Macromolecular X-ray crystallography has developed, since its first use over 50 years ago to solve the structure of myoglobin, into a widely used method with broad impact in biological sciences and in society. It is today the primary technique used to obtain structural information on biomolecules that can shed light on their function and this information is often used in biomedical applications such as drug design. As this article is written, the Protein Data Bank [1] has just reached the milestone of 100,000 deposited X-ray structures, with a continuing trend for an ever-increasing number of structures every year. The primary contribution to this success and the increasing number of X-ray structures is the broad availability of synchrotron radiation sources with many dedicated beamlines around the world providing rapid and efficient data collection along with standard data analysis tools capable of fast data interpretation.
The introduction of new X-ray sources has historically had a large impact on scientific capabilities and X-ray macromolecular crystallography has benefited greatly from the increased brilliance of new sources. Second- and third-generation synchrotron sources have successively decreased the size of crystals that can be used and reduced data collection times, allowing for increasingly challenging systems to be studied.
A new type of source has recently emerged in the hard X-ray regime for high-resolution structural biology in the form of the X-ray Free Electron Laser (FEL). X-ray FELs can deliver over 1012 X-rays in single pulses shorter than 50 fs, a revolutionary capability for X-ray sources. The Linac Coherent Light Source [2] is the first such source, in operation since 2009 at the SLAC National Accelerator Laboratory, a US national laboratory operated by Stanford University. Structural biology represented one of the key science cases promoting its construction, with the suggestion that the ultrashort intense pulses of X-ray produced could limit and potentially eliminate radiation damage issues in organic samples. This concept, now known as “diffraction-before-destruction,” can in principle lead to the ability to utilize significantly smaller crystals and even potentially single molecules for structure determination [3]. It can also remove the limits of radiation damage that continuous light sources impose on certain kinds of macromolecules; for example, the photo-reduction of high valent metal centers of metalloproteins may happen too rapidly for study at high resolution using a synchrotron source.
The use of macromolecular crystallography techniques of various kinds has been an important part of the scientific results from the first six years of LCLS and has led to plans for the expansion of the LCLS facility to enable a broader access to LCLS beamtime. In this article, we will briefly review the history of macromolecular crystallography at LCLS and how the demand has evolved and grown over the years.
We will then present the LCLS facility and how a new beamline and instrument is being built to enable further growth in the field. This new instrument is called the Macromolecular Femtosecond Crystallography (MFX) instrument and its primary intended use is made obvious by its name.
Macromolecular Crystallography Techniques at LCLS
At the start of LCLS operations in 2009, a pair of factors drove the effort in the direction of very small crystals delivered in a sample-replenishing liquid jet. The first such factor was a desire to pursue single-particle imaging. Sub-micron-sized “nanocrystals” were seen as an ideal first target sample for developing techniques at LCLS. Nanocrystals offered a path towards imaging single molecules with the possibility of gradually reducing the crystal size until the single molecule limit was reached. It was expected, from simulations of single molecule imaging, that a large number of 2D diffraction patterns were required to be merged to produce a complete 3D data set and, therefore, the need for rapid sample exchange capable of utilizing the full 120 Hz repetition rate of LCLS led to the similar need for nanocrystals; hence, fast-flowing liquid jets containing a sample slurry were chosen [4].
The second factor behind the initial pursuit of jet-based systems for very small crystals was the availability at LCLS at that time of experimental instruments that could only use soft X-rays below 2 keV. This leads to high absorption cross-sections limiting the size of the crystals that can be used. The use of soft X-rays also prohibits the beam from propagating in an atmospheric pressure environment, and this forced the development of techniques for use inside a vacuum system. This requirement was, in any case, consistent with the need for vacuum for single-particle studies where scattering from the atmospheric pressure gas would completely overwhelm the expected small signal and so vacuum operation was well aligned with the ultimate goal of imaging non-crystalline biological samples.
The dual goals of crystallography and imaging are highlighted by the simultaneous publications of results from both techniques from the same beamtime that took place in late 2009 [5, 6]. The first of these publications presented radiation-damage-free crystallographic results of Photosystem I from thousands of nanocrystals, while the second article demonstrated the capabilities to image single large viruses in two dimensions using single LCLS pulses. The initial success in crystallography and the perceived potential impact of such capabilities led to an immediate growing interest in macromolecular crystallography in general at LCLS.
