Abstract
Disease-related biomarkers are objectively measurable molecular signatures of physiological status that can serve as disease indicators or drug targets in clinical diagnosis and therapy, thus acting as a tool in support of personalized medicine. For example, the prostate-specific antigen (PSA) biomarker is now widely used to screen patients for prostate cancer. However, few such biomarkers are currently available, and the process of biomarker identification and validation is prolonged and complicated by inefficient methods of discovery and few reliable analytical platforms. Therefore, in this Perspective, we look at the advanced chemistry of aptamer molecules and their significant role as molecular probes in biomarker studies. As a special class of functional nucleic acids evolved from an iterative technology termed Systematic Evolution of Ligands by Exponential Enrichment (SELEX), these single-stranded oligonucleotides can recognize their respective targets with selectivity and affinity comparable to those of protein antibodies. Because of their fast turnaround time and exceptional chemical properties, aptamer probes can serve as novel molecular tools for biomarker investigations, particularly in assisting identification of new disease-related biomarkers. More importantly, aptamers are able to recognize biomarkers from complex biological environments such as blood serum and cell surfaces, which can provide direct evidence for further clinical applications. This Perspective highlights several major advancements of aptamer-based biomarker discovery strategies and their potential contribution to the practice of precision medicine.
INTRODUCTION
Modern healthcare has been steadily advancing toward an era of molecular medicine, personalized or precision medicine, better known as stratified or P4 (predictive, preventive, personalized, and participatory) medicine.1,2 Key tools moving this process forward are biomarkers, which are objectively measurable molecular signatures that indicate normal or pathogenic biological processes. Over the past few decades, biomarkers have become significant players in disease diagnosis and therapy, as well as clinical trials and, hence, personalized medicine.3–5 Unfortunately, despite the consistent enthusiasm among physicians and biomedical researchers, very few disease biomarkers have been put into clinical practice. This deficiency can be attributed to two major reasons: (1) lack of efficient methods for biomarker discovery and (2) lack of molecular tools to implement practical assays for biomarker validation and application.
Biomarker discovery strategies have long relied on the analysis of genes, proteins, and metabolites. Gene transcription and downstream protein expression can be greatly altered when physiological status becomes pathogenic, thus making gene transcripts and proteins useful biomarkers. Similarly, metabolic pathways can be varied during disease, and primary metabolites can therefore serve as disease-related biomarkers as well. Genomics, transcriptomics, proteomics, and metabolomics provide additional platforms for more complex analysis, where multiple biomarkers can be identified simultaneously. Researchers have long relied on these techniques as major methods in biomarker discovery.6–8 However, results from genotypic investigation do not always correlate with the phenotypic abundance and variation. And the intensive sample processing tends to cause false positive/negative results, given the complexity of the biological environment. Moreover, due to the limited sensitivity of currently available instruments, to determine biomarkers in low abundance is quite challenging.9–11 In this situation, a multiplexed, yet targeted, biomarker discovery method would be very beneficial.
Molecular tools for implementing biomarker assays are in even more need. In particular, molecular probes used for biomarker investigations must be sensitive and selective and have high binding affinities. It is also essential that such probes easily recognize their cognate targets under complex biological conditions, such as those found in body fluids or on the surfaces of cell membranes. As the probes to beat, antibodies are currently utilized in biomedical studies and clinical practices. The interactions between antibodies and antigens are well studied, and the affinities are quite satisfying. In the past decades, antibodies have become the dominating regimen for biomarker investigations.12 Nevertheless, it is difficult to produce antibodies because of their biological complexity, which, in addition to their instability and potential immunogenicity, has largely diminished the utility of antibodies as effective molecular tools. A more serious concern arises from the involvement of animal models in antibody production, which results in poor batch-to-batch consistency and thus causes the accuracy of antibody-based assays to be unreliable. For these and other reasons, alternative approaches have been suggested to rival antibodies as molecular probes.
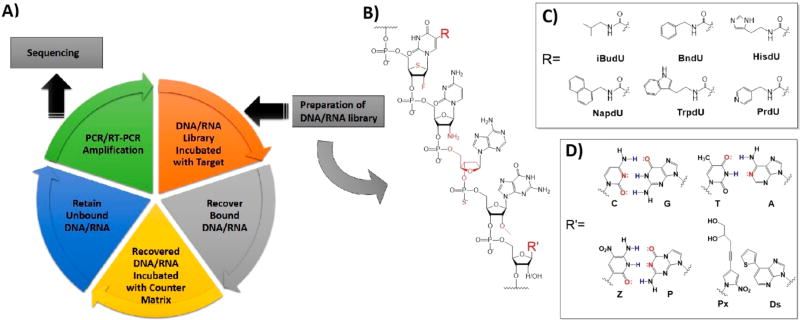
The development of aptamers has offered such an opportunity. Aptamers are single-stranded synthetic oligonucleotides composed of DNA or RNA, usually having a length of 20–100 nucleotides (nt). They demonstrate remarkable binding affinity to a variety of targets, ranging from metal ions to small molecules, proteins, and even intact cells.13,14 The molecular recognition between aptamers and their targets relies on their nucleotide sequences, as well as their unique three-dimensional folded conformations. Aptamers are generated via an in vitro selection process15 termed Systematic Evolution of Ligands by Exponential Enrichment (SELEX)16 (Figure 1A). Unlike antibodies, which are generated by an immunological process, the development of aptamers depends on chemical artistry. Briefly, a large chemically synthesized DNA/RNA library (around 1015 unique sequences) is iteratively exposed to the target of interest. Bound sequences are retained, amplified by polymerase chain reaction, and subjected to repeated rounds of selection until the binding sequences are enriched, and those that do not bind are removed. These surviving binders are sequenced, recovered, and, finally, characterized as molecular probes.
Figure 1.
Schematic of SELEX and modification of DNA/RNA library for SELEX. (A) Flowchart of SELEX procedure. (B) Modified oligonucleotide library employed in SELEX selection. Nucleotide substitution on the 2′-carbon of ribose, on the 5′-α-P-site, or artificial riboses were introduced in the modified library to generate aptamers with enhanced nuclease-resistance. Modified nucleobases and artificial nucleobases were introduced to increase functionality or information density. (C) Groups mimicking amino acid side chains are added on C5-modified deoxyuridines to increase functionality in the initial library. SomaLogic uses these modified nucleobases to generate aptamers with enhanced binding affinity. (D) Extended genetic nucleobases (Z and P, Px and Ds) and their structural comparison with natural nucleobases. Introduction of artificial nucleobases increases information density in the initial library in addition to adding functionality.
Aptamers bind with dissociation constants (Kd) in the nanomolar range and exhibit excellent selectivity, with the additional advantages of easy synthesis and manipulation, low cost of generation, and perfect batch-to-batch reproducibility. More importantly, aptamers are chemically synthesized and can be tailored to readily conjugate with other molecules, including but not limited to fluorophores, bioaffinity molecules, chemical linkers, and nanomaterials, without compromising binding abilities. These merits make aptamers valuable molecular tools for biomarker studies.17
Research on SELEX has continued in the past quarter century. With respect to targets of interest, in addition to ions and molecules, multiple living species have been used as targets in SELEX. The invention of cell-based SELEX ushered in the use of intact cells, especially diseased cells, as targets.18,19 Tissue SELEX,20 3D cell-SELEX,21 and in vivo SELEX22 have also been reported. Other than human-derived samples, parasites, bacterial cells, and viruses have also been used to generate aptamers.23 In terms of techniques, capillary electrophoresis (CE),24 surface plasmon resonance (SPR),25 microfluidic,26 and even robotic SELEX27 have all been reported.
