Abstract
Although hyperhomocysteinemia (HHcy) is an independent risk factor for cardiovascular diseases (CVD), there is a debate on whether HHcy is a risk factor or just a biomarker. Interestingly, homocysteine lowering strategies in humans had very little effect on reducing the cardiovascular risk, as compared with animals; this may suggest heterogeneity in human population and epigenetic alterations. Moreover, there are only few studies that suggest the idea that HHcy contributes to CVD in the presence of other risk factors such as inflammation, a known risk factor for CVD. Elevated levels of homocysteine have been shown to contribute to inflammation. Here, we highlight possible relationships between homocysteine, T cell immunity, and hypertension, and summarize the evidence that suggested these factors act together in increasing the risk for CVD. In light of this new evidence, we further propose that there is a need for evaluation of the causes of HHcy, defective remethylation or defective transsulfuration, which may differentially modulate hypertension progression, not just the homocysteine levels.
Keywords: hyperhomocysteinemia, T cells, inflammation, hypertension, cardiovascular disease
Introduction
Hyperhomocysteinemia (HHcy) is a clinical condition characterized by elevated plasma homocysteine levels above the normal range (5–15 μmol/L). It can be further classified into moderate and severe HHcy where the plasma homocysteine levels range between 16 and 100 μmol/L and more than 100 μmol/L, respectively (Dinavahi and Falkner 2004). Moderate elevation in plasma homocysteine levels is often caused by excess methionine intake or vitamin B deficiency or polymorphisms in the genes regulating methionine metabolism (Brattstrom et al. 1998; Davis et al. 2005). On the other hand, severe HHcy is often caused by deficiencies in the enzymes metabolizing homocysteine through demethylation and transsulfuration pathways or impaired excretion of homocysteine through kidneys (Friedman et al. 2001; Malinowska and Chmurzynska 2009; Selhub 1999). For instance, rare genetic mutations in the cystathionine-β-synthase (CBS) gene cause plasma homocysteine levels to reach as high as 200 μmol/L (Kruger et al. 2003) and these mutations are associated with several abnormalities including atherosclerosis, coronary artery diseases, venous thrombosis, birth defects, osteoporosis, and liver complications (Cattaneo 1997; Selhub 1999). Positive correlation between HHcy and cardiovascular diseases led to the postulation that homocysteine is an independent risk factor for cardiovascular diseases (McCully 2005). However, most if not all therapeutic strategies aimed at decreasing homocysteine levels had very little effect on reducing the cardiovascular risk (Cattaneo 2001; Dinavahi and Falkner 2004). Elevated oxidative stress has been shown as a key mechanism underlying the various cardiovascular pathologies including those associated with HHcy; hence, it is assumed that antioxidants could be a cure for the cardiovascular pathologies. Interestingly, antioxidant therapy in the cardiovascular patients itself yielded unconvincing or paradoxical results (Katsiki and Manes 2009; Steinhubl 2008). These findings not only revealed the complexity of cardiovascular etiology, but also indicated various confounding predisposing factors such as genetic differences, environment, and interactions between genetics and environment, which could be behind the failure of HHcy correction strategies. Altogether, the above findings have raised debate on homocysteine theory and given birth to an alternative hypothesis suggesting that homocysteine can be a risk factor in the presence of other cardiovascular disease risk factors and may cause increased cardiovascular damage in certain genetic backgrounds after a certain age and with certain habits. Supporting this notion, studies have found a positive correlation between HHcy and other known cardiovascular risk factors such as inflammation (El Oudi et al. 2011; Lazzerini et al. 2007) and hypertension (Arroliga et al. 2003; Dinavahi and Falkner 2004; Sabio et al. 2014; Sutton-Tyrrell et al. 1997), suggesting the possibility that these 3 cardiovascular risk factors may together contribute to cardiovascular disease pathology. In this review, we aimed to better understand the relationship between HHcy, hypertension, and inflammation, and proposed therapeutic approaches that target these pathways together may be beneficial in reducing the cardiovascular disease risk.
Homocysteine metabolism
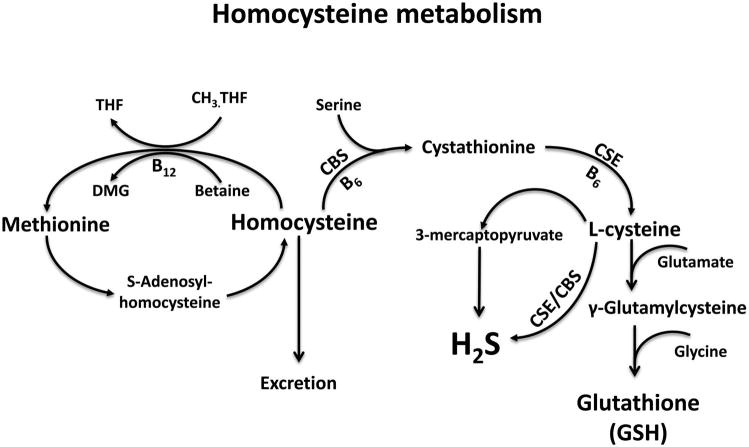
Homocysteine is a sulfhydryl containing non-protein coding amino acid formed mainly as a byproduct in the methionine metabolism (Selhub 1999). In this pathway, methionine donates its methyl group in a multi-step methyl transfer reaction and forms a stable intermediate product, S-adenosylhomocysteine (SAH), which further undergoes hydrolysis to yield homocysteine. The resulting homocysteine, at this juncture, has multiple fates (Fig. 1): (1) clearance by liver and kidneys for excretion (Friedman et al. 2001); (2) conversion back into methionine by accepting a methyl group from betaine or methyl tetrahydrofolate, although the reaction is biased towards homocysteine; or (3) entry into an irreversible transsulfuration pathway with serine to form cystathionine, which further gets converted to cysteine and α-ketobutyrate. The cysteine thus formed in the latter case can feed into pathways generating important gasotransmitter H2S and antioxidant glutathione (GSH) (Sen et al. 2014). Numerous studies have shown that disruptions in the pathways synthesizing or clearing homocysteine can contribute to HHcy.
Fig. 1.
Major steps in homocysteine metabolism. Schematic representation of key steps in homocysteine metabolism. CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; CH3. THF, 5-methyl tetrahydrofolate; DMG, dimethylglycine; THF, methenyltetrahydrofolate; B12, vitamin B12; B6, vitamin B6.
