 Our study highlights the clinical outcomes, complications, and failure rates for augmented deep sclerectomy in UG and compares these to the current published literature on both deep sclerectomy and augmented trabeculectomy.
Materials and methods
This is a retrospective, non-randomised case series. Consecutive patients undergoing DS for UG between January 2002 and July 2007 in Calderdale and Huddersfield NHS Trust were identified from a correlational ongoing glaucoma surgery database (Microsoft Access). Data entry was completed at the time of surgery and contemporaneously at each post-operative visit. In bilateral cases, only the eye operated first was included. Data extracted from the database included patient demographics, Snellen visual acuity (VA), pre- and post-operative IOP, use of MMC, spacer device implantation, post-operative complications, subsequent procedures including re-operation for glaucoma, and the use of supplemental medical therapy. Anterior chamber (AC) assessment with regards to cells and flare, as well as any vitreous activity, macular oedema, and/or posterior segment inflammation were also noted at both pre- and post-operative follow-up visits.
Forty-three eyes of 43 patients with UG were included in the study. All procedures were performed by a single-consultant glaucoma surgeon (NA) using a standardised technique which has previously been described extensively.24, 31 MMC at a dose of 0.2 or 0.4 mg/ml was applied sub-conjunctivally prior to scleral flap dissection for 2–3 min in 35 eyes (81%). The higher concentration and longer application were related to the anticipated higher failure risk in eyes with previous multiple surgeries. MMC was not used if sub-conjunctival tissues were thin or fragile. In some cases sub-conjunctival bevacizumab 5 mg was injected prior to the procedure. This was based on our previous work where no difference in IOP outcomes was found after DS with MMC or bevacizumab.32 Combined phacoemulsification and DS were done in 4 cases (9%).
The procedures were performed under local anaesthesia (sub-Tenon’s block) or general anaesthesia where indicated. The surgical site was chosen after pre-operative gonioscopy had identified an area free of peripheral anterior synechiae (PAS). This was possible in all but one case in which TDM dissection over PAS was performed by inserting an iris spatula through a paracentesis and the synechiae broken by gentle posterior pressure on the iris.
Post-operatively patients received prednisolone acetate 1% drops two hourly continued for a minimum of 8 weeks. All patients were seen on the first post-operative day, then week 1 and week 6 post-surgery. Subsequent post-operative visits were determined by clinical need. Where there was an elevation of IOP at any stage, Nd:Yag laser goniopuncture (LGP) was performed with a Magna View contact gonioscopy lens (Ocular Instruments, Bellevue, Washington, USA). Needle revision with 5-FU or MMC was subsequently performed if IOP was still elevated. The need for either or both of these interventions was not classed as a failure of the procedure. Argon and Nd:YAG laser iridoplasty was done either prophylactically to avoid iris prolapse into the LGP or to remove incarcerated iris within it. These post-operative interventions were recorded contemporaneously as part of data collection. Detailed techniques for LGP and iridoplasty have already been described in a previous publication.33
Needling procedures were all performed in the outpatient clinic. Sub-conjunctival 5-FU or MMC was injected with 2% lignocaine 10 min before the procedure. A 25-G needle was inserted in superotemporal quadrant. Initially sub-conjunctival needling was performed, if no response then needling under the flap. This was never through the TDM as all the patients had already undergone LGP. The IOP was checked immediately to ensure aqueous flow re-established.
Complete (unqualified) success criteria were defined as follows: (A) IOP <22 mm Hg and/or a 20% decrease from baseline IOP off any glaucoma medications; (B) IOP <19 mm Hg and/or a 30% drop from baseline IOP, off glaucoma medications. The IOP had to be above the predetermined level on two consecutive visits to be considered as failure. IOP <6 mm Hg on two consecutive time points 3 months after surgery was also considered as failure. Partial (qualified) success was defined as any of the above but with at least one topical IOP-lowering medication. If a patient had an unsuccessful LGP or needle revision, failure was considered to have occurred on the visit when the decision to undertake this procedure was taken.
Re-operation for glaucoma or for a complication was defined as additional surgery requiring a return to the operating theatre. Serious complications were defined as surgical complications associated with loss of two or more lines of Snellen VA for more than 6 months and/or re-operation to manage a complication. Eyes that tested Seidel positive within the first month of follow-up were classified as wound leaks whereas those occurring after 1 month were categorised as bleb leaks. Data from patients who underwent additional glaucoma surgery was censored from that time point.
The presence or absence of intraocular information was recorded at baseline and during follow up. Uveitis treatment was managed on an individual basis in the peri-operative period. Pre-operatively, topical, and systemic steroid may be added or increased, according to disease type and activity. In this cohort, no additional topical or systemic corticosteroid therapy was used pre-operatively. Any active inflammation recorded beyond 4 weeks after surgery was considered significant. Patients who had a recurrence of uveitis after surgery were treated with topical steroids and/or systemic immunosuppression if indicated. We did not collect quantitative data regarding the degree of intraocular inflammation and adjustments to post-operative uveitis treatment and acknowledge this is a limitation of the study.