Modification of libraries, the most critical aspect, is currently the very forefront in developing SELEX technologies. (Figure 1B) Specifically, efforts of researchers have been devoted to solve the problems of (1) poor nuclease resistance of aptamers and (2) limited functionality, which results in limited binding affinity, as well as low information density carried by natural oligonucleotides. Nucleotide substitution on the 2′-carbon of ribose or on the 5′-α-P-site, as well as artificial riboses, such as L-ribose and locked ribose, were introduced in the modified library to generate aptamers with enhanced nuclease resistance.28 Functional groups to mimic amino acid side chains have been added to DNA/RNA libraries to enhance binding affinity28–30 (Figure 1C). Artificial expanded nucleobases were introduced into libraries to enhance information density and/or introduce functionality.31–33 (Figure 1D) All these advances in SELEX and aptamer technologies have provided an unprecedented opportunity to improve strategies of biomarker discovery and biomarker-related investigations.
APTAMER-BASED MOLECULAR RECOGNITION FOR BIOMARKER TARGETING
Currently, numerous antibodies are being utilized for immunoassays in both life science research and clinical diagnostics. Antibody-based therapeutics have also occupied the list of popular pharmaceuticals in the past several years. As molecular probes, aptamers were initially intended to rival antibodies. Strategies of aptamer-based biomarker investigations were inspired by the antibody-based method. That is, aptamers would be generated against known biomarkers, but with much faster turnaround time and fewer tedious procedures. Advanced SELEX technology has delivered aptamers and the resultant aptamer-based theranostics strategies to target a series of disease-related biomarkers. These include mucin 1 (MUC1),34,35 carcinoembryonic antigen (CEA),36,37 thrombin,38,39 nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB),40–42 epidermal growth factor receptor (EGFR),43,44 and alpha-fetoprotein (AFP),45,46 as well as prostate-specific antigen (PSA), currently the only FDA-approved biomarker.47–49 Similar to antibodies, aptamers have also been found with the ability to regulate their target proteins.14 Hence aptamers could also serve as therapeutics for disease treatment. In fact, several aptamers regulating plateletderived growth factor (PDGF), vascular endothelial growth factor (VEGF), complement component 5 (C5), nucleolin, and other biomarkers have entered clinical trials, albeit in modified forms.50,51
In one example, Yang’s group focused on epithelial cell adhesion molecule (EpCAM) on cell surfaces.52 EpCAM is a well-recognized transmembrane glycoprotein that has been detected in adenocarcinomas, metastases, malignant effusions, and cancer stem cells. It is also a potential marker of circulating tumor cells (CTCs) and has been targeted in a FDA-approved antibody-based detection platform, named CellSearch, for metastatic breast, colorectal, and prostate cancer cells. In their SELEX experiment, His-Tag labeled recombinant EpCAM was immobilized on Ni-beads, which served as target, while Ni-beads served as negative control. This work delivered a group of DNA aptamers recognizing EpCAM protein and EpCAM-expressing cell lines, but not EpCAM-negative cell lines. In particular, aptamer SYL3C was identified and is currently widely used in the elucidation of cancer clinical samples,53immunostaining of cancer frozen tissues,54 and imaging of cancer cells.55
Another example is the development of an aptamer targeting human epidermal growth factor receptor 2 (ErbB-2/HER2).56HER2 protein belongs to the ErbB family, and this family of receptor tyrosine kinases is the very focus of drug development by its overexpression in many cancers. To date, synthetic small-molecule drugs and monoclonal antibodies (mAb) have been developed to target HER2, and several of them have become first-line therapies in the treatment of multiple cancers. More recently, Yarden’s group selected a 14-nt aptamer using antibody-immobilized native HER2 protein. They found that a trimeric version of this selected aptamer can inhibit cell growth and that the antitumor effect was nearly 2-fold that of the anti-HER2 mAb. Mechanistic studies showed that cellular internalization, cytoplasmic translocation, and lysosome degradation of the HER2 receptor were induced by aptamer binding, thus retarding tumorigenic growth. These results have inspired further research recently leading to combination with a nanocarrier to realize theranostic application.57 These works showed the potential of aptamers as alternative therapeutics by their interactions with target biomarkers.
APTAMER-BASED CELL SURFACE BIOMARKER ELUCIDATION
While aptamers have shown their utility as molecular tools targeting known biomarkers, they can also play critical roles in exploring unknown biomarkers. As previously noted, biological complexity poses a major challenge for current analytical platforms in biomarker discovery. A prime example is the cellular membrane, where a vast array of proteins are anchored to, released from, or highly interacted with membrane components like phospholipids and carbohydrates. Notably, the expression level of a membrane protein is highly correlated with the physiological or pathological status of the cell, making membrane proteins an abundant source of putative disease biomarkers. Nonetheless, lack of efficient methods to generate membrane protein-specific molecular probes, like antibodies, makes membrane protein studies and biomarker discovery from membrane proteins very difficult. After all, recombinant proteins do not always represent their natural conformations on cell surfaces in the absence of methods to mimic the complex surroundings. In the meantime, the hydrophobicity and low abundance of many membrane proteins usually restrict attempts to recover protein samples from their native states.
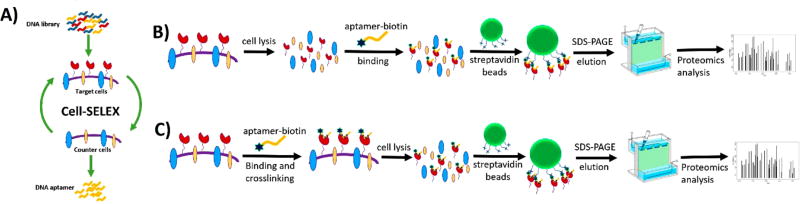
To overcome this dilemma, a modified SELEX method using intact cells as targets was invented. This research was initially motivated to prove that aptamers could recognize complex targets, and to accomplish this, red blood cells (RBCs) were used as a model.58 Subsequent work by Blank et al. using a similar strategy successfully generated an aptamer targeting microvessels of rat brain tumor. An aptamer-assisted affinity purification was then used to pull down the target proteins, which were subsequently identified by mass spectrometry.59 On the basis of these and other proofs of concept,60 a signature method, formally termed cell-SELEX, was systematically established by the Tan group.18,19 Different from traditional SELEX, cell-SELEX generates aptamers capable of distinguishing molecular differences between two types of cells, such as diseased and normal cells, two different types of diseased cells, or different subtypes of the same diseased cells (Figure 2A).
Figure 2.
Schematic of cell-SELEX and strategy of aptamer-based cell surface biomarker discovery. (A) Cell-SELEX technology selects a panel of aptamers capable of distinguishing molecular differences between two types of cells. Aptamers are selected to bind with target cells, but not counter cells. (B) Aptamer-assisted affinity purification without cross-linking aptamers and target proteins as targeted proteomics strategy for cancer cell surface biomarker discovery. (C) Aptamer-assisted affinity purification with cross-linking aptamers and target proteins as targeted proteomics strategy for cancer cell surface biomarker discovery.
This subtractive selection strategy is performed by alternately enriching bound sequences on target cells while removing those that also bind to the other cell line (counter cells). The resultant aptamers are able to selectively bind proteins upregulated on target cells. In their pioneering work establishing cell-SELEX, the Tan group incubated CCRF-CEM cells (human T-cell acute lymphoblastic leukemia, ALL) with a synthetic DNA library of 1015 randomized sequences. Ramos cells (Burkitt’s lymphoma) were used for counter selection.