Homocysteine and hypertension
Hypertension refers to a systemic increase in resistance to the arterial blood flow culminating in increased arterial blood pressure, which is a well-known risk factor for cardiovascular diseases. Current estimates predict nearly a billion people worldwide are at the risk of developing hypertension or have been diagnosed with hypertension. The etiology of hypertension is still not well defined and is a major topic of investigation, although it is accepted that the disease is multifactorial in nature and any factor that can alter the vascular properties and (or) osmotic regulation and hemodynamics can contribute to hypertension (Trott et al. 2014). HHcy is one such factor that has gained significant importance due to its ability to affect vascular endothelial cells and smooth muscle cells that maintain the integrity of the blood vessel as well as renal function (Li et al. 2002; Narayanan et al. 2014; Sen et al. 2009). Numerous studies have clearly demonstrated a strong correlation between HHcy and hypertension (Lip et al. 2001; Nygard et al. 1995; Sutton-Tyrrell et al. 1997). It has been estimated that roughly half of the systolic hypertensive individuals have HHcy values above 11 μmol/L (Sutton-Tyrrell et al. 1997). Other studies also identified positive correlation between HHcy and hypertension, especially in males (e.g., Nygard et al. 1995). However, the later longitudinal prospective cohort studies that examined the causal role of HHcy in the development of hypertension revealed that the risk of hypertension development did not vary much between high HHcy and low HHcy groups (e.g., Sundstrom et al. 2003). Other similar studies have also found non significant increase in the risk of hypertension with higher HHcy concentrations (Bowman et al. 2006; Wang et al. 2014). Although the cause and effect relationship between homocysteine and hypertension is still in its infancy, existing evidence points out several possible underlying mechanisms including: (1) loss of blood vessel vasorelaxation due to homocysteine induced cytotoxic effects on smooth muscle cells and endothelial cells (Blundell et al. 1996), which are crucial to maintain vascular contractility; (2) impaired endothelial cell mediated nitric oxide production (Jiang et al. 2005; Stuhlinger et al. 2001; Yang et al. 2013) that plays a critical role in vasorelaxation; (3) homocysteine induced alterations in the elastin/collagen ratio (Crile 1978; Steed and Tyagi 2011), which can compromise vascular elasticity or responsiveness. More recently, inflammation, in particular altered T cell immune responses, has been implicated in the pathology of hypertension (Harrison et al. 2011; Trott and Harrison 2014; Wenzel et al. 2016). Because homocysteine can also alter both inflammation and T cell immune response, it is possible that homocysteine may contribute to the hypertension through modulating immune response.
Homocysteine-mediated regulation of inflammation
Numerous studies have demonstrated a positive correlation between HHcy and increased inflammatory markers in various disease conditions (El Oudi et al. 2011; Lazzerini et al. 2006; Oudi et al. 2010; Zhu et al. 2015). In addition, these studies also prompted new investigations mainly focusing on understanding the underlying mechanisms for homocysteine regulation of inflammation. In vitro studies have implicated the existence of multiple mechanisms through which homocysteine can modulate inflammatory responses. For instance, homocysteine can induce inflammatory cytokine or chemokine production in monocyte and endothelial cultures (Meng et al. 2013; Poddar et al. 2001; Postea et al. 2008; Wang and O 2001). In these studies, homocysteine seems to mediate its effects indirectly through reactive oxygen species generation (ROS) and (or) PPAR-γ activation, both of which are known modulators of inflammation. In addition to these indirect mechanisms, homocysteine can also activate NFκB transcription factor (Wang et al. 2000), which is known to drive inflammatory gene expression in immune cells. It should be noted that, while these studies shed light on the potential pathways that can be targeted by HHcy, it remains to be tested whether all these pathways play a major role in driving inflammation in vivo. Indeed, there is some progress in animal models of inflammation with regard to the role of HHcy in causation of inflammation (Flannigan et al. 2014; Zhu et al. 2015). For instance, HHcy was shown to aggravate the colon inflammation through the enhancement in the IL-17 cytokine levels, which was also observed during hypertension with abnormally activated T cell presence (Kirabo et al. 2014; Zhu et al. 2015). Nonetheless, it remains to be seen whether such HHcy-induced inflammatory changes also contribute to the development of hypertension.
Metabolic requirements of activated T cell proliferation and putative regulation by homocysteine levels in causation of hypertension
Proper activation and function of T cells are crucial for the body’s ability to fight infections without causing significant damage to the host tissue. One of the hallmarks of T cell immune response is the activation of a rare population of antigen specific T cells and their massive expansion and differentiation to produce effector cells and memory cells that can clear the pathogen and establish long-term protection against re-infections (Kaech et al. 2002). Although antigen recognition is sufficient to initiate T cell receptor signaling on T cells, the fate of antigen-stimulated T cells is dictated by a complex network of co-stimulatory, co-inhibitory, and cytokine signals that precisely direct the T cell function, which is meant to protect the host (Grossman et al. 2004; Smith-Garvin et al. 2009). Studies have shown that these complex signaling pathways are highly sensitive to additional signals in the microenvironment including metabolites, cell stressors, and ROS, etc.
