Abstract
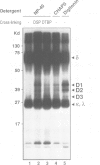
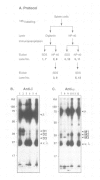
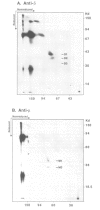
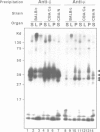
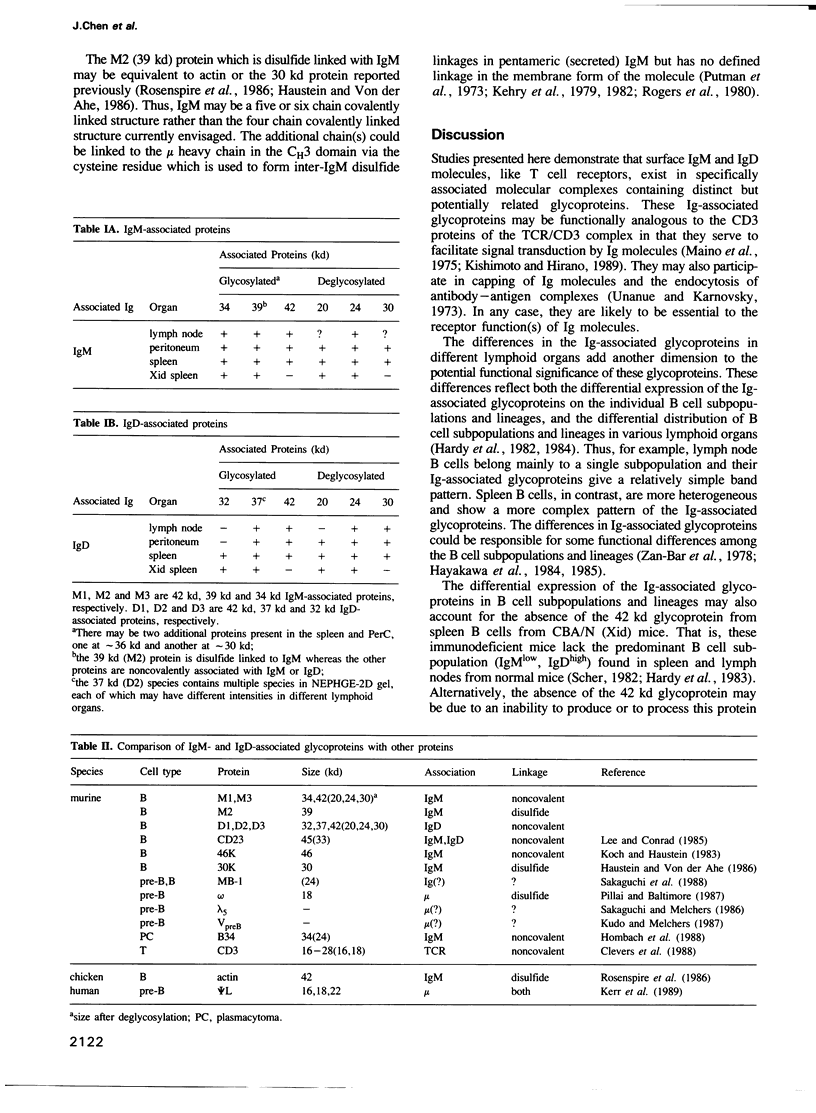
Studies presented here demonstrate that IgM and IgD molecules on normal murine B lymphocytes exist in different, noncovalently associated molecular complexes containing distinct but potentially related glycoproteins. The glycoproteins in these complexes, particularly those associated with IgD, show striking differences in various lymphoid organs and in X-linked immunodeficient (Xid) mice. These differences are due in part to post-translational processing. They apparently reflect the differential expression of the Ig-associated glycoproteins in the various B cell subpopulations and lineages and the differential distribution of the subpopulations and lineages in the various lymphoid organs. In addition, they reflect structural differences in the IgM and IgD complexes which, we suggest, permit differential signal transduction by IgM and IgD on the same B cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A., Scher I., Sharrow S. O., Smith A. H., Paul W. E., Sachs D. H., Sell K. W. B-lymphocyte heterogeneity: development and characterization of an alloantiserum which distinguishes B-lymphocyte differentiation alloantigens. J Exp Med. 1977 Jan 1;145(1):101–110. doi: 10.1084/jem.145.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier J. C., Ransom J. T. Molecular mechanisms of transmembrane signaling in B lymphocytes. Annu Rev Immunol. 1987;5:175–199. doi: 10.1146/annurev.iy.05.040187.001135. [DOI] [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Cohen D. I., Hedrick S. M., Nielsen E. A., D'Eustachio P., Ruddle F., Steinberg A. D., Paul W. E., Davis M. M. Isolation of a cDNA clone corresponding to an X-linked gene family (XLR) closely linked to the murine immunodeficiency disorder xid. 1985 Mar 28-Apr 3Nature. 314(6009):369–372. doi: 10.1038/314369a0. [DOI] [PubMed] [Google Scholar]
- Cohen D. I., Steinberg A. D., Paul W. E., Davis M. M. Expression of an X-linked gene family (XLR) in late-stage B cells and its alteration by the xid mutation. 1985 Mar 28-Apr 3Nature. 314(6009):372–374. doi: 10.1038/314372a0. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Jorgensen H., Brink R. A., Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989 Nov 23;342(6248):385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Haaijman J., Herzenberg L. A. B-cell subpopulations identified by two-colour fluorescence analysis. Nature. 1982 Jun 17;297(5867):589–591. doi: 10.1038/297589a0. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Parks D. R., Herzenberg L. A. Demonstration of B-cell maturation in X-linked immunodeficient mice by simultaneous three-colour immunofluorescence. Nature. 1983 Nov 17;306(5940):270–272. doi: 10.1038/306270a0. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Parks D. R., Herzenberg L. A., Herzenberg L. A. Murine B cell differentiation lineages. J Exp Med. 1984 Apr 1;159(4):1169–1188. doi: 10.1084/jem.159.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haustein D., Von der Ahe D. A 30-kDa protein is disulfide linked to IgM on normal and neoplastic murine B lymphocytes. Eur J Immunol. 1986 Jan;16(1):113–115. doi: 10.1002/eji.1830160122. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach J., Leclercq L., Radbruch A., Rajewsky K., Reth M. A novel 34-kd protein co-isolated with the IgM molecule in surface IgM-expressing cells. EMBO J. 1988 Nov;7(11):3451–3456. doi: 10.1002/j.1460-2075.1988.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber B., Gershon R. K., Cantor H. Identification of a B-cell surface structure involved in antigen-dependent triggering: absence of this structure on B cells from CBA/N mutant mice. J Exp Med. 1977 Jan 1;145(1):10–20. doi: 10.1084/jem.145.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehry M. R., Fuhrman J. S., Schilling J. W., Rogers J., Sibley C. H., Hood L. E. Complete amino acid sequence of a mouse mu chain: homology among heavy chain constant region domains. Biochemistry. 1982 Oct 26;21(22):5415–5424. doi: 10.1021/bi00265a006. [DOI] [PubMed] [Google Scholar]
- Kehry M., Sibley C., Fuhrman J., Schilling J., Hood L. E. Amino acid sequence of a mouse immunoglobulin mu chain. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2932–2936. doi: 10.1073/pnas.76.6.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr W. G., Cooper M. D., Feng L., Burrows P. D., Hendershot L. M. Mu heavy chains can associate with a pseudo-light chain complex (psi L) in human pre-B cell lines. Int Immunol. 1989;1(4):355–361. doi: 10.1093/intimm/1.4.355. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Mosher D. F., Vaheri A. Dimeric character of fibronectin, a major cell surface-associated glycoprotein. Biochem Biophys Res Commun. 1977 Jan 24;74(2):699–706. doi: 10.1016/0006-291x(77)90359-x. [DOI] [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Sun L., Watanabe T. Monoclonal rat antibodies to murine IgM determinants. J Immunol Methods. 1981;42(1):17–26. doi: 10.1016/0022-1759(81)90220-9. [DOI] [PubMed] [Google Scholar]
- Koch N., Haustein D. Association of surface IgM with two membrane proteins on murine B lymphocytes detected by chemical crosslinking. Mol Immunol. 1983 Jan;20(1):33–37. doi: 10.1016/0161-5890(83)90102-5. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Kudo A., Melchers F. A second gene, VpreB in the lambda 5 locus of the mouse, which appears to be selectively expressed in pre-B lymphocytes. EMBO J. 1987 Aug;6(8):2267–2272. doi: 10.1002/j.1460-2075.1987.tb02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Federspiel N. A., Ruitenberg J. J., Phillips J. H., Allison J. P., Littman D., Weiss A. The T cell antigen receptor complex expressed on normal peripheral blood CD4-, CD8- T lymphocytes. A CD3-associated disulfide-linked gamma chain heterodimer. J Exp Med. 1987 Apr 1;165(4):1076–1094. doi: 10.1084/jem.165.4.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Gutman G. A., Lewis D. E., Griswold S. T., Warner N. L. Monoclonal antibodies against rat immunoglobulin kappa chains. Hybridoma. 1982;1(2):125–131. doi: 10.1089/hyb.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- Lee W. T., Conrad D. H. The murine lymphocyte receptor for IgE. III. Use of chemical cross-linking reagents to further characterize the B lymphocyte Fc epsilon receptor. J Immunol. 1985 Jan;134(1):518–525. [PubMed] [Google Scholar]