It took one year after the initial soft X-ray experiments before hard X-rays (above 2 keV and below 10 keV) could be used at LCLS. Hard X-rays allow for efficient beam propagation out of vacuum and this led to the simultaneous pursuit of atmospheric and in-vacuum techniques. The in-vacuum techniques primarily continued along the path of using small liquid jets to deliver a replenishing stream of crystals [7]. Over the years since, multiple variations of liquid jets have been developed and used [8-11] and the techniques of what is now known as Serial Femtosecond Crystallography (SFX) are well-established [12-17], with important results in membrane protein studies such as G-Protein Coupled Receptors (GPCR) [18-20] and time-resolved mechanistic studies [21-23].
While the in-vacuum techniques may be best suited for smaller crystals, the use of small crystals does not represent the only case where LCLS can be useful. Originally, the Serial Femtosecond Crystallography technique was called primarily nano-crystallography. However, over time, much of the focus shifted from pushing the limits of crystal size towards single molecules to using LCLS for scientific discoveries with an optimal crystal size for a particular sample. This drove the typical crystal size to be in the 2-5 micron range or more, rather than sub-micron. In many cases, even larger crystals are the ideal option for multiple reasons and these are comparable in size to what is usable at state-of-the-art synchrotron beamlines. This allows for the use of many of the techniques developed over the last couple of decades at second- and third-generation sources. With slightly larger crystals, a small X-ray focus and hard X-rays, atmospheric pressure data collection using instrumentation essentially identical to what is in use at synchrotrons is practical. These techniques were implemented over the years at LCLS [24], and recent publications demonstrate their capabilities for new scientific discovery [25, 26].
Many advantages may exist in using atmospheric pressure data collection. A vacuum environment is not a natural fit for biological samples requiring hydration. This typically limits what can be done in vacuum since the time the sample is exposed to the vacuum must be short. Liquid jets can be used in vacuum, because the time spent by the sample in vacuum before it is hit by the FEL beam is short enough to prevent evaporation and dehydration of the sample. However, the use of jets, in many cases, leads to sample waste with the sample flowing constantly between X-ray pulses. The ability to mount crystals at atmospheric pressure, potentially know the location and orientation of each of them, and hit all of them without waste represents a key potential advantage, leading to a reduced sample quantity for a given experiment. For precious and difficult-to-express samples, a few crystals may be all that is available and controlled data collection using goniometer-based techniques or other fixed target sample translation methods may be the only option.
Also, performing experiments at atmospheric pressure is simpler in general, and not just for biological samples. Even liquid jets can, in many cases, be used more easily at atmospheric pressure, and for slow-flowing jets such as the Lipidic Cubic Phase (LCP) jet used for many studies on GPCRs, operation in air may be advantageous. Jets in air are now in use at synchrotrons in a technique called Serial Microsecond Crystallography [8, 9].
Additionally, operation in vacuum typically requires special custom detectors, which can have limitations. For example, they often have a limited number of pixels, below what is regularly desirable for macromolecular crystallography. They can also suffer from a limited dynamic range. Operation at atmospheric pressure opens the door to the use of commercially available detectors which are often not usable in-vacuum.
As the multiple femtosecond crystallography techniques utilizing X-Ray FEL sources mature, a broad range of needs and desires are emerging that indicate a need for diverse sample handling and delivery methods that can be selected for each specific sample. Versatility is one of the main goals pursued by LCLS in building a new instrument primarily aimed at crystallography.
Growing Demand in Structural Biology at LCLS
The initial success and publication of results in 2011 [5] led to a rapid increase in interest in femtosecond diffraction methods by the structural biology community. Combined with the availability of hard X-rays, which are more suitable for macromolecular crystallography, LCLS saw a rapid increase in user proposals in the fields of structural biology. LCLS is a user facility which awards beamtime to a particular field of study based on a pro-rata system, which means the quantity of beamtime awarded to structural biology research is proportional to the demand relative to other fields of science. The ultrashort pulses of LCLS represent a unique opportunity for novel science in many fields, which are reviewed separately by panels of experts.