The metabolic requirements of T cells are dynamic and change dramatically with the activation, where the biosynthetic pathways are biased over ATP generating pathways that dominate in naïve T cells. Transport of certain amino acids is also significantly upregulated in T cells following activation (Garg et al. 2011) to meet the biosynthetic demand. Hence, activation of T cells is highly susceptible to micro-nutrient/metabolite availability in the T cell microenvironment. For instance, limiting tryptophan during T cell activation significantly affects the T cell proliferation following activation (Gmunder et al. 1991). T cells are one of the few cells that exhibit dependency on cysteine supply for their metabolic needs. Naïve T cells have relatively low intracellular cysteine mainly due to the lack of cystine transporters to bring external cysteine and due to the lack of efficient endogenous transsulfuration pathways to produce cysteine; thus making cysteine a limiting factor during T cell activation. It has been shown that antigen presenting cells fulfill the cysteine requirements of T cells during activation (Garg et al. 2011). However, following stimulation, T cells overcome the cysteine dependency through upregulation of cystine transporters and the components of transsulfuration pathway. The main requirement of cysteine for T cells is to make glutathione, which has been shown to be essential for optimal T cell expansion following stimulation (Garg et al. 2011). In addition, numerous studies have shown that glutathione plays an important role in modulating T cell proliferation following activation (Fidelus et al. 1987; Hadzic et al. 2005). Glutathione is a tripeptide, which plays an important role in protecting cells from oxidative damage caused by the hydroperoxides produced during redox reactions. Under physiological conditions, a majority of the glutathione in the cells exists as reduced form, which rapidly gets utilized by enzymatic redox reactions, thus leading to the formation of oxidized glutathione (GSSG). This oxidized form glutathione can be reduced back to GSH through NAPDH-mediated pathways that resupply the cells with GSH to maintain the redox balance. Alternatively, cells can de novo synthesize GSH through enzymatic reactions utilizing glycine, cysteine, and glutamate. It is counterintuitive in the sense that during T cell activation, there is an upregulation of xC–cystine transporter (imports one cystine for every one glutamate export) to bring cystine into the cells at the expense of glutamate, which is equally necessary for the synthesis of GSH along with cysteine and glycine. It is possible that availability of cysteine rather than glutamate is the rate limiting step in the synthesis of GSH especially in T cells (Garg et al. 2011; Srivastava et al. 2010). Though not yet clear, the apparent lack of glutamate dependency for GSH synthesis could be because glutamate is present at much higher levels intracellularly in T cells or there are other transporters, which might compensate for glutamate exit through xC–cystine transporter.
Although homocysteine has been implicated in inflammatory responses, involvement of homocysteine on modulating T cell immune response is still emerging. However, based on the metabolic requirements of T cells, it may not be surprising that homocysteine can also influence T cell immune responses and, hence, may contribute to T cell mediated hypertension development. Consistent with this idea, Zhang et al. (2002) saw that homocysteine indeed increased the expansion of lectin stimulated T cells and increased their ability to produce IFN-γ. In addition, other studies have shown that homocysteine can increase (1) T cell adhesion on endothelial cells (Koga et al. 2002); (2) alter co-stimulatory signals on T cells (Ma et al. 2013); and (3) affect total lymphocyte counts in the circulation (Fefelova et al. 2015). One of the major factors driving the T cell proliferation following activation is the availability of IL-2 that is produced by activated T cells themselves. The Zhang et al. (2002) study, which tested the homocysteine’s ability to drive IL-2 production by the T cells, showed no significant difference, suggesting homocysteine may act through IL-2 independent mechanisms.
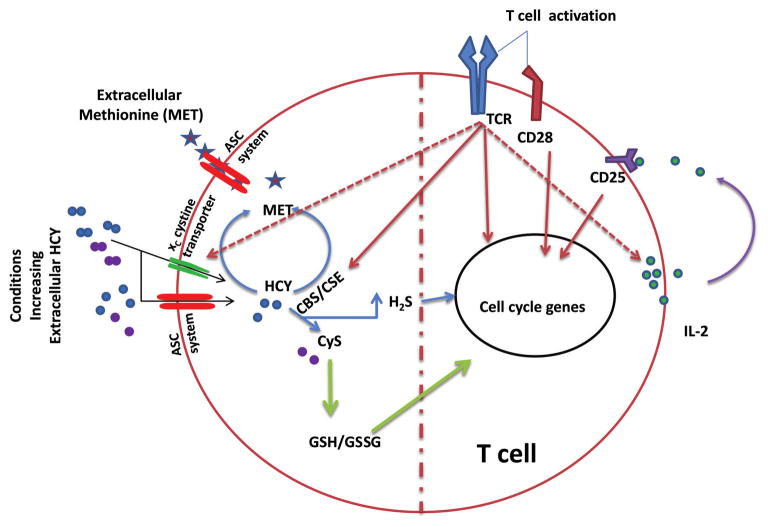
Potential mechanisms underlying homocysteine-mediated proliferative effects on activated T cells may involve cysteine import and de novo cysteine synthesis. Interestingly, homocysteine and homocystine also use the cysteine (ASC transporter: alanine–serine–cysteine transporter) and cystine (xC–cystine transporter) transporters, respectively, to gain entry into the endothelial cells (Budy et al. 2006), which may be the case in the T cells as well (Fig. 2), as these cells also express these transporters (Garg et al. 2011). Hence, it is possible that HHcy may competitively block cysteine/cystine import in a concentration dependent manner. Once inside the cell, homocysteine may either stimulate or inhibit cysteine synthesis based on the presence of intact or deficient CBS/cystathionine γ-lyase (CSE) enzyme function, respectively. Hence, whether HHcy is stimulatory or inhibitory to the activated T-cell proliferation and development of hypertension may depend on the presence of intact CBS/CSE enzyme function, which is necessary for conversion of homocysteine through the transsulfuration pathway to produce cysteine and H2S (Figs. 1 and 2). Suppression of these enzymes (CBS/CSE) not only inhibits cystine entry through xC–cystine transporter by creating HHcy and inhibiting cystine entry into the cells (competitive inhibition), but also compromises de novo cysteine synthesis through the transsulfuration pathway, thereby inhibiting activated T cell proliferation and prevention of hypertension development. In naïve T cells, HHcy resulting from mutations in CBS/CSE enzymes may enhance oxidative stress susceptibility, by inhibiting de novo cysteine synthesis and cystine/cysteine import. However, with the intact CBS/CSE enzyme function, HHcy resulting from the deficiencies in remethylation pathway (Veeranki and Tyagi 2013) may actually promote activated T cell proliferation by stimulating transsulfuration pathway, which may be the case in the previous study (Zhang et al. 2002). This possible duality of HHcy, suppression or activation of T cell proliferation depending on the intact CBS/CSE enzyme function, could be the main reason behind the observed discrepancies with the HHcy mediated hypertension development. Further support for such a hypothesis comes from the fact that production of H2S, which also stimulates T cell proliferation (please see below), is potentially limited only in HHcy resulting from inhibition of CBS/CSE function but not in HHcy resulting from the other causes. Moreover, the type of vitamin B deficiency also influences which of the 2 pathways, the transsulfuration pathway or the remethylation pathway, that clear HHcy are compromised (Veeranki and Tyagi 2013). Though B6, B9, and B12 deficiencies can all produce HHcy, only the B6 deficiency can limit the transsulfuration pathway resulting in cysteine and H2S production suppression; the B9 and B12 deficiency limits remethylation pathway, possibly without decline in cysteine and H2S production. In light of the new evidence that underscored T cell dependent regulation of hypertension and HHcy mediated T cell proliferation and possible duality of HHcy in T cell expansion (as proposed above), careful assessment of underlying HHcy causes (which pathway affected: transsulfuration or remethylation) not just homocysteine levels in patients progressing into the hypertension state may solve the conundrum of HHcy-mediated hypertension: a bystander or the cause. Future studies are necessary to test these possibilities.