After 20 rounds of selection, bound sequences were enriched in the library pool, as clearly indicated by flow cytometry monitoring and by confocal microscope imaging. Cloning and DNA sequencing determined sequences of a panel of aptamers, having Kd values in the subnanomolar range.
In essence, cell-SELEX technology circumvents issues related to the complex milieu of cell membrane surfaces, where no prior knowledge of the cell surface molecular signature is required. Those proteins that could be identified as potential biomarkers retain their native states. Meanwhile living cells serve to support facile separation of the bound and unbound sequences. Moreover, after several repeated rounds of enrichment, the “survival” (bound) sequences can recognize their cognate target biomarkers in very low abundance, or even elucidate the trace differences of expression levels of certain biomarkers found on target cells compared to counter cells. Additionally, cell-SELEX is essentially a multiplexed strategy that probes the cohort of surface proteins. In this process, a panel of molecular probes can be generated, after which a panel of corresponding biomarkers can be targeted in the same series of experiments.
Upon binding to their cognate target cells, aptamers have nearly fulfilled their primary role as molecular probes. The Tan group then moved forward to identify the target proteins, with the intention of identifying potential biomarkers for ALL. The target protein of aptamer sgc8 (specific for ALL cells) was pulled down using aptamer-assisted affinity purification, wherein biotinylated sgc8 and streptavidin magnetic beads were employed to capture and isolate the target proteins. The recovered proteins were resolved with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and subsequent liquid chromatography-mass spectrometry (LC-MS) analysis (Figure 2B). A transmembrane protein, protein tyrosine kinase 7 (PTK7), that was found overexpressed in other tumors but previously unknown in ALL, was finally identified as the target.61 Aptamer sgc8 is now used in a wide range of biomedical studies as a molecular recognition moiety and, in combination with various nanomaterials, for targeted delivery,62–65cancer therapy,54,66–68cancer detection,[poo]69,70 and tumor imaging.71–73
This aptamer-assisted affinity purification method, as a form of targeted proteomics, has proven to be useful. Similar strategies have been widely used to identify several other cell-specific protein targets (Table 1). Nonetheless, the efficiency of this method is far from satisfying. The removal of nonspecifi-cally bound proteins from the capture beads requires stringent washing conditions; yet those aptamers with inferior binding affinity are typically insufficient to capture detectable amount of proteins in such harsh conditions, especially when confronting rarely expressed protein targets. This largely impedes use of this aptamer-based method from being generally adopted.
Table 1.
Cell Surface Proteins Elucidated as Potential Biomarkers Using Cell-SELEX and Aptamer-Based Targeted Proteomics
| target | aptamer | cell line | method | application |
|---|---|---|---|---|
| pigpen | III. I59 | YPEN-1, epithelial | aptamer-based affinity purification + SDS-PAGE + MS/MS59 | glioblastoma angiogenesis detection59 |
| TN-C | GBI-1077 | U251, glioblastoma | aptamer-based affinity purification + SDS-PAGE + LC-MS/MS77 | tumor diagnosis by MRI,78 cancer imaging79 |
| IGHM | TD0580 | Ramos, Burkitt’s lymphoma | 5-dUI photocross-linking + aptamer-based affinity purification + SDS-PAGE + LC-MS/MS74 | aptamer—micelles for cancer detection/drug delvery,81 cancer cell detection82, aptamer particles for circulating tumor cell detection,83 F-apt/SWNT for cancer cell probing,84 aptamer-mediated cell targeting68 |
| PTK7 | Sgc818 | CCRF-CEM, acute lymphoblastic leukemia | aptamer-based affinity purification + SDS-PAGE + LC-MS/MS61 | activatable aptamer probe for in vivo cancer imaging,71 logic-gate based cancer theranostics,64,69,70 aptamer—drug conjugates for targeted therapy67 |
| CXorfl7, Gal-3, GPNMB, LPL, SGP-1, LACTB/CD80, CD40, CPNE2 | not identified17 | immature dendritic/mature dendritic | aptamer-based affinity purification + SDS-PAGE + LC-MS/MS17 | immunological studies and clinical applications of DC-based cancer vaccines 17 |
| hemagglutinin | PP385 | vaccinia virus infected HeLa | AlphaScreen85 | diagnostic and/or therapeutic tools for infectious disease85 |
| Axl | GL21.T86 | U87MG, glioma | phospho-receptor tyrosine kinase array analysis + filter binding analysis87 | Axl inhibitor,87 aptamer-miRNA conjugate for targeted delivery88 and therapy89 |
| ALPPL-2 | SQ-290 | Panc-1 and Capan-1, pancreatic cancer | aptamer-based affinity purification + SDS-PAGE + LC-MS/MS90 | 5-fluoro-2′-deoxyuridine targeted delivery91 |
| troponin T | AraHH00192 | mTECs, mouse tumor endothelial | aptamer-based affinity purification + SDS-PAGE + MALDI-TOF93 | antiangiogenic therapy techniques93 |
| STIP1 | TOV694 | TOV-21G, ovarian cancer | formaldehyde cross-linking + aptamer-based affinity purification + SDS-PAGE + LC-MS75 | therapeutic tool75 |
| Siglec-5 | K1995 | NB4, acute myelogenous leukemia | aptamer-based affinity purification + SDS-PAGE + LC-MS/MS95 | detection of small numbers of AML cells in human bone marrow specimens95 |
| CD 109 | S396 | 5–8F, nasopharyngeal carcinoma | aptamer-based affinity purification + SDS-PAGE + LC-MS/MS96 | cellular imaging96 |
| Selectin L/integrin α 4 | sgc-3b/sgc-4e18 | Jurkat 6 × 10−1, acute lymphoblastic leukemia | formaldehyde cross-linking + SILAC + aptamer-based affinity purification + SDS-PAGE + LC-MS76 | cellular detection76 |
Covalent cross-linking between target protein and aptamer was therefore introduced to increase the efficiency of aptamer-based isolation of proteins (Figure 2C). In an early trial, chemically modified photoactive 5-dUI (5-iodo-deoxyuridine) was used to replace several dTs (deoxythymidine) in the biotinylated aptamer TD05, along with a disulfide bridge incorporated, to interact with the target protein of Ramos cells (Burkitt’s lymphoma). In this method, cells were lysed only after binding and UV-induced covalent cross-linkage formed between the modified aptamer and its target protein. Subsequently, aptamer-tagged protein was captured by streptavidin beads and then released by cleaving the disulfide bond. Results from SDS-PAGE and LC-MSMS analysis identified the target protein as the immunoglobulin heavy mu chain (IGHM) of B-cell receptor, which was known to be highly associated with the development of Burkitt’s lymphoma.[poo]74
The cross-linking strategy allows stringent washing conditions during affinity purification of target protein, thus largely avoiding off-target interferences. However, replacing dT with 5-dUI requires labor-intensive position optimization to avoid diminishing the binding ability of the aptamer, which limited the general usage of this method.
Recently, inspired by the method of chromatin immunoprecipitation (ChIP) for studying intracellular interactions between DNA and proteins, another cross-linking approach was explored using formaldehyde as the cross-linker.75 The interaction between desthiobiotin-labeled aptamer TOV6 and targeted ovarian cancer TOV21 cells was fixed by introducing formaldehyde. Subsequent cell lysis, affinity purification, and stringent washing allowed the DNA-protein conjugate to be isolated by biotin competition. This experiment identified stress-induced phosphoprotein 1 (STIP) as a potential biomarker for ovarian cancer cells.