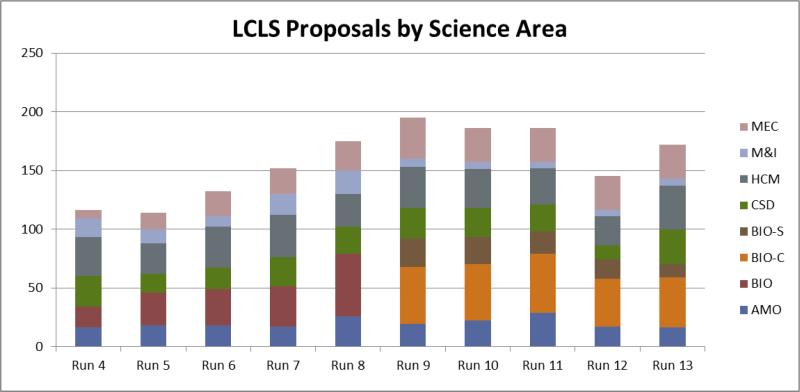
There are currently six main fields of research at LCLS: Atomic, Molecular and Optical (AMO) science, Chemistry and Soft Condensed matter (CSD), Hard Condensed Matter (HCM), Methods & Instrumentation (M&I), Matter in Extreme Conditions (MEC) and biology (BIO). The demand for each type of science can be seen in Figure 1, starting in Run 4, which took place between June and October 2011. Each subsequent run took place roughly six months later. Over the years, the demand in biology increased significantly, primarily due to increased demand in crystallography, which led, in Run 9 (March-August 2014), to a splitting of the biology panel into a BIO-C panel for Crystallography only and BIO-S for spectroscopy, imaging and anything else, revealing which fraction of the proposals aim to use crystallographic techniques. It can be seen how the demand in biology increased rapidly, not only in absolute terms, but the biology share of the proposals also increased dramatically as results were published and interest in LCLS grew. Today, approximately 25% of the proposals LCLS receives are for biological crystallography.
Figure 1.
LCLS demand by science field over the years, starting in Run 4 (June-October 2011) when the current review approach was adopted, up to Run 12 (Oct 2015 – March 2016). Each “run” is roughly six months in duration. The growing demand in the fields of biology (red) led to the splitting into two biology review panels: crystallography (orange) and spectroscopy/imaging (brown). See text for description of acronyms.
This demand for biological crystallography is for two separate systems used at LCLS: the Coherent X-ray Imaging (CXI) instrument [27], which operates in vacuum, and the atmospheric pressure capabilities of the X-ray Pump-Probe (XPP) instrument [28]. The primary use for CXI is with liquid jets of various forms, while the main crystallography use of XPP is for goniometer-based techniques using hardware similar to that of typical synchrotron macromolecular crystallography beamlines, including robotic sample pin exchange [24]. At LCLS, this goniometer-based set-up has been combined with specialized data collection software and high-density sample mounts such as grids [24] or microfluidic traps [29] to increase sample throughput and support efficient serial crystallography experiments of crystals in limited supply. However, these are not the exclusive use of instruments for macromolecular crystallography and other chip-based approaches [14] have been proposed and pursued both in air and in vacuum, as have liquid jets and drop delivery systems, with the emphasis at atmospheric pressure and towards systems delivering samples on demand [30].
There are many similarities between the in-air and in-vacuum approaches for macromolecular crystallography at LCLS and, given the increased demand and a desire to serve more users, further integration of the approaches is necessary. LCLS is now constructing a new beamline to deliver the beam to the new instrument that will greatly increase the access to beamtime for all users.
LCLS Overview
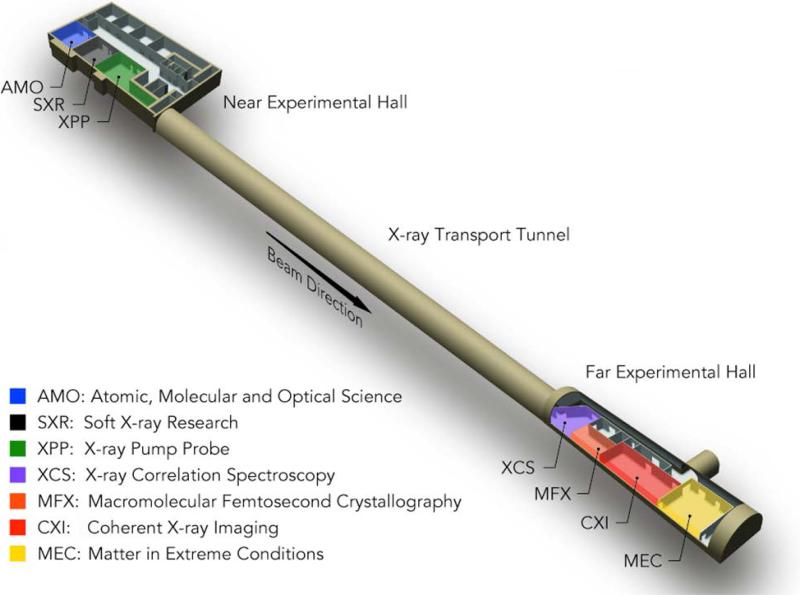
The LCLS facility, due to its required linear design, currently has a single source which must be shared between instruments arranged in-line. Using mirrors to deflect the beam can allow for a small number of separate branch lines to be built, but only a small ultimate number of beam endpoints can exist. The original plan for the facility included six experimental hutches which now house six X-ray instruments [31]. These can be seen in Figure 2. They are located in two separate experimental halls. The Near Experimental Hall (NEH) includes the two soft X-ray instruments: the Atomic, Molecular and Optical (AMO) science instrument in blue and the Soft X-ray Research (SXR) instrument in black. One hard X-ray instrument is also located in the NEH, the XPP instrument in green.