Fig. 2.
Homocysteine mediated modulation of T cell immune response. Under physiological conditions, antigen stimulation of T cell receptor (TCR), engagement of cluster of differentiation 28 (CD28) by co-stimulatory molecules on antigen presenting cells provide the necessary signals for full activation of T cells that enter into the cell cycle. IL-2 produced by the activated T cells in autocrine fashion further influence the extent to which T cells divide. Interestingly, hyperhomocysteinemia, although it enhances the proliferation of activated T cells, does not augment IL-2 secretion by the T cells. Activation of T cells also upregulates important enzymes, cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), in the methionine (MET) cycle. Under these conditions, we propose extracellular MET or homocysteine (HCY) can feed into MET metabolism in T cells and result in the production of L-cysteine (CyS) intracellularly. Intracellular cysteine thus formed feeds in to the pathways generating H2S and glutathione (GSH), which enhances the T cell expansion. ASC, alanine-serine-cysteine; CD25, cluster of differentiation 25; GSSG, oxidized glutathione. [Color online.]
H2S, a byproduct of transsulfuration pathway, also stimulates T cell proliferation
H2S, an important gasotransmitter, has been long known for its cytotoxicity at higher concentrations. However, at low concentrations, it is an important signaling molecule that is crucial in several physiological functions such as vasodilation, reducing oxidative stress, neuromodulation, angiogenesis, and inflammation (Chen et al. 2007). In addition, more recent studies have shown that H2S also plays an important role in modulating T cell immune responses, by demonstrating H2S ability in enhancing T cell proliferation (Kaur et al. 2015; Miller et al. 2012). Because homocysteine can be readily catabolized to L-cysteine endogenously through the transsulfuration pathway and produces H2S as a byproduct, it is very likely that homocysteine can modulate T cell proliferation by targeting availability of cysteine and H2S (Fig. 2).
T cell immunity in causation of hypertension and possible interactions with HHcy
T lymphocytes are essential for the development of adaptive immune responses that provide long-term memory to the host against infections (Park and Kupper 2015). Abnormalities in T cell activation and function not only limit the body’s ability to fight infections but, under certain conditions, leads to inflammation, autoimmunity, and cancer (Dejaco et al. 2006; Gajewski et al. 2013; Harrison et al. 2011). Furthermore, more recent studies have shown that T cells also play an important role in driving the pathology of metabolic disorders including atherosclerosis, hypertension, and obesity (Bordon 2011; Tse et al. 2013). In particular, the T cell’s role in contributing to the pathology of hypertension is intriguing. Although early studies have hinted at the involvement of lymphocytes in hypertension (e.g., Okuda and Grollman 1967), the importance of T cells in hypertension was brought back to light by studies from Guzik et al. (2007), who clearly demonstrated, using lymphocyte-deficient mice and T cell adoptive transfer assay, that T cells can increase the blood pressure. This has led to a series of new studies by others as well, who focused on further understanding the mechanisms, resulting in further characterization of T cell mechanisms involved in hypertension (Abais-Battad et al. 2015; Harrison et al. 2011; Singh et al. 2014; Trott and Harrison 2014). While the detailed underlying mechanisms are still elusive and several interesting hypotheses are currently being tested, it is clear that strategies that may prevent inadvertent activation of T cells are beneficial in treating hypertension.
As dendritic cells (DCs) and macrophages (antigen presenting cells, APC) play a crucial role in T cell activation, studies have also examined the role of these immune cells in causation of hypertension via T cells activation and consequent hypertensive cytokine secretion. It was shown that accumulation of isoketal modified proteins, signature of oxidative damage, in the DCs can cause inadvertent T cell activation and proliferation thereby leading to secretion of IFN-γ and IL-17A cytokines and concomitant development of hypertension (Kirabo et al. 2014). Although adaptive transfer of DCs after oxidative damage is sufficient enough to activate T cells and cause hypertension in the receiving mice, the inducers of oxidative damage in the DCs that lead to hypertension needs further elaboration. Apart from angiotensin II (Ang II; Kirabo et al. 2014), another potential inducer of DCs oxidative damage is HHcy. Though the direct evidence for oxidative damage in DCs by HHcy is yet to be produced, given its role in induction of oxidative stress in macrophages, T cells, and endothelial cells (Chernyavskiy et al. 2016; Winchester et al. 2015; Zhang et al. 2002), such a role for HHcy in the context of hypertension is a possibility. It was recently noted that macrophage polarization can also influence the T cell polarization (Arnold et al. 2015). For instance, M1 polarization with lipopolysaccharide (LPS) in the presence or absence of IFNγ can substantially determine the T helper cell polarization to Th1 or Th17, respectively (Arnold et al. 2015). Such differential T helper cell polarization assumes utmost significance in hypertension development with the studies that implicated IL-17 producing Th17 cells in hypertension development (Harrison 2014; Madhur et al. 2010). Given that HHcy can induce M1 polarization in the presence of LPS (Chernyavskiy et al. 2016) and also enhances IFNγ secretion by T cells after activation (Zhang et al. 2002), it is interesting to study whether HHcy’s presence affects the course of hypertension development. In addition, as antigen presenting cells fulfill the cysteine requirements of T cells during activation (Garg et al. 2011), it is plausible that HHcy may effectively influence cysteine supply by these APCs, thereby also affecting T cell activation (Fig. 2).