Based on the concept of formaldehyde cross-linkage, very recently, Shangguan and Wang et al. reported an improved strategy, by which protein selectin L and integrin α4 were identified as target proteins of aptamer sgc3b and sgc4e, another two aptamers selected along with sgc8, in a parallel mode.76 They used a quantitative proteomic method, termed stable-isotope labeling by amino acids in cell culture (SILAC), combined with cross-linked aptamer-based affinity purification. A fluorophore was labeled on the aptamer to allow them monitor and optimize the binding conditions using flow cytometry. Briefly, the two aptamers dual labeled with fluorescein and biotin were incubated separated with heavy Jurkat 6 × 10−1 cells (cultured with heavy K, [13C6, 15N2]-l-lysine, and heavy R, [13C6]-l-arginine) and normal Jurkat 6 × 10−1 cells. These aptamers were originally selected against CCRF-CEM cells but were also positive for binding Jurkat 6 × 10−1 cells. Following previous method, similar steps of binding, cross-linking with formaldehyde, cell lysis, and affinity purification using streptavidin agarose resin were conducted, followed by mixing the two bead portions in a 1:1 ratio. The proteins were eluted by heating at 95 °C for 1 h followed by analysis with SDS-PAGE and LC-MS/MS. As a result, 71 proteins were identified from the mixture. Quantitative analysis was achieved by calculating heavy/light (H/L) ratios, and potential protein targets were thus determined by meticulous comparison. The authors suggested that this method could be developed for general protein target elucidation, either two aptamers at a time or a single aptamer, using a random or scrambled sequence as the control bait.
APTAMER-BASED MULTIPLEXED BIOMARKER DISCOVERY PLATFORM
Aptamers selected against all kinds of protein targets have been reported and used in bioanalysis and biomedicine. However, proteins positively identified as biomarkers remain scarce. In addition, biomarkers expressed in complex biological environments, such as serum, cell surface matrix, or tissue slice, are much more clinically relevant. Therefore, a multiplexed biomarker discovery platform is very much needed. Based on their previous decade of aptamer research, SomaLogic developed such an aptamer-based platform, named SOMAscan, that can screen thousands of proteins in a small volume of biological sample, such as serum or plasma. Aptamers, called “SOMAmers”, used on this platform are composed of functionalized nucleotides with modifications (Figure 1D).32 With their increased functionality, combined with a strategy of slow off-rate selection, SOMAmers typically have binding affinity in the sub-nanomolar or even picomolar range.
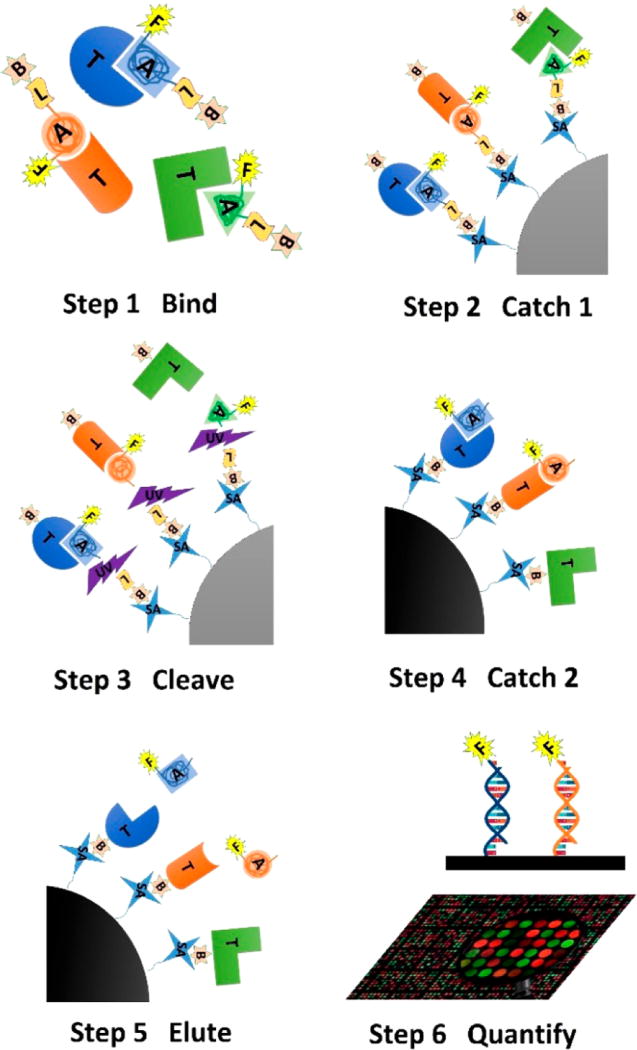
This biomarker discovery strategy combines aptamer and DNA microarray technologies.97–99 (Figure 3) Briefly, a mixture of biotinylated aptamer having a photocleavable group and a fluorescent tag is incubated with the test samples, followed by capture using streptavidin beads (Catch-1). The beads are stringently washed to remove unbound protein before a secondary biotin is labeled on each aptamer-captured protein. The aptamer–protein complex is released from the beads by UV light irradiation, and then an anionic competitor, dextran sulfate, is added to elute the nonspecifically bound proteins. Next, secondary streptavidin beads are incubated with the aptamer–protein complex, and the aptamer is released by subsequent elution with basic solution. The eluted aptamers are then subjected to DNA microarray to determine the identities and corresponding proteins.
Figure 3.
Schematic of SomaLogic proteomics assay.97 Step 1: Biotin (B), photolinker (L), and fluorophore (F)-labeled SOMAmers (A) bind with cognate target proteins (T). Step 2: SOMAmer–protein complexes are captured by streptavidin (SA)-coated magnetic beads, followed by washing unbound proteins and addition of secondary biotin on captured proteins. Step 3: UV irradiation cleaves the photolinker, and non-specifically bound protein is removed using competitive buffer. Step 4: Biotin-labeled proteins are captured by secondary SA-coated magnetic beads. Step 5: Aptamers are eluted. Step 6: DNA microarray analysis is used to quantitate the protein.
In their earliest trial, this strategy was used to screen biomarkers for plasma from subjects with chronic kidney disease (CKD).97 Two well-known CKD biomarkers plus 58 potential biomarkers were identified, demonstrating the potential utility of this technology to rapidly discover unique protein signatures characteristic of various disease states. This technology has now been used to identify biomarkers from bronchoalveolar lavage fluid of pediatric patients with cystic fibrosis (CF) lung disease,100 from serum of malignant pleural mesothelioma (MM) patients,101 from blood of non-small cell lung cancer (NSCLC) patients,102 from blood of myocardial injury patients,103 from serum of tuberculosis (TB) patients,104 and from serum of Duchenne muscular dystrophy (DMD) patients.105 This platform was even used to identify biomarkers from cancerous exosomes (cell-derived vesicles that are present in many, or potentially all, biological fluids, including blood, urine, and cultured medium of cell cultures).106 At this time, 1129 proteins can be screened using this platform.103
PERSPECTIVE
A quarter century has elapsed since aptamer and SELEX technologies were first reported. It was anticipated that these single-stranded oligonucleotide probes would rival antibodies in affinity assays and therapeutic uses, and excel in biomarker discovery for disease theranostics. This demand is growing along with the trend of current medical practice toward emphasizing molecular-level and personalized modes. Personalized medicine takes into account individual variability, such as individual disease-related biomarkers, so that medical decisions can be tailored based on their predicted response or relative risk. This thus requires technologies with fast turnaround time to identify individual biomarkers, as well as molecular tools for disease theranostics.