MedCalc Software, (Broekstraat 52, 9030 Mariakerke, Belgium) was used for statistical analysis. Statistical analyses were done on an intent-to-treat basis and eyes with intra-operative perforations were included in the analyses. A multivariate Cox’s regression analysis was performed to assess the association of various factors to survival outcomes. The log-rank test was used to check for differences in success rates between eyes with or without previous intraocular surgery. Non-parametric comparisons were made with the χ2 test with Yates correction or Fisher’s test where appropriate.
Results
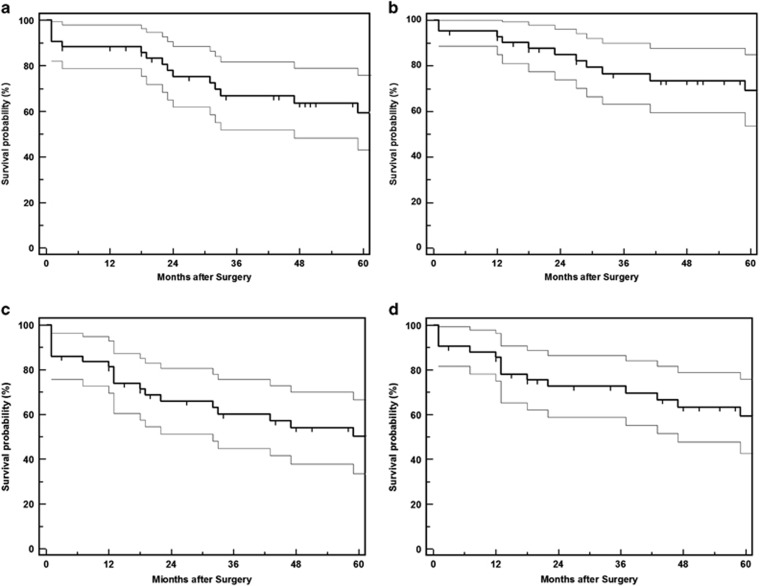
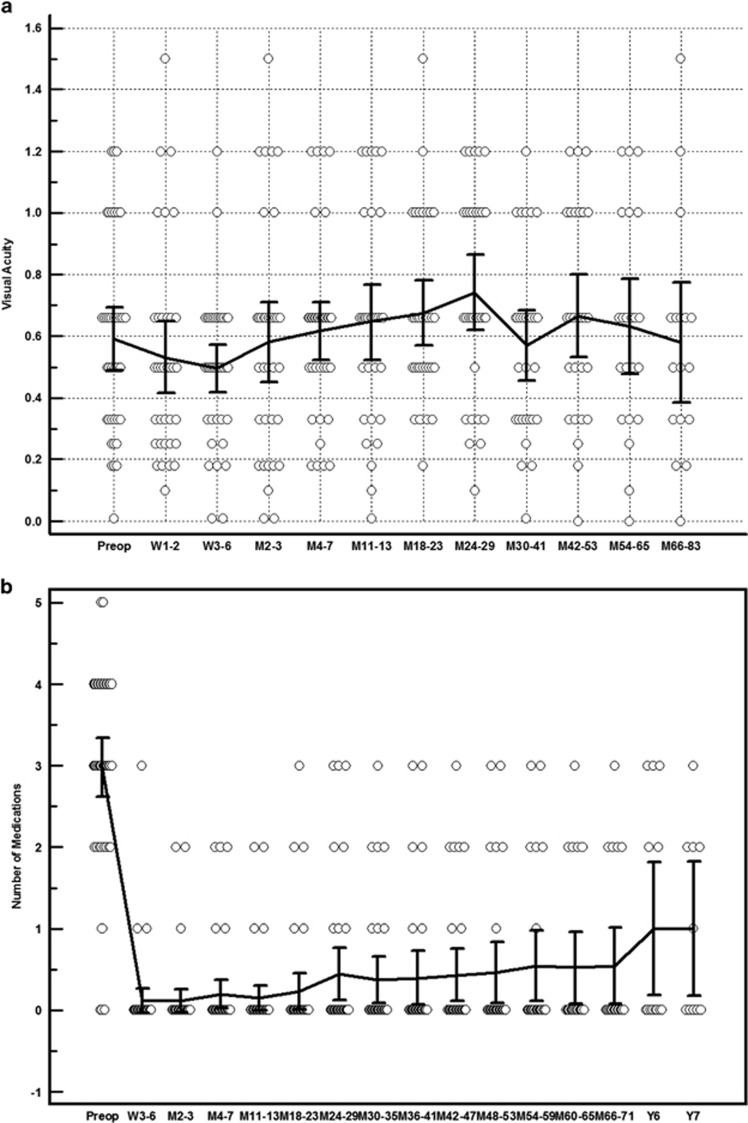
Patient demographics, anatomical classification and uveitis diagnosis are detailed in Table 1. The mean post-operative follow-up was 68.5±33.5 months (range 12–144 months). Twenty-three eyes (53.5%) had previous intraocular surgery, the details of which are described in Table 2A. The mean pre-operative IOP was 33.6±12.0 mm Hg. Mean IOP at 1, 3, and 5 years after surgery was 15.5±5.0 mm Hg, 16.9±6.7 mm Hg, and 16.4±5.2 mm Hg, respectively. The probability of IOP <22 and <19 mm Hg with needle revision and LGP but without medications (unqualified success) or further glaucoma procedures was 69 and 62%, respectively, at 3 years and 60 and 51% at 5 years. Figure 1 shows Kaplan–Meier curves for both unqualified and qualified success rates at <22 mm Hg and <19 mm Hg. Mean visual acuity remained stable and the number of glaucoma medications decreased from 3.0±1.2 to 0.8±1.2 (P<0.001) by last follow-up (Figure 2). Cox’s regression analyses showed no significant association of age, sex, pre-operative IOP, use of MMC, recurrence of anterior uveitis, post-operative LGP, and needle revision with complete success rates (IOP <19 mm Hg without medications).