Figure 2.
The LCLS experimental instruments are located in two separate experimental halls, with six existing instruments currently in operation and a seventh under construction: the Macromolecular Femtosecond Crystallography (MFX) instrument in orange.
The Far Experimental Hall (FEH) can only receive the hard X-ray beam, and it contains the X-ray Correlation Spectroscopy (XCS) instrument in purple, the CXI instrument in red, and the Matter in Extreme Conditions (MEC) instrument in gold, in Hutches 4, 5, and 6, respectively. A new instrument, the Macromolecular Femtosecond Crystallography (MFX) instrument, is shown in orange in Figure 2 and will be described in the following sections.
LCLS Operations and Beam Distribution
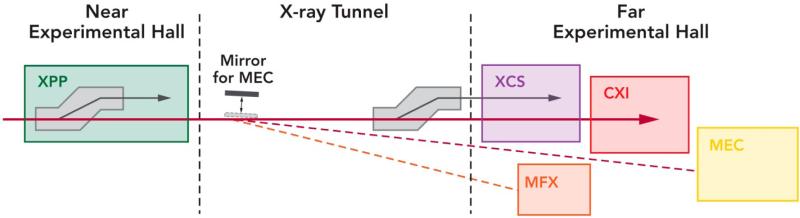
LCLS typically operates two instruments on any given day, each receiving the single beam for a 12-hour shift. The beam can be steered via mirrors or crystals from any instrument to any other in a few seconds, except for the cases when experiments at XPP or XCS use “pink” beam, which at LCLS is meant to represent the full bandwidth of the LCLS beam used on the main beam axis with no monochromator. This typically leads to a bandwidth of up to 1% [32, 33], but can also represent special modes of operation of LCLS such as two-color modes [34] where no monochromator can be used. The hard X-ray beam distribution system is shown in Figure 3 and illustrates the operational challenges of LCLS. The XPP instrument is equipped with a Large Offset Double Cystal Monochromator (LODCM), which allows monochromatic beam experiments to be performed on an axis offset by 600 mm horizontally. This allows a vacuum beam transport pipe to be installed to take the beam to the FEH. The same can be done with an LODCM for the XCS instrument, allowing the beam to propagate to CXI via the motorized removal of the first crystal in the monochromator. However, when XPP or XCS need to use the pink beam, then the entire instrument is moved into the direct beam path and the vacuum pipe taking the beam further downstream is removed. In the case of XCS, use of the pink beam blocks the path to CXI and thus prevents its use without manual reconfiguration of the beamline, which takes several hours. The case of XPP using the pink beam is more problematic, as it blocks the beam completely from going into the Far Experimental Hall hutches (half of the LCLS facility and the entirety of the remaining hard X-ray hutches). With approximately 75% of the demand for LCLS being for the use of hard X-rays, blocking the beam to the FEH is an undesirable limitation.
Figure 3.
Layout of the LCLS hard X-ray beam distribution system. A pair of Large Offset Double Crystal Monochromators (LODCM) can allow the use of the monochromatic beam on a beam axis offset by 600 mm at XPP and XCS. The use of a thin first crystal on these monochromators allows for multiplexing the beam. The MEC mirror will serve the dual use of sending the beam to MEC in Hutch 6 as well as MFX in Hutch 4.5.