This challenge has been partially met by the generation of thousands of aptamers and numerous aptamer-based methods for disease diagnosis and therapy. For example, only within the past decade, a panel of aptamers have been developed targeting PSA.47,107,108 These alternative molecular probes have been developed into a variety of biosensors,49,109 which largely enriched the diagnosis approaches that used to merely rely on antibodies. However, the goal of aptamers rivaling antibodies is far from being realized. In fact, the overall number of well-validated aptamer probes remains limited, which restricts aptamer-based technology to the “proof-of-concept” phase. Several major barriers are hampering general applications of aptamer as molecular probes, thereby impairing the development of aptamer-based biomarker strategies. Generation of aptamers need a more effective way that requires improvements in SELEX technology, specifically to further shorten turnaround time and to enhance the binding affinity of selected aptamers.
One concern is associated with the natural shortcomings of DNA/RNA molecules as binding moieties. Aromatic ring stacking by nucleobases, hydrogen bonding between nucleo-bases and electrostatic interactions with the negatively charged backbone are the only significant interactions that can be derived from DNA/RNA molecules. Moreover, the presence of only two pairs of building blocks (A-T and C-G) provides very low information density, which corresponds to a very limited number of folding patterns. SOMAmers set an impressive precedent of how introduction of functional groups can help generate aptamers with enhanced binding affinity. A very recent publication by the Mayer group introduced an intriguing method exploiting click-chemistry to add functionality to oligonucleotide libraries for SELEX,30 which could further expand the possibility of introducing various functional groups in a versatile way. Hirao’s group introduced a fifth nucleotide appended with a hydrophilic group into a library for selecting aptamers with enhanced affinity.30 Another expanded genetic system used in SELEX was jointly reported by the Benner and Tan groups, in which a pair of artificial nucleotides was incorporated into the DNA library to select aptamers toward living cells31,32 and cell surface targets.110 ntroduction of this base pair not only added functionality to the library reservoir but also largely increased information density, providing much larger folding possibility of oligonucleotides, eventually making the entire selection process more efficient. These new generations of SELEX will increase in popularity and effectiveness when the related chemical biological technologies are fully equipped, especially the engineering of more effective polymerases to better accept artificial nucleobases and more efficient sequencing technologies for artificial nucleobase-containing DNA/RNA molecules.
Of course, every technical success relies on advances in device engineering, analytical method development, and instrument design. Particularly, with regard to SELEX, more efficient separation methods, PCR with higher fidelity and efficiency, and improved sequencing technology are always in great need. Toward this end, Soh’s group displayed every single sequence of a DNA library on particle surfaces by emulsion PCR, and employed advanced FACS sorting to screen aptamers with high-affinity to provide a rapid and economical method to generate high-quality aptamers.111 Many other advances utilizing novel technologies, such as microfluidic devices and DNA microarray technologies, for SELEX have already been reported by the Soh group and others.24,112 All of these efforts redound to greater control of the chemistry/biology interface, such that the resultant molecular tools can be put into clinical use to expand the practice of precision medicine.
When it comes to cell-SELEX, the phenotypic heterogeneity between passages of cells is always one of the greatest reasons to hinder the successful delivery of aptamers. Plus, the massive amount of cells required for SELEX experiment limited the cell targets to those immortalized cells that are suitable for being cultured in laboratories. It is thus urgent to develop improved cell-SELEX method with reduced selection cycles, while applying miniaturized selection strategies and devices that requires much smaller scale of cells.
Efforts to improve strategies related to the biomarker process are also being made, in particular to increase the efficiency of purification while also lowering the limit of detection. The cross-linking methods effectively enhance the affinity between an aptamer and its protein, but a means to avoid off-target conjugation is still urgently needed. The Famulok group reported a strategy utilizing a phenyl azide as photo-cross-linker and validated the chemistry using three structurally different aptamers.113 The Bertozzi group reported a proximity-enhanced biorthogonal ligation method for cross-linking between an aptamer conjugated to cyclooctyne and azidosu-gar-labeled glycoproteins.114 Most recently, the Tan group reported a protein–aptamer template-directed cross-linking method with aptamers carrying an F-carboxyl group.115 Of course, lowering the limit of detection also requires innovations in downstream proteomic analytical methods, including mass spectrometry or DNA microarrays, which are not the focus of this Perspective and have recently been reviewed elsewhere. These efforts all give evidence of continuous progress in the field of cell-surface biomarker discovery and will, of course, make a significant contribution to the overall goal of personalized medicine.
Acknowledgments
The authors thank Dr. Kathryn R Williams for manuscript review. This work is supported by grants awarded by the National Institutes of Health (GM079359 and CA133086) and the NSFC (21505032, 21325520, and 1327009).
Footnotes
The authors declare no competing financial interest.
References
- 1.Galli SJ. J. Allergy Clin. Immunol. 2016;137:1289–300. doi: 10.1016/j.jaci.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weston AD, Hood L. J. Proteome. Res. 2004;3:179. doi: 10.1021/pr0499693. [DOI] [PubMed] [Google Scholar]
- 3.Buyse M, Sargent DJ, Grothey A, Matheson A, de Gramont A. Nat. Rev. Clin. Oncol. 2010;7:309. doi: 10.1038/nrclinonc.2010.43. [DOI] [PubMed] [Google Scholar]