Table 1. Baseline characteristics, uveitis classification and diagnosis, spacer, and anti-metabolite use.
| Demographics | |
| Mean age, years (±SD) | 52.8 (±16.9) |
| Sex (male:female) | 23:20 |
| Ethnic origin | |
| White Caucasian | 35 |
| Asian Indian | 6 |
| African | 1 |
| Middle Eastern | 1 |
| IOP and baseline treatment | |
| Mean pre-operative IOP, mmHg (±SD) | 33.6 (±12.0) |
| Mean pre-operative medications (±SD) | 3.0 (±1.2) |
| Uveitis anatomical classification | |
| Anterior | 23 |
| Intermediate | 3 |
| Posterior | 16 |
| Panuveitis | 4 |
| Uveitis phenotypic diagnosis | |
| Undifferentiated | 15 |
| Fuch’s heterochromic cyclitis | 14 |
| Herpes zoster | 4 |
| Sarcoidosis | 3 |
| Juvenile idiopathic arthritis | 2 |
| Herpes simplex | 1 |
| HLA-B27 associated | 1 |
| Tuberculosis related | 1 |
| Sympathetic | 1 |
| Brucellosis | 1 |
| Spacer device | |
| None | 8 |
| SK Gel | 22 |
| Aquaflow | 8 |
| Esnoper | 5 |
| Anti-metabolite used | |
| None | 4 |
| Subconjunctivalbevacizumab 5 mg | 3 |
| Mitomycin 0.2 mg/ml for 2 min | 32 |
| Mitomycin 0.4 mg/ml for 3 min | 4 |
Table 2A. Surgical interventions prior to index DS.
| Number of eyes | |
|---|---|
| Cataract surgery | |
| Phacoemulsification+IOL | 8 |
| Extra-capsular+IOL | 4 |
| Lensectomy | 1 |
| Trabeculectomy | |
| No augmentation | 0 |
| MMC augmentation | 1 |
| 5-FU augmentation | 2 |
| Others | |
| 5-FU needling | 1 |
| PPV, endolaser, silicone oil | 1 |
| Cyclodiode | 1 |
| Intravitreal triamcinolone | 2 |
| Choroidal drainage | 1 |
Figure 1.
Kaplan–Meier survival plots with 95% confidence intervals for maintaining IOP. (a) <22 mm Hg with laser goniopuncture and needle revision but no glaucoma medications and/or further glaucoma surgery; (b) <22 mm Hg with laser goniopuncture and needle revision and one or more glaucoma medications; (c) <19 mm Hg with laser goniopuncture and needle revision but no glaucoma medications and/or further glaucoma surgery; (d) <19 mm Hg with laser goniopuncture and needle revision and one or more glaucoma medications.
Figure 2.
(a) Mean visual acuity (logMar) changes over time. (b) Mean number of medications at the pre-operative (pre-op) time point and subsequent pre-op reviews. M, months; W, weeks; Y, years.
The effect of various factors on complete success (IOP<19) was investigated in a stepwise Cox’s regression model. There was no significant effect of age, sex, pre-operative IOP, previous intraocular surgery, intra-operative MMC application, recurrence of iritis, and post-operative needle revision. Laser goniopuncture had a significant favourable effect on success rate (hazard ratio 0.3, 95% CI 0.1–0.9, P=0.03). However, this has to be interpreted with caution in view of the wide confidence intervals of the hazard ratio.
Intra-operatively, poor to moderate flow through the TDM, presumably due to trabecular meshwork fibrosis, was noted to have occurred in 13 cases. Four eyes had an intra-operative perforation with a peripheral iridectomy being performed in one of these cases. Table 2B lists all post-operative complications which occurred in our cohort. Recurrence of intraocular inflammation (anterior uveitis) was observed in 16 eyes. Serious complications included hypotony with macular folds and adherence of iris to the TDM, each developing in two eyes. In the cases with hypotony, one eye developed chronic cystoid macular oedema (CMO) unresponsive to treatment and consequent poor vision; the other eye underwent conjunctival compression sutures, followed by a scleral patch graft and further patch graft using Tutoplast. In the latter case hypotony resolved and the patient ultimately achieved a VA of 6/6 and a final IOP of 15 mm Hg.