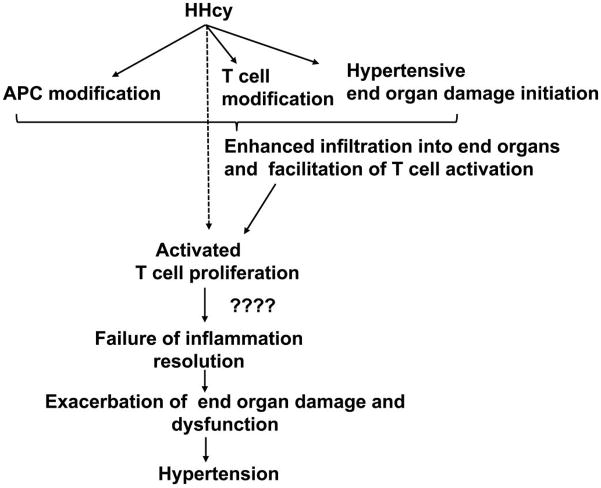
Apart from oxidative damage, phagocytic cell (dendritic cells and macrophages) infiltration into hypertensive target organs such as kidney, brain, and vasculature, which when damaged contributes to hypertension; have also been demonstrated as a potential mechanism for inflammation-induced hypertension (Gooch and Sharma 2014; Harrison 2014; Hilgers 2002; Singh et al. 2014; Trott and Harrison 2014). Both direct modifications in the phagocytic cells as well as changes in the endothelial cells together contribute to the enhanced infiltration of phagocytic cells (Nguyen et al. 2007). Interestingly, T cell infiltration was also observed along with phagocytic cell infiltration in the target organs and mice lacking lymphocytes exhibited attenuated hypertensive responses. These observations are consistent with a model where the inducers of hypertension (such as Ang II and possibly HHcy) cause initial assault and (or) alterations in the phagocytic cells and target organs leading to phagocytic cell localization in the target organs; and subsequent secretion of chemoattractants which not only calls for further infiltration but also facilitates T cell invasion, interaction with phagocytic cells (activation), proliferation and secretion of hypertensive cytokines leading to hypertension. Consistent with this model, HHcy was demonstrated to enhance adhesion of both monocytes and T cells to the aortic endothelium (Koga et al. 2002), which may facilitate T cell activation and proliferation contributing to hypertension development. Further studies are necessary to clarify such a possibility (Fig. 3).
Fig. 3.
T cell immunity in hyperhomocysteinemia (HHcy)-mediated hypertension. The hypothesis is that HHcy-mediated oxidative damage in end organs (kidney, brain, and vasculature), APC (antigen presenting cells), and T cells leads to their accumulation in the end organs. Such accumulation facilitates T cell activation through interactions with APC. HHcy can promote activated T cell proliferation, which may lead to skewed T cell subpopulations and heightened inflammation. These events further cause exacerbation of end-organ damage and hypertension.
In the recent past, there has been progress in identification of lymphocyte-mediated hypertensive end-organ damage. It was observed that lymphocyte deficiency, which enhances natriuresis, is renoprotective in a model of Ang II induced hypertension (Crowley et al. 2010). Studies that focused on identification of a specific type of lymphocyte associated with hypertension development found that T cells can regulate hypertension development. Further, it was also observed that different T cell subtypes could have opposite roles in causation and (or) prevention of hypertension and associated end-organ damage. The T cells can be subdivided into 3 major subtypes: cytotoxic (CD8+), helper (CD4+), and regulatory (Treg) cells. It was observed that adaptive transfer of Treg cells (CD4+CD25+) but not T helper cells (CD4+CD25−) protected Ang II induced vascular damage such as vascular stiffness, impaired vascular responses, and inflammatory cell infiltration (Barhoumi et al. 2011). The causal role for T cells in hypertension was recently established by the studies that used mice lacking specific T cell subtypes (e.g., Trott et al. 2014). It was noted that oligoclonal cytotoxic T cells (CD8+) but not T helper cells (CD4+) mediate sodium retention and renal vascular damage, thereby promoting Ang II induced hypertension (Trott et al. 2014). These studies have identified T cell polarization as critical for hypertension development. For instance, lack of proper Treg cell response or heightened cytotoxic T cell response is detrimental and leads to hypertension. The factors that cause skewness in the T cell subtypes during hypertension development and whether HHcy is one among them need further investigation.
More recently, T cell mediated cytokine release has been implicated in the pathology of hypertension (Harrison et al. 2011; Trott and Harrison 2014; Wenzel et al. 2016). Especially the T helper cell subtype, TH17 cells, which produce IL-17 when activated, have been implicated in hypertension. While overexpression of IL-17 cytokine resulted in elevation of hypertension (Karbach et al. 2014), its neutralization through a specific antibody was ineffective in controlling blood pressure (Marko et al. 2012), suggesting a complex web of time- and dose-dependent events and a need for more studies for further clarification. Interestingly, the TH17 helper cell subtype is also responsible for cell-mediated auto-immunity, further suggesting a role for chronic tissue injury induced autoimmunity in causation of hypertension. Further support for this hypothesis comes from the studies that showed the involvement of Treg cells, which downregulate autoimmune responses, in controlling blood pressure and (or) in amelioration of hypertension-induced injury (Harrison et al. 2011). Because homocysteine can also alter both inflammation and T cell immune response, it is possible that homocysteine may contribute to the hypertension through modulating immune responses (Fig. 3). For instance, homocysteine was shown to enhance Con A induced T cell proliferation by elevating ROS through thiolmediated auto-oxidation (Zhang et al. 2002). However, it is unknown whether there is any differential response among different T cell subsets to homocysteine inflicted proliferation enhancement in different models of hypertension. A possibility for such differential T cell proliferation enhancement comes from the studies that demonstrated enhancement in IL-17 production (a characteristic feature with TH17 cell proliferation) concurrent with Treg cell suppression (also reduces IL-10 secretion) in a HHcy model of atherogenesis (e.g., Feng et al. 2009). How HHcy might lead to differential activation of different T cell subsets is currently unknown. It is plausible that modulation of ROS levels might play a critical role, as it has been proposed that different levels of ROS regulate T cell proliferation differently and the ROS excess leads to T cell apoptosis (Kesarwani et al. 2013). Remarkably, ROS have also been implicated in development of hypertension through chronic injury in the central nervous system (CNS) and renal and vascular systems (Wenzel et al. 2016). It is possible that the oxidative stress inflicted damage further leads to immune cell infiltration and inflammatory reaction. From that point onward, whether the inflammation is resolved or not resolved perhaps determines the outcome, i.e., hypertension (Fig. 3).