- 4.Dalton WS, Friend SH. Science. 2006;312:1165. doi: 10.1126/science.1125948. [DOI] [PubMed] [Google Scholar]
- 5.Gutman S, Kessler LG. Nat. Rev. Cancer. 2006;6:565. doi: 10.1038/nrc1911. [DOI] [PubMed] [Google Scholar]
- 6.Wasinger VC, Zeng M, Yau Y. Int. J. Proteomics. 2013;2013:180605. doi: 10.1155/2013/180605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamandis EP. Mol. Cell. Proteomics. 2004;3:367. doi: 10.1074/mcp.R400007-MCP200. [DOI] [PubMed] [Google Scholar]
- 8.Reid JD, Parker CE, Borchers CH. Curr. Opin. Mol. Ther. 2007;9:216. [PubMed] [Google Scholar]
- 9.Horvatovich P, Govorukhina N, Bischoff R. Analyst. 2006;131:1193. doi: 10.1039/b607833h. [DOI] [PubMed] [Google Scholar]
- 10.Drucker E, Krapfenbauer K. EPMA J. 2013;4:7. doi: 10.1186/1878-5085-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rifai N, Gillette MA, Carr SA. Nat. Biotechnol. 2006;24:971. doi: 10.1038/nbt1235. [DOI] [PubMed] [Google Scholar]
- 12.Kingsmore SF. Nat. Rev. Drug Discovery. 2006;5:310. doi: 10.1038/nrd2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayasena SD. Clin. Chem. 1999;45:1628. [PubMed] [Google Scholar]
- 14.Keefe AD, Pai S, Ellington A. Nat. Rev. Drug Discovery. 2010;9:537. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellington AD, Szostak JW. Nature. 1990;346:818. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 16.Tuerk C, Gold L. Science. 1990;249:505. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 17.Berezovski MV, Lechmann M, Musheev MU, Mak TW, Krylov SN. J. Am. Chem. Soc. 2008;130:9137. doi: 10.1021/ja801951p. [DOI] [PubMed] [Google Scholar]
- 18.Shangguan D, Li Y, Tang Z, Cao ZC, Chen HW, Mallikaratchy P, Sefah K, Yang CJ, Tan W. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11838. doi: 10.1073/pnas.0602615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sefah K, Shangguan D, Xiong X, O’Donoghue MB, Tan W. Nat. Protoc. 2010;5:1169. doi: 10.1038/nprot.2010.66. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Xu H, Ding H, Huang Y, Cao X, Yang G, Li J, Xie Z, Meng Y, Li X, Zhao Q, Shen B, Shao N. J. Pathol. 2009;218:327. doi: 10.1002/path.2543. [DOI] [PubMed] [Google Scholar]
- 21.Souza AG, Marangoni K, Fujimura PT, Alves PT, Silva MJ, Bastos VA, Goulart LR, Goulart VA. Exp. Cell Res. 2016;341:147. doi: 10.1016/j.yexcr.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Cheng C, Chen YH, Lennox KA, Behlke MA, Davidson BL. Mol. Ther.-Nucleic Acids. 2013;2:e67. doi: 10.1038/mtna.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamah SM, Healy JM, Cload ST. Acc. Chem. Res. 2008;41:130. doi: 10.1021/ar700142z. [DOI] [PubMed] [Google Scholar]
- 24.Mosing RK, Bowser MT. Methods Mol. Biol. 2009;535:33. doi: 10.1007/978-1-59745-557-2_3. [DOI] [PubMed] [Google Scholar]
- 25.Ngubane NA, Gresh L, Pym A, Rubin EJ, Khati M. Biochem. Biophys. Res. Commun. 2014;449:114. doi: 10.1016/j.bbrc.2014.04.163. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Yang X, Wang E. Anal. Chim. Acta. 2010;683:12. doi: 10.1016/j.aca.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Glokler J, Schutze T, Konthur Z. Molecules. 2010;15:2478. doi: 10.3390/molecules15042478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tolle F, Mayer G. Chem. Sci. 2013;4:60. [Google Scholar]
- 29.Zichi D, Eaton B, Singer B, Gold L. Curr. Opin. Chem. Biol. 2008;12:78. doi: 10.1016/j.cbpa.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Tolle F, Brandle GM, Matzner D, Mayer G. Angew. Chem., Int. Ed. 2015;54:10971. doi: 10.1002/anie.201503652. [DOI] [PubMed] [Google Scholar]
- 31.Sefah K, Yang Z, Bradley KM, Hoshika S, Jimenez E, Zhang L, Zhu G, Shanker S, Yu F, Turek D, Tan W, Benner SA. Proc. Natl. Acad. Sci. U. S. A. 2014;111:1449. doi: 10.1073/pnas.1311778111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Yang Z, Sefah K, Bradley KM, Hoshika S, Kim MJ, Kim HJ, Zhu G, Jimenez E, Cansiz S, Teng IT, Champanhac C, McLendon C, Liu C, Zhang W, Gerloff DL, Huang Z, Tan W, Benner SA. J. Am. Chem. Soc. 2015;137:6734. doi: 10.1021/jacs.5b02251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimoto M, Yamashige R, Matsunaga K, Yokoyama S, Hirao I. Nat. Biotechnol. 2013;31:453. doi: 10.1038/nbt.2556. [DOI] [PubMed] [Google Scholar]
- 34.Hu Y, Duan J, Zhan Q, Wang F, Lu X, Yang XD. PLoS One. 2012;7:e31970. doi: 10.1371/journal.pone.0031970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen HY, Zhao J, Zhang M, Yang HB, Ma YX, Gu YQ. Mol. Imaging. Biol. 2015;17:38. doi: 10.1007/s11307-014-0763-y. [DOI] [PubMed] [Google Scholar]
- 36.Park JW, Na W, Jang J. RSC Adv. 2016;6:14335. [Google Scholar]
- 37.Wen W, Huang JY, Bao T, Zhou J, Xia HX, Zhang XH, Wang SF, Zhao YD. Biosens. Bioelectron. 2016;83:142. doi: 10.1016/j.bios.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 38.Bock LC, Griffin LC, Latham JA, Vermaas EH, Toole JJ. Nature. 1992;355:564. doi: 10.1038/355564a0. [DOI] [PubMed] [Google Scholar]
- 39.Deng B, Lin YW, Wang C, Li F, Wang ZX, Zhang HQ, Li XF, Le XC. Anal. Chim. Acta. 2014;837:1. doi: 10.1016/j.aca.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 40.Lebruska LL, Maher LJ., 3rd Biochemistry. 1999;38:3168. doi: 10.1021/bi982515x. [DOI] [PubMed] [Google Scholar]
- 41.Wurster SE, Maher LJ., 3rd RNA. 2008;14:1037. doi: 10.1261/rna.878908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Metelev VG, Kubareva EA, Oretskaya TS. Biochemistry (Moscow) 2013;78:867. doi: 10.1134/S0006297913080026. [DOI] [PubMed] [Google Scholar]
- 43.Li N, Nguyen HH, Byrom M, Ellington AD. PLoS One. 2011;6:e20299. doi: 10.1371/journal.pone.0020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esposito CL, Passaro D, Longobardo I, Condorelli G, Marotta P, Affuso A, de Franciscis V, Cerchia L. PLoS One. 2011;6:e24071. doi: 10.1371/journal.pone.0024071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang CJ, Lin HI, Shiesh SC, Lee GB. Biosens. Bioelectron. 2012;35:50. doi: 10.1016/j.bios.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 46.Dong LL, Tan QW, Ye W, Liu DL, Chen HF, Hu HW, Wen D, Liu Y, Cao Y, Kang JW, Fan J, Guo W, Wu WZ. Sci. Rep. 2015;5:15552. doi: 10.1038/srep15552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savory N, Abe K, Sode K, Ikebukuro K. Biosens. Bioelectron. 2010;26:1386. doi: 10.1016/j.bios.2010.07.057. [DOI] [PubMed] [Google Scholar]