Table 2B. Post-operative complications.
| Number of eyes | |
|---|---|
| Uveitis activity | |
| Activity increase | 16 |
| Anterior segment | |
| Shallow anterior chamber | 3 |
| Hyphaema | 3 |
| Conjunctival edge leak | 1 |
| Late iris incarceration | |
| Into goniopuncture | 2 |
| Into perforation | 2 |
| Scleral flap thinning | 2 |
| Posterior segment | |
| Hypotonous maculopathy (transient) | 2 |
| Decompression retinopathy | 1 |
| Others | |
| Ptosis | 1 |
| Vision loss (> 2 lines Snellen) | 1 |
Table 2C highlights all subsequent laser and surgical procedures performed following the index DS. The probability of performing LGP was 42% at 1 year, 53% at 3 years and 60% at 5 years. Seven eyes (16.3%) underwent a total of 13 needling revision procedures; two were supplemented with 5-FU and eleven with MMC. Needle revision was deemed successful (IOP <19 without medications) in four of the seven eyes. Seven eyes (16.3%) had multiple subsequent glaucoma procedures including trabeculectomy with MMC in two eyes, DS with MMC in two eyes and Baerveldt tube implantation in six eyes. The latter procedure was successful in lowering IOP in all six cases; in two eyes it was performed after phaco-vitrectomy for floaters resulted in loss of previously stable IOP control; in another two eyes it was performed after a second filtration procedure failed. In the remaining two eyes it was done after the index DS had failed.
Table 2C. Procedures after index DS procedure.
| % eyes | |
|---|---|
| Nd:YAG laser | |
| Goniopuncture | 27 |
| Nd:YAG and argon laser iridoplasty | 2 |
| Posterior capsulotomy | 2 |
| Surgery | |
| DS with MMC | 2 |
| Revision of DS with MMC and trabeculectomy with MMC | 1 |
| Baerveldt tube implant | 5 |
| Phacoemulsification | 8 |
| Phacoemulsification and vitrectomy | 2 |
| Others | |
| Removal of plomb | 1 |
| Compression sutures | 1 |
| Scleral patch graft | 1 |
| Tutoplast patch graft | 1 |
Discussion
The aim of this study was to follow-up the long-term outcomes for patients with UG who underwent non-penetrating glaucoma surgery (NPGS) from the only previously published UK-based cohort of its kind.28 We included 43 eyes of 43 UG patients with a mean follow-up of 68.5±33.5 months. To our knowledge this is the longest follow-up series for non-tube glaucoma drainage surgery in the literature.
The main indication for surgery in UG is uncontrolled IOP on maximal medication in the absence of pupillary block.34 The pre-operative mean IOP of 33.6±12.0 mm Hg in our study reflects the relatively high IOP prior to surgery compared to that in other types of chronic open angle glaucoma. In this respect, an IOP of <19 mm Hg, or even 22 mm Hg, would be deemed a success based on a significant percentage drop in IOP. Thus the mean IOPs at 1, 3, and 5 years after surgery, 15.5± 5.0 mm Hg, 16.9±6.7 mm Hg, and 16.4± 5.2 mm Hg, respectively, represent excellent values of pressure reduction when compared to those before surgery. The decrease in number of glaucoma medications from 3.0±1.2 to 0.8±1.2 by last follow-up was also significant (P<0.001).
The unqualified success rates for IOP <22 and <19 mm Hg (69 and 62%, respectively, at 3 years, 61 and 51% at 5 years) compare quite favourably with previous studies of both trabeculectomy and deep sclerectomy for UG which all show good initial outcomes but have mostly limited long-term data (Table 3). Studies with longer follow-up include that by Kaburaki et al which had mean results of more than 5 years for augmented (MMC) trabeculectomy and reported mean unqualified and qualified success rates of 57.1±7.5% and 64.7±7.0 mm Hg, respectively, for IOP <16 mm Hg.7 This retrospective non-randomised comparative study showed results similar to trabeculectomy for POAG in the same series but bleb survival was shorter in UG patients (59% at 5 years) and post-operative inflammation, particularly if present between 2 weeks and 3 months after surgery, was associated with worse IOP control and increased bleb failure. Furthermore, post-operative hypotony rates for UG were very high (28.3%) and significantly more frequent than the POAG group.