- Maino V. C., Hayman M. J., Crumpton M. J. Relationship between enhanced turnover of phosphatidylinositol and lymphocyte activation by mitogens. Biochem J. 1975 Jan;146(1):247–252. doi: 10.1042/bj1460247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettgen H. C., Pettey C. L., Maloy W. L., Terhorst C. A T3-like protein complex associated with the antigen receptor on murine T cells. Nature. 1986 Mar 20;320(6059):272–275. doi: 10.1038/320272a0. [DOI] [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulin spots on the surface of rabbit lymphocytes. J Exp Med. 1970 Nov;132(5):1001–1018. doi: 10.1084/jem.132.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai S., Baltimore D. Formation of disulphide-linked mu 2 omega 2 tetramers in pre-B cells by the 18K omega-immunoglobulin light chain. Nature. 1987 Sep 10;329(6135):172–174. doi: 10.1038/329172a0. [DOI] [PubMed] [Google Scholar]
- Putnam F. W., Florent G., Paul C., Shinoda T., Shimizu A. Complete amino acid sequence of the Mu heavy chain of a human IgM immunoglobulin. Science. 1973 Oct 19;182(4109):287–291. doi: 10.1126/science.182.4109.287. [DOI] [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Rosenspire A. J., Lee M. S., Pollak S. V., Choi Y. S. Disulfide linkages between antigen-binding receptors on chicken B-lymphocytes. Mol Immunol. 1986 Jan;23(1):1–13. doi: 10.1016/0161-5890(86)90166-5. [DOI] [PubMed] [Google Scholar]
- SELL S., GELL P. G. STUDIES ON RABBIT LYMPHOCYTES IN VITRO. I. STIMULATION OF BLAST TRANSFORMATION WITH AN ANTIALLOTYPE SERUM. J Exp Med. 1965 Aug 1;122:423–440. doi: 10.1084/jem.122.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N., Kashiwamura S., Kimoto M., Thalmann P., Melchers F. B lymphocyte lineage-restricted expression of mb-1, a gene with CD3-like structural properties. EMBO J. 1988 Nov;7(11):3457–3464. doi: 10.1002/j.1460-2075.1988.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N., Melchers F. Lambda 5, a new light-chain-related locus selectively expressed in pre-B lymphocytes. Nature. 1986 Dec 11;324(6097):579–582. doi: 10.1038/324579a0. [DOI] [PubMed] [Google Scholar]
- Scher I. The CBA/N mouse strain: an experimental model illustrating the influence of the X-chromosome on immunity. Adv Immunol. 1982;33:1–71. doi: 10.1016/s0065-2776(08)60834-2. [DOI] [PubMed] [Google Scholar]
- Sidman C. L., Unanue E. R. Receptor-mediated inactivation of early B lymphocytes. Nature. 1975 Sep 11;257(5522):149–151. doi: 10.1038/257149a0. [DOI] [PubMed] [Google Scholar]
- Stall A. M., Loken M. R. Allotypic specificities of murine IgD and IgM recognized by monoclonal antibodies. J Immunol. 1984 Feb;132(2):787–795. [PubMed] [Google Scholar]
- Subbarao B., Ahmed A., Paul W. E., Scher I., Lieberman R., Mosier D. E. Lyb-7, a new B cell alloantigen controlled by genes linked to the IgCH locus. J Immunol. 1979 Jun;122(6):2279–2285. [PubMed] [Google Scholar]
- Tedder T. F., Schlossman S. F. Phosphorylation of the B1 (CD20) molecule by normal and malignant human B lymphocytes. J Biol Chem. 1988 Jul 15;263(20):10009–10015. [PubMed] [Google Scholar]
- Unanue E. R., Karnovsky M. J. Redistribution and fate of Ig complexes on surface of B lymphocytes: functional implications and mechanisms. Transplant Rev. 1973;14:184–210. doi: 10.1111/j.1600-065x.1973.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Zan-Bar I., Vitetta E. S., Assisi F., Strober S. The relationship between surface immunoglobulin isotype and immune function of murine B lymphocytes. III. Expression of a single predominant isotype on primed and unprimed B cells. J Exp Med. 1978 May 1;147(5):1374–1394. doi: 10.1084/jem.147.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]