- 48.Xiang B, Dong DW, Shi NQ, Gao W, Yang ZZ, Cui Y, Cao DY, Qi XR. Biomaterials. 2013;34:6976. doi: 10.1016/j.biomaterials.2013.05.055. [DOI] [PubMed] [Google Scholar]
- 49.Tzouvadaki I, Jolly P, Lu X, Ingebrandt S, de Micheli G, Estrela P, Carrara S. Nano Lett. 2016;16:4472. doi: 10.1021/acs.nanolett.6b01648. [DOI] [PubMed] [Google Scholar]
- 50.Lao YH, Phua KK, Leong KW. ACS Nano. 2015;9:2235. doi: 10.1021/nn507494p. [DOI] [PubMed] [Google Scholar]
- 51.Kanwar JR, Roy K, Maremanda NG, Subramanian K, Veedu RN, Bawa R, Kanwar RK. Curr. Med. Chem. 2015;22:2539. doi: 10.2174/0929867322666150227144909. [DOI] [PubMed] [Google Scholar]
- 52.Song Y, Zhu Z, An Y, Zhang W, Zhang H, Liu D, Yu C, Duan W, Yang CJ. Anal. Chem. 2013;85:4141. doi: 10.1021/ac400366b. [DOI] [PubMed] [Google Scholar]
- 53.Liu ZX, Lu Y, Pu Y, Liu J, Liu B, Yu B, Chen K, Fu T, Yang CJ, Liu HX, Tan WH. Sci. Rep. 2015;5:18516. doi: 10.1038/srep18516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pu Y, Liu ZX, Lu Y, Yuan P, Liu J, Yu B, Wang GD, Yang CJ, Liu HX, Tan WH. Anal. Chem. 2015;87:1919. doi: 10.1021/ac504175h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subramanian N, Sreemanthula JB, Balaji B, Kanwar JR, Biswas J, Krishnakumar S. Chem. Commun. 2014;50:11810. doi: 10.1039/c4cc02996h. [DOI] [PubMed] [Google Scholar]
- 56.Mahlknecht G, Maron R, Mancini M, Schechter B, Sela M, Yarden Y. Proc. Natl. Acad. Sci. U. S. A. 2013;110:8170. doi: 10.1073/pnas.1302594110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee H, Dam DH, Ha JW, Yue J, Odom TW. ACS Nano. 2015;9:9859. doi: 10.1021/acsnano.5b05138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morris KN, Jensen KB, Julin CM, Weil M, Gold L. Proc. Natl. Acad. Sci. U. S. A. 1998;95:2902. doi: 10.1073/pnas.95.6.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blank M, Weinschenk T, Priemer M, Schluesener HJ. J. Biol. Chem. 2001;276:16464. doi: 10.1074/jbc.M100347200. [DOI] [PubMed] [Google Scholar]
- 60.Wang C, Zhang M, Yang G, Zhang D, Ding H, Wang H, Fan M, Shen B, Shao NJ. J. Biotechnol. 2003;102:15. doi: 10.1016/s0168-1656(02)00360-7. [DOI] [PubMed] [Google Scholar]
- 61.Shangguan D, Cao Z, Meng L, Mallikaratchy P, Sefah K, Wang H, Li Y, Tan W. J. Proteome. Res. 2008;7:2133. doi: 10.1021/pr700894d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu GZ, Hu R, Zhao ZL, Chen Z, Zhang XB, Tan WH. J. Am. Chem. Soc. 2013;135:16438. doi: 10.1021/ja406115e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu G, Zheng J, Song E, Donovan M, Zhang K, Liu C, Tan W. Proc. Natl. Acad. Sci. U. S. A. 2013;110:7998. doi: 10.1073/pnas.1220817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Douglas SM, Bachelet I, Church GM. Science. 2012;335:831. doi: 10.1126/science.1214081. [DOI] [PubMed] [Google Scholar]
- 65.Wang RW, Zhu GZ, Mei L, Xie Y, Ma HB, Ye M, Qing FL, Tan WH. J. Am. Chem. Soc. 2014;136:2731. doi: 10.1021/ja4117395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu C, Han D, Chen T, Peng L, Zhu G, You M, Qiu L, Sefah K, Zhang X, Tan W. J. Am. Chem. Soc. 2013;135:18644. doi: 10.1021/ja4094617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Niu W, Chen X, Tan W, Veige AS. Angew. Chem., Int. Ed. 2016;55:8889. doi: 10.1002/anie.201602702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiong X, Liu H, Zhao Z, Altman MB, Lopez-Colon D, Yang CJ, Chang LJ, Liu C, Tan W. Angew. Chem., Int. Ed. 2013;52:1472. doi: 10.1002/anie.201207063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.You M, Zhu G, Chen T, Donovan MJ, Tan W. J. Am. Chem. Soc. 2015;137:667. doi: 10.1021/ja509263k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.You M, Peng L, Shao N, Zhang L, Qiu L, Cui C, Tan W. J. Am. Chem. Soc. 2014;136:1256. doi: 10.1021/ja4114903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi H, He X, Wang K, Wu X, Ye X, Guo Q, Tan W, Qing Z, Yang X, Zhou B. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3900. doi: 10.1073/pnas.1016197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang L, Jacobson O, Avdic D, Rotstein BH, Weiss ID, Collier L, Chen XY, Vasdev N, Liang SH. Angew. Chem., Int. Ed. 2015;54:12777. doi: 10.1002/anie.201505927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao Z, Fan H, Zhou G, Bai H, Liang H, Wang R, Zhang X, Tan W. J. Am. Chem. Soc. 2014;136:11220. doi: 10.1021/ja5029364. [DOI] [PubMed] [Google Scholar]
- 74.Mallikaratchy P, Tang Z, Kwame S, Meng L, Shangguan D, Tan W. Mol. Cell. Proteomics. 2007;6:2230. doi: 10.1074/mcp.M700026-MCP200. [DOI] [PubMed] [Google Scholar]
- 75.Van Simaeys D, Turek D, Champanhac C, Vaizer J, Sefah K, Zhen J, Sutphen R, Tan W. Anal. Chem. 2014;86:4521. doi: 10.1021/ac500466x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bing T, Shangguan D, Wang YS. Mol. Cell. Proteomics. 2015;14:2692. doi: 10.1074/mcp.M115.051243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Daniels DA, Chen H, Hicke BJ, Swiderek KM, Gold L. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15416. doi: 10.1073/pnas.2136683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gu MJ, Li KF, Zhang LX, Wang H, Liu LS, Zheng ZZ, Han NY, Yang ZJ, Fan TY. Int. J. Nanomed. 2015;10:5187. doi: 10.2147/IJN.S84351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li ZM, Huang P, He R, Lin J, Yang S, Zhang XJ, Ren QS, Cui DX. Mater. Lett. 2010;64:375. [Google Scholar]
- 80.Tang Z, Shangguan D, Wang K, Shi H, Sefah K, Mallikratchy P, Chen HW, Li Y, Tan W. Anal. Chem. 2007;79:4900. doi: 10.1021/ac070189y. [DOI] [PubMed] [Google Scholar]
- 81.Wu Y, Sefah K, Liu H, Wang R, Tan W. Proc. Natl. Acad. Sci. U. S. A. 2010;107:5. doi: 10.1073/pnas.0909611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ho LC, Wu WC, Chang CY, Hsieh HH, Lee CH, Chang HT. Anal. Chem. 2015;87:4925. doi: 10.1021/acs.analchem.5b00569. [DOI] [PubMed] [Google Scholar]
- 83.Zheng FY, Cheng Y, Wang J, Lu J, Zhang B, Zhao YJ, Gu ZZ. Adv. Mater. 2014;26:7333. doi: 10.1002/adma.201403530. [DOI] [PubMed] [Google Scholar]
- 84.Yan LA, Shi H, He XX, Wang KM, Tang JL, Chen MA, Ye XS, Xu FZ, Lei YL. Anal. Chem. 2014;86:9271. doi: 10.1021/ac5024149. [DOI] [PubMed] [Google Scholar]