Table 3. Comparison of our outcomes with previous results of augmented trabeculectomy and deep sclerectomy in uveitis.
| Reference | Procedure | Mean follow up (months) | Eyes (n) | Main IOP outcome | Success at 1 year (% eyes) | Success at 5 years (% eyes) | Long-term hypotony (% eyes) |
|---|---|---|---|---|---|---|---|
| Noble et al6 | MMC trab | 52 | 21 | <30% pre-op | 90.0 | NA | 9.5 |
| Kaburaki et al7 | MMC trab | 65 | 53 | <21 mm Hg | NA | 79.1 (Q) | 28.3 |
| Prata et al34 | MMC trab | 10 | 24 | <21 mm Hg | 91.7 | NA | 8.3 |
| Ceballos et al11 | MMC trab | 29 | 44 | <21 mm Hg | 78.0 | NA | 7.0 |
| Towler et al35 | 5-FU trab | 43 | 50 | <21 mm Hg | 88.0 (Q) | 67.0 (Q) | 0 |
| Chawla et al12 | 5-FU trab | 61 | 31 | <21 mm Hg | 90.0 (Q) | 76.5 (Q) | 0 |
| Al Obeidan26 | DS only | 21 | 13 | ‘IOP control’ at last visit | 84.6 (U) 92.3 (Q) | NA | 7.7 |
| Al Obeidan29 | DS MMC | 33 | 33 | <23 mm Hg | 72.7(U)a 93.9 (Q)a | NA | 3.0 |
| <18 mm Hg | 69.7 (U) 81.8 (Q) | NA | |||||
| Auer et al27 | DS only | 12 | 14 | <21 mm Hg | 45.4 (U) 91.0 (Q) | NA | 7.1 |
| Anand et al28 | DS MMC | >46 | 26 | <21 mm Hg | 89.0 (U/Q)b | NA | NA |
| This study | DS MMC | >68 | 43 | <22 mm Hg | 88.0 (U) 93.0 (Q) | 60.0 (U) 74.0 (Q) | 4.7 |
| <19 mm Hg | 81.0 (U) 86.0 (Q) | 51.0 (U) 60.0 (Q) | 4.7 |
Abbreviations: MMC, mitomycin C; trab, trabeculectomy; 5-FU, 5-fluorouracil; DS, deep sclerectomy; IOP, intraocular pressure; Q, qualified success; UQ, unqualified success.
Outcome data reported at<3 years.
Outcome data reported at 3 years.
In common with Kaburaki’s findings, other studies on MMC augmented trabeculectomy in UG have largely shown improved outcomes but also higher incidences of bleb leakage and long-term hypotony.35, 36, 37 Noble et al compared MMC trabeculectomy in uveitic eyes to a control group and found that uveitis was a negative predictor for success on multivariate analysis.6 After 2 years, a 30% decrease in IOP from baseline without medications was achieved in 51% of uveitic eyes compared to 70% of the control eyes. Hypotony was observed in 9% of cases and endophthalmitis in one eye within a very small uveitic group. In contrast to these studies, our MMC enhanced DS results showed that recurrence of inflammation had no bearing on post-operative IOP control and that hypotony rates were very low within a relatively larger group of uveitis patients.
Stavrou and Murray also reported similar complete success rates for IOP <21 mm Hg in unaugmented trabeculectomy (53% at 5 years) but their results compared less well to their non-uveitic control group (67% at 5 years) and their study failed to detail intra- and post-operative complications.38 Chawla et al published one of the longest follow-up data for 5-FU augmented trabeculectomy with very high qualified success rates (76.5%) but much lower unqualified success (47.1%) for IOP <21 mm Hg at the 5-year mark.12 Although quoting no long-term hypotony, the authors reported post-operative hypotony in 19.4% of cases with rates of 6.5% for AC reformation and 3.2% for bleb re-suturing. Towler et al reported similar results for 5-FU augmented trabeculectomy with a 67% 5-year success rate for IOP <21 mm Hg although 26% of these eyes were also on a topical beta-blocker by then.39 No major complications such as long-term hypotony or endophthalmitis were seen in this cases series. Our unqualified 5 year results are superior to the papers mentioned above and qualified results are very similar. We consider unqualified results to be a better depiction of surgical procedure success as the introduction of topical IOP-lowering medications produces additional variables.
In uveitic patients, NPGS offers the postulated benefits of minimal post-operative AC inflammation and a reduced risk of delayed complications such as hypotony and bleb-related infections which are more common with trabeculectomy.40, 41 The absence of an iridectomy and AC penetration should reduce the inflammatory response while the presence of a TDM may act as a barrier to infectious organisms entering the eye. The restriction of aqueous flow through the TDM may also be advantageous in uveitic eyes which have a tendency to reduced aqueous secretion. As summarised in Table 3, we identified three case series specific to NPGS in UG after an extensive online search using Medline and EMBASE.26, 27, 28, 29 The mean follow-up in these case series varied from 12 to 46 months with unqualified success (for IOP <21 mm Hg) ranging from 45 to 85% of cases within those time frames. No cases of bleb-related infection or delayed hypotony were reported in these series. In addition, a retrospective, comparative case series published by Dupas et al showed similar IOP outcomes at 1 year for both MMC augmented trabeculectomy and DS; the latter, however, required more post-operative manipulations such as needle revision and LGP.42 As expected, post-operative inflammation measured by laser flare-cell metre at 1 week was significantly less in the DS group. The IOP outcomes of our cohort compare favourably with those reported in both other case series of augmented DS and trabeculectomy for UG. As with previous DS studies, there were also no long-term, sight-threatening complications such as endophthalmitis or persisting hypotonous maculopathy.