While XPP and CXI comprise the vast majority of hard X-ray experiments, they simply cannot operate on the same day and, in practice, cannot typically operate on the same week. The consequence of this limitation is that only one pink beam experiment can be run per week at the most for XPP, XCS, and CXI combined, capping the total fraction of hard X-ray pink beamtime effectively at 25%. Macromolecular crystallography represents by itself 25% of the demand, with another ~12% for other types of biology. Also, many proposals in other fields of science outside biology use the pink beam at XPP or XCS, which, as a consequence, imposes a hard limit to the amount of beamtime that can be allocated to biology and crystallography. The only path to increasing the amount of crystallography and beamtime using the pink beam in general is to remove the operational issues with XPP and XCS blocking the beam to other hutches. This is part of what the MFX project will accomplish.
The MFX Project
The MFX project is adding a new dedicated beamline to deliver the beam into a new Hutch 4.5. As shown in Figure 3, an existing X-ray mirror is used to deflect the beam into a dedicated beamline to the MEC instrument in Hutch 6. This same mirror has been modified to allow a dual use at the existing MEC angle as well as at twice this angle to send the beam to the MFX instrument. A new beamline has been designed and, as of late October 2015, its installation was almost complete. The size of the XCS Hutch 4 was reduced during construction from August to October 2015 to make space for the MFX hutch called Hutch 4.5. MFX will provide a permanent location for a stable and flexible endstation supporting X-ray diffraction experiments in atmosphere. MFX will allow for the optimization of the operations of LCLS in general, but also specifically as it relates to structural biology by relocating many of the experiments from the XPP and XCS instruments.
The intent is to provide a new hutch and beamline by the end of calendar year 2015, allowing experiments to start in mid-2016. This new beamline will allow simultaneous use of XPP in monochromatic mode while using MFX in pink beam mode using thin crystal multiplexing techniques [35]. This will increase the availability of beamtime for users in all science fields by making structural biology experiments previously performed at XPP multiplexable when operated at MFX.
Additionally, rapid switching between MFX and CXI will be possible at the press of a button and possibly automated, removing the current limitations on the number of biology experiments possible at LCLS imposed by the use of XPP in pink beam mode, which prevents such rapid switching to CXI. MFX will also represent a permanent location for a stable endstation dedicated to X-ray diffraction experiments in atmosphere, allowing a system to be in a ready state for opportunistic beamtime should a chance arise. To this end, goniometer-based diffraction experiments will be supported using robotically mounted samples’ cryogenic temperatures, amenable to longer-term storage and mounting at a moment's notice. The endstation will be very flexible to also support experiments at physiological temperatures as well as a variety of other experimental set-ups, including crystal injectors.
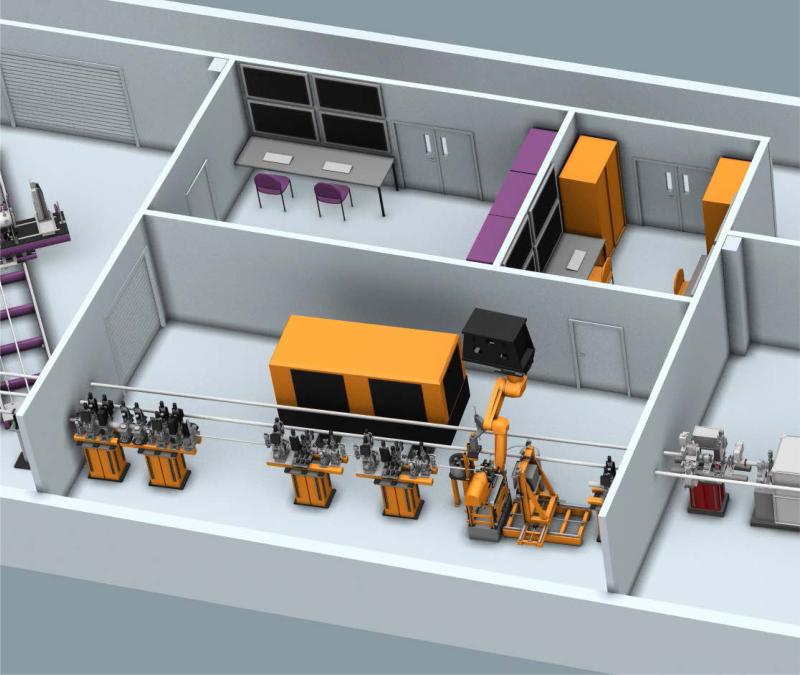
Work is underway to develop the MFX endstation. The first stage of this process is to provide a versatile platform for a broad range of experiments. This will include a six-degrees-of-freedom table to support multiple sample environments. It will also include a large detector mover capable of accurate positioning of a large area detector typical for macromolecular crystallography beamlines. It will also include a ceiling-mounted detector robot similar to that used in the XPP instrument [28]. This detector robot will provide additional flexibility to the MFX instrument. A conceptual design of the endstation system is shown in Figure 4.