Summary
While there is convincing evidence pointing towards the involvement of T cell immunity in hypertension, the underlying mechanisms for T cell activation, factors that cause selective proliferation of certain T cell subtypes, and the nature of hypertensive cytokine profile are still not clear. Under normal physiological conditions, inadvertent activation of T cells is prevented by several regulatory mechanisms that are in place to prevent potential autoimmunity. It is also known that T cell immune responses are sensitive to external factors such as oxidative stress, inflammatory cytokine, and cellular metabolites, which can alter the T cell activation and may contribute to the development of immune disorders. While it is still not clear, it is plausible that hypertension may also involve similar mechanisms. We propose that elevated homocysteine levels may contribute to altered T cell immune responses and can contribute to the pathology of hypertension. In this regard, it is imperative to carefully assess the causes of HHcy, which of the 2 pathways that clear homocysteine (remethylation or transsulfuration) is defective, to better understand the HHcy-mediated hypertension development, not just the homocysteine levels. It is plausible that these pathways that clear HHcy may differentially regulate T cell proliferation when affected. Further studies testing these possibilities will be useful in better understanding the relation between cardiovascular risk factors, homocysteine, immune response, and hypertension.
Acknowledgments
The manuscript is supported by funding from the following National Institutes of Health grants: HL108621, HL074185, and DK104653.
Footnotes
This Review is part of a Special Issue of selected papers from the 3rd Cardiovascular Forum for Promoting Centers of Excellence and Young Investigators held in Omaha, Nebraska, USA, on 10–12 September 2015.
References
- Abais-Battad JM, Rudemiller NP, Mattson DL. Hypertension and immunity: mechanisms of T cell activation and pathways of hypertension. Curr Opin Nephrol Hypertens. 2015;24(5):470–474. doi: 10.1097/MNH.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold CE, Gordon P, Barker RN, Wilson HM. The activation status of human macrophages presenting antigen determines the efficiency of Th17 responses. Immunobiology. 2015;220(1):10–19. doi: 10.1016/j.imbio.2014.09.022. [DOI] [PubMed] [Google Scholar]
- Arroliga AC, Sandur S, Jacobsen DW, Tewari S, Mustafa M, Mascha EJ, Robinson K. Association between hyperhomocysteinemia and primary pulmonary hypertension. Respir Med. 2003;97(7):825–829. doi: 10.1016/S0954-6111(03)00038-6. [DOI] [PubMed] [Google Scholar]
- Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, et al. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension. 2011;57(3):469–476. doi: 10.1161/HYPERTENSIONAHA.110.162941. [DOI] [PubMed] [Google Scholar]
- Blundell G, Jones BG, Rose FA, Tudball N. Homocysteine mediated endothelial cell toxicity and its amelioration. Atherosclerosis. 1996;122(2):163–172. doi: 10.1016/0021-9150(95)05730-7. [DOI] [PubMed] [Google Scholar]
- Bordon Y. Regulatory T cells: Weight watchers. Nat Rev Immunol. 2011;11(2):72. doi: 10.1038/nri2920. [DOI] [PubMed] [Google Scholar]
- Bowman TS, Gaziano JM, Stampfer MJ, Sesso HD. Homocysteine and risk of developing hypertension in men. J Hum Hypertens. 2006;20(8):631–634. doi: 10.1038/sj.jhh.1002052. [DOI] [PubMed] [Google Scholar]
- Brattstrom L, Wilcken DE, Ohrvik J, Brudin L. Common methylenetetrahydrofolate reductase gene mutation leads to hyperhomocysteinemia but not to vascular disease: the result of a meta-analysis. Circulation. 1998;98(23):2520–2526. doi: 10.1161/01.CIR.98.23.2520. [DOI] [PubMed] [Google Scholar]
- Budy B, O’Neill R, DiBello PM, Sengupta S, Jacobsen DW. Homocysteine transport by human aortic endothelial cells: identification and properties of import systems. Arch Biochem Biophys. 2006;446(2):119–130. doi: 10.1016/j.abb.2005.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo M. Hyperhomocysteinemia: a risk factor for arterial and venous thrombotic disease. Int J Clin Lab Res. 1997;27(3):139–144. doi: 10.1007/BF02912449. [DOI] [PubMed] [Google Scholar]
- Cattaneo M. Is hyperhomocysteinemia a risk factor or a consequence of coronary heart disease? Arch Intern Med. 2001;161(21):2628–2629. doi: 10.1001/archinte.161.21.2628. [DOI] [PubMed] [Google Scholar]
- Chen CQ, Xin H, Zhu YZ. Hydrogen sulfide: third gaseous transmitter, but with great pharmacological potential. Acta Pharmacol Sin. 2007;28(11):1709–1716. doi: 10.1111/j.1745-7254.2007.00629.x. [DOI] [PubMed] [Google Scholar]
- Chernyavskiy I, Veeranki S, Sen U, Tyagi SC. Atherogenesis: hyperhomocysteinemia interactions with LDL, macrophage function, paraoxonase 1, and exercise. Ann NY Acad Sci. 2016;1363:138–154. doi: 10.1111/nyas.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crile G., Jr Struma lymphomatosa and carcinoma of the thyroid. Surg Gynecol Obstet. 1978;147(3):350–352. [PubMed] [Google Scholar]
- Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;298(4):R1089–R1097. doi: 10.1152/ajpregu.00373.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SR, Quinlivan EP, Shelnutt KP, Ghandour H, Capdevila A, Coats BS, et al. Homocysteine synthesis is elevated but total remethylation is unchanged by the methylenetetrahydrofolate reductase 677C->T polymorphism and by dietary folate restriction in young women. J Nutr. 2005;135(5):1045–1050. doi: 10.1093/jn/135.5.1045. [DOI] [PubMed] [Google Scholar]
- Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117(3):289–300. doi: 10.1111/j.1365-2567.2005.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinavahi R, Falkner B. Relationship of homocysteine with cardiovascular disease and blood pressure. J Clin Hypertens (Greenwich) 2004;6(9):494–498. doi: 10.1111/j.1524-6175.2004.03643.x. quiz 499–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Oudi M, Bouguerra C, Aouni Z, Mazigh C, Bellaaj R, Machghoul S. Homocysteine and inflammatory biomarkers plasma levels, and severity of acute coronary syndrome. Ann Biol Clin (Paris) 2011;69(2):175–180. doi: 10.1684/abc.2011.0533. [DOI] [PubMed] [Google Scholar]