Intra-operatively, poor to moderate flow through the TDM, presumably due to trabecular meshwork fibrosis, was noted in 13 cases. However, there was no difference in IOP outcomes on regression analyses (see Results section). The intra-operative perforation rate was just under 10%. This is important from a consent point of view as the advantages of a closed ‘non-penetrating’ system are lost with the subsequent increased risks of hypotony, failure, and endophthalmitis. Post-operatively, the LGP rates of 42% at 1 year and 60% at 5 years were similar to those reported for long-term DS studies in non-uveitic eyes.23, 43 However, late iris prolapse into the LGP site is a limitation of DS as it may result in loss of IOP control.44 A higher incidence of bleb needling is well recognised in UG patients undergoing trabeculectomy with reported rates varying from 33 to 48.4%.6, 12 The presence of a sub-scleral lake and the use of spacer devices favour reduced scleral flap scarring in DS. However, needling can still be attempted to reduce the degree of sub-conjunctival and sub-scleral fibrosis. Needle revisions were performed in seven eyes (16.3%) from our cohort with a total number of 13 procedures. Needling was deemed successful (IOP <19 mm Hg without medications) in four of these seven eyes. Although effective in more than half the cases in which it was performed, needle revision did not guarantee success. Additional fibrosis of the TDM and scarring of supra-choroidal and Schlemm’s canal drainage pathways also make this operation less responsive to needling procedures. A total of seven eyes subsequently required multiple glaucoma procedures including redo augmented DS, MMC augmented trabeculectomy, and/or Baerveldt tube implantation. The latter was performed in six eyes and was successful in lowering IOP in all cases.
Two retrospective studies of trabeculectomy in UG patients have suggested that surgical success is dependent on post-operative inflammation, but not on inflammation at the time of surgery.9, 16 Recurrence of intraocular inflammation was observed in 16 eyes at some point in our cohort. Details of the severity and exact type of episode were not recorded and this is another limitation of our study. However, the recurrences did not have an impact on long-term IOP and complication outcomes. In a previous study we showed that young patient age at surgery (<30 years) resulted in statistically significant reduced survival rates for trabeculectomy in UG patients with 50% needing a subsequent tube or cyclodiode laser procedure to control their IOP.12 These patients did well with tube surgery and consideration for GDI as a primary procedure was suggested in that study. In our current study, age as a continuous variable had no effect on success rates and only six patients were under 30 years of age making it difficult to extrapolate any meaningful conclusions.
This study has similar limitations to our previously published report.28 Its retrospective nature implies that the number of complications may have been undetected or under-reported and the number of cases diminished with increasing follow-up duration. There was also a limited number of patients (n=43) and uveitic eyes with diverse aetiologies were included. A total of 23 eyes (53.5%) had undergone previous intraocular surgery, 5 of which had undergone previous trabeculectomy, and 8 eyes were pseudophakic. Some cases were therefore at higher risk of failure and/or carried varying prognoses. Outcomes were also unpredictable in some instances, for example, one eye had persisting high IOP following trabeculectomy but subsequently developed hypotony after DS and LGP. On the other hand, a few eyes undergoing primary DS with no previous surgery or active inflammation failed. Statistically, there was no significant difference in success rates between eyes with or without a previous history of intraocular surgery (P=0.07).
With these limitations taken into consideration, we conclude that, in the medium to long-term, DS augmented with MMC appears to be a safe and reasonably effective procedure to lower IOP in UG. The significant long-term failure rate is in keeping with most studies of glaucoma drainage surgery in uveitis. The overall incidence of intra- and post-operative complications in DS is lower when compared to MMC augmented trabeculectomy. When compared to the latter, DS has the additional benefits of less frequent post-operative follow-up with no dependence on suture manipulation, removal, or lysis. This study is a real-life, retrospective, descriptive report of prospectively collected, long-term data for DS in a single NPGS-experienced surgeon practice. A prospective randomised case-control study comparing DS to trabeculectomy in the surgical management of UG would be needed to provide more definitive data comparing the efficacy and safety of these two treatment modalities.
Footnotes
The authors declare no conflict of interest.