Figure 4.
Conceptual schematic of the MFX endstation with adjacent control room (top right). The beam travels from the left. Four stands support beamline components followed by the endstation. A versatile table will support various sample environments, which will include capabilities for goniometer-based crystallography and robotic sample exchange. A detector mover will be capable of positioning a large format detector and a ceiling mounted robot will provide ultimate flexibility in detector positioning for various applications. A future laser enclosure is planned for time-resolved experiments.
Once the support table, detector mover, and detector robot are operational, the LCLS facility will begin to schedule suitable user experiments in the MFX hutch, and the likely scenario is for some experiments historically performed at XPP to move to MFX. For example, goniometer-based crystallography experiments have, to date, taken place in the XPP hutch. A system developed and supported by the Structural Molecular Biology (SMB) group at the Stanford Synchrotron Radiation Lightsource (SSRL) has been installed, aligned and used at XPP for every LCLS run since late 2010 [24]. A schematic of the system is shown in Figure 5. Each time this system is in use, typically for one to two weeks in duration each experimental run, it needs to be completely removed to make way for different experimental setups at XPP. MFX is being designed to allow these or similar components to remain in the experimental hutch as permanently as possible, greatly reducing the amount of effort to perform any given experiment. Furthermore, it will remove scheduling limitations that allowed for only one or two weeks of use of the system for every five months of LCLS operations. Greater access to beamtime will be achieved not only for users who wish to use goniometer-based macromolecular crystallography techniques, but also to all users via multiplexing and more efficient use of beamtime owing to a fixed set-up.
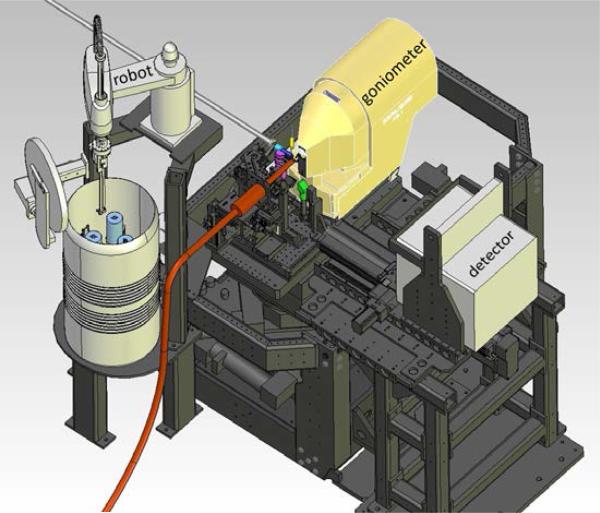
Figure 5.
Schematic representation of the SSRL-SMB goniometer-based macromolecular crystallography system showing the micro-crystal goniometer, SAM sample exchange robot [36], cryogenic cooler nozzle (orange), and large area X-ray detector. A similar system will eventually be permanently installed in the MFX hutch. Temporary installation of this system is likely at MFX.
Beyond goniometer-based crystallography, MFX will also provide possibilities for using liquid jets at atmospheric pressure. In due course, all of these capabilities are expected to be fully supported and maintained by the LCLS facility for easy access to all users. The system is being designed with versatility in mind to allow multiple techniques such as small- and wide-angle scattering, X-ray emission spectroscopy, and eventually, with the addition of a laser system, a variety of time-resolved techniques.
The MFX instrument is expected to begin operation in the second half of 2016 and will have a phased operations program. The end result will make MFX a state-of-the-art X-ray FEL instrument capable of most techniques in use in structural biology, allowing for LCLS to be used to solve unique, challenging problems that cannot be solved using traditional methods.