- Fefelova EV, Tereshkov PP, Dutov AA, Tsybikov NN. Lymphocyte Subpopulations and Cytokine Levels in Experimental Hyperhomocysteinemia. Bull Exp Biol Med. 2015;159(3):358–360. doi: 10.1007/s10517-015-2962-1. [DOI] [PubMed] [Google Scholar]
- Feng J, Zhang Z, Kong W, Liu B, Xu Q, Wang X. Regulatory T cells ameliorate hyperhomocysteinaemia-accelerated atherosclerosis in apoE−/− mice. Cardiovasc Res. 2009;84(1):155–163. doi: 10.1093/cvr/cvp182. [DOI] [PubMed] [Google Scholar]
- Fidelus RK, Ginouves P, Lawrence D, Tsan MF. Modulation of intracellular glutathione concentrations alters lymphocyte activation and proliferation. Exp Cell Res. 1987;170(2):269–275. doi: 10.1016/0014-4827(87)90305-3. [DOI] [PubMed] [Google Scholar]
- Flannigan KL, Agbor TA, Blackler RW, Kim JJ, Khan WI, Verdu EF, et al. Impaired hydrogen sulfide synthesis and IL-10 signaling underlie hyperhomocysteinemia-associated exacerbation of colitis. Proc Natl Acad Sci USA. 2014;111(37):13559–13564. doi: 10.1073/pnas.1413390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AN, Bostom AG, Selhub J, Levey AS, Rosenberg IH. The kidney and homocysteine metabolism. J Am Soc Nephrol. 2001;12(10):2181–2189. doi: 10.1681/ASN.V12102181. [DOI] [PubMed] [Google Scholar]
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg SK, Yan Z, Vitvitsky V, Banerjee R. Differential dependence on cysteine from transsulfuration versus transport during T cell activation. Antioxid Redox Signal. 2011;15(1):39–47. doi: 10.1089/ars.2010.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmunder H, Eck HP, Droge W. Low membrane transport activity for cystine in resting and mitogenically stimulated human lymphocyte preparations and human T cell clones. Eur J Biochem. 1991;201(1):113–117. doi: 10.1111/j.1432-1033.1991.tb16263.x. [DOI] [PubMed] [Google Scholar]
- Gooch JL, Sharma AC. Targeting the immune system to treat hypertension: where are we? Curr Opin Nephrol Hypertens. 2014;23(5):473–479. doi: 10.1097/MNH.0000000000000052. [DOI] [PubMed] [Google Scholar]
- Grossman Z, Min B, Meier-Schellersheim M, Paul WE. Concomitant regulation of T-cell activation and homeostasis. Nat Rev Immunol. 2004;4(5):387–395. doi: 10.1038/nri1355. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadzic T, Li L, Cheng N, Walsh SA, Spitz DR, Knudson CM. The role of low molecular weight thiols in T lymphocyte proliferation and IL-2 secretion. J Immunol. 2005;175(12):7965–7972. doi: 10.4049/jimmunol.175.12.7965. [DOI] [PubMed] [Google Scholar]
- Harrison DG. The immune system in hypertension. Trans Am Clin Climatol Assoc. 2014;125:130–138. discussion 138–140. [PMC free article] [PubMed] [Google Scholar]
- Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57(2):132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgers KF. Monocytes/macrophages in hypertension. J Hypertens. 2002;20(4):593–596. doi: 10.1097/00004872-200204000-00010. [DOI] [PubMed] [Google Scholar]
- Jiang X, Yang F, Tan H, Liao D, Bryan RM, Jr, Randhawa JK, et al. Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler Thromb Vasc Biol. 2005;25(12):2515–2521. doi: 10.1161/01.ATV.0000189559.87328.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- Karbach S, Croxford AL, Oelze M, Schuler R, Minwegen D, Wegner J, et al. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler Thromb Vasc Biol. 2014;34(12):2658–2668. doi: 10.1161/ATVBAHA.114.304108. [DOI] [PubMed] [Google Scholar]
- Katsiki N, Manes C. Is there a role for supplemented antioxidants in the prevention of atherosclerosis? Clin Nutr. 2009;28(1):3–9. doi: 10.1016/j.clnu.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Kaur S, Schwartz AL, Miller TW, Roberts DD. CD47-dependent regulation of H(2)S biosynthesis and signaling in T cells. Methods Enzymol. 2015;555:145–168. doi: 10.1016/bs.mie.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesarwani P, Murali AK, Al-Khami AA, Mehrotra S. Redox regulation of T-cell function: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal. 2013;18(12):1497–1534. doi: 10.1089/ars.2011.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirabo A, Fontana V, de Faria APC, Loperena R, Galindo CL, Wu J, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124(10):4642–4656. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, Claycombe K, Meydani M. Homocysteine increases monocyte and T-cell adhesion to human aortic endothelial cells. Atherosclerosis. 2002;161(2):365–374. doi: 10.1016/S0021-9150(01)00670-0. [DOI] [PubMed] [Google Scholar]
- Kruger WD, Wang L, Jhee KH, Singh RH, Elsas LJ., II Cystathionine beta-synthase deficiency in Georgia (U.S.A.): correlation of clinical and biochemical phenotype with genotype. Hum Mutat. 2003;22(6):434–441. doi: 10.1002/humu.10290. [DOI] [PubMed] [Google Scholar]
- Lazzerini PE, Selvi E, Lorenzini S, Capecchi PL, Ghittoni R, Bisogno S, et al. Homocysteine enhances cytokine production in cultured synoviocytes from rheumatoid arthritis patients. Clin Exp Rheumatol. 2006;24(4):387–393. [PubMed] [Google Scholar]
- Lazzerini PE, Capecchi PL, Selvi E, Lorenzini S, Bisogno S, Galeazzi M, Laghi Pasini F. Hyperhomocysteinemia, inflammation and auto-immunity. Autoimmun Rev. 2007;6(7):503–509. doi: 10.1016/j.autrev.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Li N, Chen YF, Zou AP. Implications of hyperhomocysteinemia in glomerular sclerosis in hypertension. Hypertension. 2002;39(2):443–448. doi: 10.1161/hy02t2.102992. [DOI] [PubMed] [Google Scholar]
- Lip GY, Edmunds E, Martin SC, Jones AF, Blann AD, Beevers DG. A pilot study of homocyst(e)ine levels in essential hypertension: relationship to von Willebrand factor, an index of endothelial damage. Am J Hypertens. 2001;14(7):627–631. doi: 10.1016/S0895-7061(00)01321-2. [DOI] [PubMed] [Google Scholar]