References
- Panek WC, Holland GN, Lee DA, Christensen RE. Glaucoma in patients with uveitis. Br J Ophthalmol 1990; 74: 223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merayo-Lloves J, Power WJ, Rodriguez A, Pedroza-Seres M, Foster CS. Secondary glaucoma in patients with uveitis. Ophthalmologica 1999; 213: 300–304. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Ohtani S, Miyata K, Miyata N, Shirato S, Mochizuki M. A clinical evaluation of uveitis-associated secondary glaucoma. Jpn J Ophthalmol 2002; 46: 556–562. [DOI] [PubMed] [Google Scholar]
- Neri P, Azuara-Blanco A, Forrester JV. Incidence of glaucoma in patients with uveitis. J Glaucoma 2004; 13: 461–465. [DOI] [PubMed] [Google Scholar]
- Panek WC, Holland GN, Lee DA, Christensen RE. Glaucoma in patients with uveitis. Br J Ophthalmol 1990; 74: 223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble J, Derzko-Dzulynsky L, Rabinovitch T, Birt C. Outcomes of trabeculectomy with intraoperative mitomycin C for uveitic glaucoma. Can J Ophthalmol 2007; 42: 89–94. [PubMed] [Google Scholar]
- Kaburaki T, Koshino T, Kawashima H, Numaga J, Tomidokoro A, Shirato S et al. Initial trabeculectomy with mitomycin C in eyes with uveitic glaucoma with inactive uveitis. Eye (Lond) 2009; 23: 1509–1517. [DOI] [PubMed] [Google Scholar]
- Elgin U, Berker N, Batman A, Soykan E. Trabeculectomy with mitomycin C in secondary glaucoma associated with Behcet disease. J Glaucoma 2007; 16: 68–72. [DOI] [PubMed] [Google Scholar]
- Park UC, Ahn JK, Park KH, Yu HG. Phacotrabeculectomy with mitomycin C in patients with uveitis. Am J Ophthalmol 2006; 142: 1005–1012. [DOI] [PubMed] [Google Scholar]
- Novak-Laus K, Mandic Z, Ivekovic R, Korsić J, Tedeschi-Reiner E, Masnec-Paskvalin S et al. Trabeculectomy with mitomycin C in glaucoma associated with uveitis. Coll Antropol 2005; 29(Suppl 1): 17–20. [PubMed] [Google Scholar]
- Ceballos EM, Beck AD, Lynn MJ. Trabeculectomy with antiproliferative agents in uveitic glaucoma. J Glaucoma 2002; 11: 189–196. [DOI] [PubMed] [Google Scholar]
- Chawla A, Mercieca K, Fenerty C, Jones NP. Outcomes and complications of trabeculectomy enhanced with 5-fluorouracil in adults with glaucoma secondary to uveitis. J Glaucoma 2013; 22(8): 663–666. [DOI] [PubMed] [Google Scholar]
- Rachmiel R, Trope GE, Buys YM, Flanagan JG, Chipman ML. Ahmed glaucoma valve implantation in uveitic glaucoma versus open-angle glaucoma patients. Can J Ophthalmol 2008; 43: 462–467. [DOI] [PubMed] [Google Scholar]
- Papadaki TG, Zacharopoulos IP, Pasquale LR, Christen WB, Netland PA, Foster CS. Long-term results of Ahmed glaucoma valve implantation for uveitic glaucoma. Am J Ophthalmol 2007; 144: 62–69. [DOI] [PubMed] [Google Scholar]
- Ozdal PC, Vianna RN, Deschenes J. Ahmed valve implantation in glaucoma secondary to chronic uveitis. Eye 2006; 20: 178–183. [DOI] [PubMed] [Google Scholar]
- Kafkala C, Hynes A, Choi J, Topalkara A, Foster CS. Ahmed valve implantation for uncontrolled pediatric uveitic glaucoma. J AAPOS 2005; 9: 336–340. [DOI] [PubMed] [Google Scholar]
- Budenz DL, Scott IU, Nguyen QH, Feuer W, Singh K, Nicolela MT et al. Combined Baerveldt glaucoma drainage implant and trabeculectomy with mitomycin C for refractory glaucoma. J Glaucoma 2002; 11: 439–445. [DOI] [PubMed] [Google Scholar]
- Ceballos EM, Parrish RK, Schiffman JC. Outcome of Baerveldt glaucoma drainage implants for the treatment of uveitic glaucoma. Ophthalmology 2002; 109: 2256–2260. [DOI] [PubMed] [Google Scholar]
- Molteno AC, Sayawat N, Herbison P. Otago glaucoma surgery outcome study: long-term results of uveitis with secondary glaucoma drained by Molteno implants. Ophthalmology 2001; 108: 605–613. [DOI] [PubMed] [Google Scholar]
- Nguyen QH. Primary surgical management refractory glaucoma: tubes as initial surgery. Curr Opin Ophthalmol 2009; 20(2): 122–125. [DOI] [PubMed] [Google Scholar]
- Gedde SJ, Heuer DK, Parrish RK 2nd, Tube versus Trabeculectomy Study Group. Review of the results of the Tube Versus Trabeculectomy Study. Curr Opin Ophthalmol 2010; 21(2): 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou AG, Mermoud A, Jewelewicz DA. Post-operative inflammation following deep sclerectomy with collagen implant versus standard trabeculectomy. Graefes Arch Clin Exp Ophthalmol 1998; 236: 593–596. [DOI] [PubMed] [Google Scholar]