- 85.Parekh P, Tang Z, Turner PC, Moyer RW, Tan W. Anal. Chem. 2010;82:8642. doi: 10.1021/ac101801j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cerchia L, Esposito CL, Jacobs AH, Tavitian B, de Franciscis V. PLoS One. 2009;4:e7971. doi: 10.1371/journal.pone.0007971. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Cerchia L, Esposito CL, Camorani S, Rienzo A, Stasio L, Insabato L, Affuso A, de Franciscis V. Mol. Ther. 2012;20:2291. doi: 10.1038/mt.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iaboni M, Russo V, Fontanella R, Roscigno G, Fiore D, Donnarumma E, Esposito CL, Quintavalle C, Giangrande PH, de Franciscis V, Condorelli G. Mol. Ther.–Nucleic Acids. 2016;5:e289. doi: 10.1038/mtna.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Esposito CL, Cerchia L, Catuogno S, De Vita G, Dassie JP, Santamaria G, Swiderski P, Condorelli G, Giangrande PH, de Franciscis V. Mol. Ther. 2014;22:1151. doi: 10.1038/mt.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dua P, Kang HS, Hong SM, Tsao MS, Kim S, Lee DK. Cancer Res. 2013;73:1934. doi: 10.1158/0008-5472.CAN-12-3682. [DOI] [PubMed] [Google Scholar]
- 91.Dua P, S S, Kim S, Lee DK. Nucleic Acid Ther. 2015;25:180. doi: 10.1089/nat.2014.0516. [DOI] [PubMed] [Google Scholar]
- 92.Ara MN, Hyodo M, Ohga N, Hida K, Harashima H. PLoS One. 2012;7:e50174. doi: 10.1371/journal.pone.0050174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ara MN, Hyodo M, Ohga N, Akiyama K, Hida K, Hida Y, Shinohara N, Harashima H. Cancer Med. 2014;3:825. doi: 10.1002/cam4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van Simaeys D, Lopez-Colon D, Sefah K, Sutphen R, Jimenez E, Tan WH. PLoS One. 2010;5:e13770. doi: 10.1371/journal.pone.0013770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang M, Jiang G, Li W, Qiu K, Zhang M, Carter CM, Al-Quran SZ, Li Y. J. Hematol. Oncol. 2014;7:5. doi: 10.1186/1756-8722-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jia W, Ren C, Wang L, Zhu B, Jia W, Gao M, Zeng F, Zeng L, Xia X, Zhang X, Fu T, Li S, Du C, Jiang X, Chen Y, Tan W, Zhao Z, Liu W. Oncotarget. 2016;7:55328. doi: 10.18632/oncotarget.10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, Carter J, Dalby AB, Eaton BE, Fitzwater T, Flather D, Forbes A, Foreman T, Fowler C, Gawande B, Goss M, Gunn M, Gupta S, Halladay D, Heil J, Heilig J, Hicke B, Husar G, Janjic J, Jarvis T, Jennings S, Katilius E, Keeney TR, Kim N, Koch TH, Kraemer S, Kroiss L, Le N, Levine D, Lindsey W, Lollo B, Mayfield W, Mehan M, Mehler R, Nelson SK, Nelson M, Nieuwlandt D, Nikrad M, Ochsner U, Ostroff RM, Otis M, Parker T, Pietrasiewicz S, Resnicow DI, Rohloff J, Sanders G, Sattin S, Schneider D, Singer B, Stanton M, Sterkel A, Stewart A, Stratford S, Vaught JD, Vrkljan M, Walker JJ, Watrobka M, Waugh S, Weiss A, Wilcox SK, Wolfson A, Wolk SK, Zhang C, Zichi D. PLoS One. 2010;5:15004. [Google Scholar]
- 98.Brody EN, Gold L, Lawn RM, Walker JJ, Zichi D. Expert Rev. Mol. Diagn. 2010;10:1013. doi: 10.1586/erm.10.89. [DOI] [PubMed] [Google Scholar]
- 99.Kraemer S, Vaught JD, Bock C, Gold L, Katilius E, Keeney TR, Kim N, Saccomano NA, Wilcox SK, Zichi D, Sanders GM. PLoS One. 2011;6:e26332. doi: 10.1371/journal.pone.0026332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harris JK, Wolfson A, Walker J, Wagner B, Deterding R, Sagel SD, Accurso FJ. Pediatr. Pulmonol. 2010;45(S33):300. doi: 10.1002/ppul.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ostroff RM, Mehan MR, Stewart A, Ayers D, Brody EN, Williams SA, Levin S, Black B, Harbut M, Carbone M, Goparaju C, Pass HI. PLoS One. 2012;7:e46091. doi: 10.1371/journal.pone.0046091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mehan MR, Williams SA, Siegfried JM, Bigbee WL, Weissfeld JL, Wilson DO, Pass HI, Rom WN, Muley T, Meister M, Franklin W, Miller YE, Brody EN, Ostroff RM. Clin. Proteomics. 2014;11:32. doi: 10.1186/1559-0275-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ngo D, Sinha S, Shen D, Kuhn EW, Keyes MJ, Shi X, Benson MD, O’Sullivan JF, Keshishian H, Farrell LA, Fifer MA, Vasan RS, Sabatine MS, Larson MG, Carr SA, Wang TJ, Gerszten RE. Circulation. 2016;134:270. doi: 10.1161/CIRCULATIONAHA.116.021803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nahid P, Bliven-Sizemore E, Jarlsberg LG, De Groote MA, Johnson JL, Muzanyi G, Engle M, Weiner M, Janjic N, Sterling DG, Ochsner UA. Tuberculosis. 2014;94:187. doi: 10.1016/j.tube.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hathout Y, Brody E, Clemens PR, Cripe L, DeLisle RK, Furlong P, Gordish-Dressman H, Hache L, Henricson E, Hoffman EP, Kobayashi YM, Lorts A, Mah JK, McDonald C, Mehler B, Nelson S, Nikrad M, Singer B, Steele F, Sterling D, Sweeney HL, Williams S, Gold L. Proc. Natl. Acad. Sci. U. S. A. 2015;112:7153. doi: 10.1073/pnas.1507719112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Webber J, Stone TC, Katilius E, Smith BC, Gordon B, Mason MD, Tabi Z, Brewis IA, Clayton A. Mol. Cell. Proteomics. 2014;13:1050. doi: 10.1074/mcp.M113.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jeong S, Han SR, Lee YJ, Lee SW. Biotechnol. Lett. 2010;32:379. doi: 10.1007/s10529-009-0168-1. [DOI] [PubMed] [Google Scholar]
- 108.Park JW, Lee SJ, Ren S, Lee S, Kim S, Laurell T. Sci. Rep. 2016;6:27121. doi: 10.1038/srep27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rahi A, Sattarahmady N, Heli H. Talanta. 2016;156–157:218. doi: 10.1016/j.talanta.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 110.Zhang L, Yang Z, Trinh TL, Teng IT, Wang S, Bradley KM, Hoshika S, Wu Q, Cansiz S, Rowold DJ, McLendon C, Kim MS, Wu Y, Cui C, Liu Y, Hou W, Stewart K, Wan S, Liu C, Benner SA, Tan W. Angew. Chem., Int. Ed. 2016;55:12372. doi: 10.1002/anie.201605058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang J, Gong Q, Maheshwari N, Eisenstein M, Arcila ML, Kosik KS, Soh HT. Angew. Chem., Int. Ed. 2014;53:4796. doi: 10.1002/anie.201309334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gotrik MR, Feagin TA, Csordas AT, Nakamoto MA, Soh HT. Acc. Chem. Res. 2016;49:1903. doi: 10.1021/acs.accounts.6b00283. [DOI] [PubMed] [Google Scholar]
- 113.Vinkenborg JL, Mayer G, Famulok M. Angew. Chem., Int. Ed. 2012;51:9176. doi: 10.1002/anie.201204174. [DOI] [PubMed] [Google Scholar]
- 114.Robinson PV, de Almeida-Escobedo G, de Groot AE, McKechnie JL, Bertozzi CR. J. Am. Chem. Soc. 2015;137:10452. doi: 10.1021/jacs.5b04279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang RW, Lu DQ, Bai HR, Jin C, Yan GB, Ye M, Qiu LP, Chang RS, Cui C, Liang H, Tan WH. Chem. Sci. 2016;7:2157. doi: 10.1039/c5sc02631h. [DOI] [PMC free article] [PubMed] [Google Scholar]