Acknowledgments
Funding for the MFX beamline construction was provided by the U.S. Department of Energy, Office of Science, Biological and Environmental Research via the SLAC Mesoscale Integrated Biology Pilot Project. Further funding was provided by the Linac Coherent Light Source (LCLS), SLAC National Accelerator Laboratory, supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract no. DE-AC02-76SF00515. The initial funding for the MFX endstation was provided by the National Institute of General Medical Sciences (NIGMS). The Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (including P41GM103393). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
References
- 1.Bernstein FC, et al. Journal of Molecular Biology. 1977;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- 2.Emma P, et al. Nature Photonics. 2010;4(9):641–647. [Google Scholar]
- 3.Neutze R, et al. Nature. 2000;406(6797):752–757. doi: 10.1038/35021099. [DOI] [PubMed] [Google Scholar]
- 4.DePonte DP, et al. Journal of Physics D-Applied Physics. 2008;41(19) [Google Scholar]
- 5.Chapman HN, et al. Nature. 2011;470(7332):73–U81. doi: 10.1038/nature09750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seibert MM, et al. Nature. 2011;470(7332):78–U86. doi: 10.1038/nature09748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutet S, et al. Science. 2012;337(6092):362–364. doi: 10.1126/science.1217737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Botha S, et al. Acta Crystallographica Section D-Biological Crystallography. 2015;71:387–397. doi: 10.1107/S1399004714026327. [DOI] [PubMed] [Google Scholar]
- 9.Nogly P, et al. IUCrJ. 2015;2(2) [Google Scholar]
- 10.Sierra RG, et al. Acta Crystallographica Section D-Biological Crystallography. 2012;68:1584–1587. doi: 10.1107/S0907444912038152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weierstall U, et al. Nature Communications. 2014;5 doi: 10.1038/ncomms4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redecke L, et al. Science. 2013;339(6116):227–230. doi: 10.1126/science.1229663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barends TRM, et al. Nature. 2014;505(7482):244. doi: 10.1038/nature12773. [DOI] [PubMed] [Google Scholar]
- 14.Hunter MS, et al. Scientific Reports. 2014;4 doi: 10.1038/srep06026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawaya MR, et al. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(35):12769–12774. doi: 10.1073/pnas.1413456111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uervirojnangkoorn M, et al. Elife. 2015;4 doi: 10.7554/eLife.05421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlichting I. IUCRJ. 2015;2:246–255. doi: 10.1107/S205225251402702X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang YY, et al. Nature. 2015;523(7562):561. doi: 10.1038/nature14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W, et al. Science. 2013;342(6165):1521–1524. doi: 10.1126/science.1244142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang HT, et al. Cell. 2015;161(4):833–844. doi: 10.1016/j.cell.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kern J, et al. Nature Communications. 2014;5 [Google Scholar]
- 22.Kupitz C, et al. Nature. 2014;513(7517):261. doi: 10.1038/nature13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenboer J, et al. Science. 2014;346(6214):1242–1246. doi: 10.1126/science.1259357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen AE, et al. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(48):17122–17127. doi: 10.1073/pnas.1418733111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou QJ, et al. Nature. 2015;525(7567):62. doi: 10.1038/nature14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keedy DA, et al. Mapping the Conformational Landscape of a Dynamic Enzyme by Multitemperature and XFEL Crystallography. eLife. 2015 doi: 10.7554/eLife.07574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang M, et al. Journal of Synchrotron Radiation. 2015;22(3):514–519. doi: 10.1107/S160057751500449X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chollet M, et al. Journal of Synchrotron Radiation. 2015;22(3):503–507. doi: 10.1107/S1600577515005135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyubimov AY, et al. Acta Crystallographica Section D-Biological Crystallography. 2015;71:928–940. doi: 10.1107/S1399004715002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roessler CG, et al. Journal of Synchrotron Radiation. 2013;20:805–808. doi: 10.1107/S0909049513020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White WE, Robert A, Dunne M. Journal of Synchrotron Radiation. 2015;22:472–476. doi: 10.1107/S1600577515005196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu D, et al. Applied Physics Letters. 2012;101(3):034103. [Google Scholar]
- 33.Feng Y, et al. A Hard X-ray Transmissive Single-shot Spectrometer for FEL Sources. 2012 [Google Scholar]
- 34.Marinelli A, et al. Nature Communications. 2015;6 doi: 10.1038/ncomms7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng Y, et al. Journal of Synchrotron Radiation. 2015;22(3):626–633. doi: 10.1107/S1600577515003999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen AE, et al. Journal of Applied Crystallography. 2002;35:720–726. doi: 10.1107/s0021889802016709. [DOI] [PMC free article] [PubMed] [Google Scholar]