- Kozobolis VP, Christodoulakis EV, Tzanakis N, Zacharopoulos I, Pallikaris IG. Primary deep sclerectomy versus primary deep sclerectomy with the use of mitomycin C in primary open-angle glaucoma. J Glaucoma 2002; 11: 287–293. [DOI] [PubMed] [Google Scholar]
- Anand N, Atherley C. Deep sclerectomy augmented with mitomycin C. Eye 2005; 19: 442–450. [DOI] [PubMed] [Google Scholar]
- Suominen S, Harju M, Ihanamaki T, Vesti E. The effect of deep sclerectomy on intraocular pressure of normal-tension glaucoma patients: 1-year results. Acta Ophthalmol 2010; 88: 27–32. [DOI] [PubMed] [Google Scholar]
- Al Obeidan SA, Osman EA, Al-Muammar AM, Abu El-Asrar AM. Efficacy and safety of deep sclerectomy in uveitic glaucoma. Int Ophthalmol 2008; 29: 367–372. [DOI] [PubMed] [Google Scholar]
- Auer C, Mermoud A, Herbort CP. Deep sclerectomy for the management of uncontrolled uveitic glaucoma: preliminary data. Klin Monatsbl Augenheilkd 2004; 221: 339–342. [DOI] [PubMed] [Google Scholar]
- Anand N. Deep sclerectomy with mitomycin C for glaucoma secondary to uveitis. Eur J Ophthalmol 2011; 21(6): 708–714. [DOI] [PubMed] [Google Scholar]
- Al Obeidan SA, Osman EA, Mousa A, Al-Muammar AM, Abu El-Asrar AM. Long-term evaluation of efficacy and safety of deep sclerectomy in uveitic glaucoma. Ocular Immunol Inflamm 2015; 23(1): 82–89. [DOI] [PubMed] [Google Scholar]
- Miserocchi E, Carassa RG, Bettin P, Brancato R. Viscocanalostomy in patients with glaucoma secondary to uveitis: preliminary report. J Cataract Refract Surg 2004; 30: 566–570. [DOI] [PubMed] [Google Scholar]
- Mercieca K, Shevade B, Anand N. Outcomes of combined phacoemulsification and deep sclerectomy: a 10-year UK single-centre study. Eye 2015; 29(11): 1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand N, Bong C. Deep sclerectomy with bevacizumab and mitomycin C: a comparative study. J Glaucoma 2015; 24(1): 25–31. [DOI] [PubMed] [Google Scholar]
- Anand N, Pilling R. Nd:YAG laser goniopuncture after deep sclerectomy: outcomes. Acta Ophthalmol 2009; 88: 110–115. [DOI] [PubMed] [Google Scholar]
- Sung VC, Barton K. Management of inflammatory glaucomas. Curr Opin Ophthalmol 2004; 15: 136–140. [DOI] [PubMed] [Google Scholar]
- Prata JA Jr, Neves RA, Minckler DS, Mermoud A, Heuer DK. Trabeculectomy with mitomycin C in glaucoma associated with uveitis. Ophthalmic Surg 1994; 25: 616–620. [PubMed] [Google Scholar]
- Chen CW, Huang HT, Bair JS, Lee CC. Trabeculectomy with simultaneous application of mitomycin C in refractory glaucoma. J Ocul Pharmacol 1990; 6: 175–182. [DOI] [PubMed] [Google Scholar]
- Skuta GL, Beeson CC, Higginbotham EJ, Lichter PR, Musch DC, Bergstrom TJ et al. Intraoperative mitomycin versus postoperative 5-fluorouracil in high risk glaucoma filtering surgery. Ophthalmology 1992; 99: 438–444. [DOI] [PubMed] [Google Scholar]
- Stavrou P, Murray PI. Long-term follow-up of trabeculectomy without antimetabolites in patients with uveitis. Am J Ophthalmol 1999; 128: 434–439. [DOI] [PubMed] [Google Scholar]
- Towler HM, McCluskey P, Shaer B, Lightman S. Long-term follow up of trabeculectomy with intraoperative 5-fluorouracil for uveitis-related glaucoma. Ophthalmology 2000; 107(10): 1822–1828. [DOI] [PubMed] [Google Scholar]
- Bindlish R, Condon GP, Schlosser JD, D'Antonio J, Lauer KB, Lehrer R. Efficacy and safety of mitomycin-C in primary trabeculectomy: five-year follow-up. Ophthalmology 2002; 109: 1336–1341. [DOI] [PubMed] [Google Scholar]
- DeBry PW, Perkins TW, Heatley G, Kaufman P, Brumback LC. Incidence of late-onset bleb-related complications following trabeculectomy with mitomycin. Arch Ophthalmol 2002; 120: 297–300. [DOI] [PubMed] [Google Scholar]
- Dupas B, Fardeau C, Cassoux N, Bodaghi B, LeHoang P. Deep sclerectomy and trabeculectomy in uveitic glaucoma. Eye (Lond) 2010; 24: 310–314. [DOI] [PubMed] [Google Scholar]
- Mendrinos E, Mansouri K, Mermoud A, Shaarawy T. Long-term results of deep sclerectomy with collagen implant in exfoliative glaucoma. J Glaucoma 2009; 18: 361–367. [DOI] [PubMed] [Google Scholar]
- Vuori ML. Complications of Neodymium:YAG laser goniopuncture after deep sclerectomy. Acta Ophthalmol Scand 2003; 81: 573–576. [DOI] [PubMed] [Google Scholar]