- Ma K, Lv S, Liu B, Liu Z, Luo Y, Kong W, et al. CTLA4-IgG ameliorates homocysteine-accelerated atherosclerosis by inhibiting T-cell overactivation in apoE(−/−) mice. Cardiovasc Res. 2013;97(2):349–359. doi: 10.1093/cvr/cvs330. [DOI] [PubMed] [Google Scholar]
- Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55(2):500–507. doi: 10.1161/.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska A, Chmurzynska A. Polymorphism of genes encoding homocysteine metabolism-related enzymes and risk for cardiovascular disease. Nutr Res. 2009;29(10):685–695. doi: 10.1016/j.nutres.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Marko L, Kvakan H, Park JK, Qadri F, Spallek B, Binger KJ, et al. Interferon-gamma signaling inhibition ameliorates angiotensin II-induced cardiac damage. Hypertension. 2012;60(6):1430–1436. doi: 10.1161/HYPERTENSIONAHA.112.199265. [DOI] [PubMed] [Google Scholar]
- McCully KS. Hyperhomocysteinemia and arteriosclerosis: historical perspectives. Clin Chem Lab Med. 2005;43(10):980–986. doi: 10.1515/CCLM.2005.172. [DOI] [PubMed] [Google Scholar]
- Meng S, Ciment S, Jan M, Tran T, Pham H, Cueto R, et al. Homocysteine induces inflammatory transcriptional signaling in monocytes. Front Biosci (Landmark Ed) 2013;18:685–695. doi: 10.2741/4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TW, Wang EA, Gould S, Stein EV, Kaur S, Lim L, et al. Hydrogen sulfide is an endogenous potentiator of T cell activation. J Biol Chem. 2012;287(6):4211–4221. doi: 10.1074/jbc.M111.307819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan N, Pushpakumar SB, Givvimani S, Kundu S, Metreveli N, James D, et al. Epigenetic regulation of aortic remodeling in hyperhomocysteinemia. FASEB J. 2014;28(8):3411–3422. doi: 10.1096/fj.14-250183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282(48):35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- Nygard O, Vollset SE, Refsum H, Stensvold I, Tverdal A, Nordrehaug JE, et al. Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. JAMA. 1995;274(19):1526–1533. doi: 10.1001/jama.274.19.1526. [DOI] [PubMed] [Google Scholar]
- Okuda T, Grollman A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex Rep Biol Med. 1967;25(2):257–264. [PubMed] [Google Scholar]
- Oudi ME, Aouni Z, Mazigh C, Khochkar R, Gazoueni E, Haouela H, Machghoul S. Homocysteine and markers of inflammation in acute coronary syndrome. Exp Clin Cardiol. 2010;15(2):e25–e28. [PMC free article] [PubMed] [Google Scholar]
- Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med. 2015;21(7):688–697. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddar R, Sivasubramanian N, DiBello PM, Robinson K, Jacobsen DW. Homocysteine induces expression and secretion of monocyte chemoat-tractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation. 2001;103(22):2717–2723. doi: 10.1161/01.CIR.103.22.2717. [DOI] [PubMed] [Google Scholar]
- Postea O, Koenen RR, Hristov M, Weber C, Ludwig A. Homocysteine upregulates vascular transmembrane chemokine CXCL16 and induces CXCR6+ lymphocyte recruitment in vitro and in vivo. J Cell Mol Med. 2008;12(5A):1700–1709. doi: 10.1111/j.1582-4934.2008.00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio JM, Vargas-Hitos JA, Martinez-Bordonado J, Navarrete-Navarrete N, Diaz-Chamorro A, Olvera-Porcel C, et al. Relationship between homocysteine levels and hypertension in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2014;66(10):1528–1535. doi: 10.1002/acr.22340. [DOI] [PubMed] [Google Scholar]
- Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- Sen U, Basu P, Abe OA, Givvimani S, Tyagi N, Metreveli N, et al. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am J Physiol Renal Physiol. 2009;297(2):F410–F419. doi: 10.1152/ajprenal.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen U, Pushpakumar SB, Amin MA, Tyagi SC. Homocysteine in renovascular complications: hydrogen sulfide is a modulator and plausible anaerobic ATP generator. Nitric Oxide. 2014;41:27–37. doi: 10.1016/j.niox.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MV, Chapleau MW, Harwani SC, Abboud FM. The immune system and hypertension. Immunol Res. 2014;59(1–3):243–253. doi: 10.1007/s12026-014-8548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70(1):68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steed MM, Tyagi SC. Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxid Redox Signal. 2011;15(7):1927–1943. doi: 10.1089/ars.2010.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol. 2008;101(10):S14–S19. doi: 10.1016/j.amjcard.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Stuhlinger MC, Tsao PS, Her JH, Kimoto M, Balint RF, Cooke JP. Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation. 2001;104(21):2569–2575. doi: 10.1161/hc4601.098514. [DOI] [PubMed] [Google Scholar]
- Sundstrom J, Sullivan L, D’Agostino RB, Jacques PF, Selhub J, Rosenberg IH, et al. Plasma homocysteine, hypertension incidence, and blood pressure tracking: the Framingham Heart Study. Hypertension. 2003;42(6):1100–1105. doi: 10.1161/01.HYP.0000101690.58391.13. [DOI] [PubMed] [Google Scholar]
- Sutton-Tyrrell K, Bostom A, Selhub J, Zeigler-Johnson C. High homocysteine levels are independently related to isolated systolic hypertension in older adults. Circulation. 1997;96(6):1745–1749. doi: 10.1161/01.CIR.96.6.1745. [DOI] [PubMed] [Google Scholar]
- Trott DW, Harrison DG. The immune system in hypertension. Adv Physiol Educ. 2014;38(1):20–24. doi: 10.1152/advan.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, et al. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension. 2014;64(5):1108–1115. doi: 10.1161/HYPERTENSIONAHA.114.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse K, Tse H, Sidney J, Sette A, Ley K. T cells in atherosclerosis. Int Immunol. 2013;25(11):615–622. doi: 10.1093/intimm/dxt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranki S, Tyagi SC. Defective homocysteine metabolism: potential implications for skeletal muscle malfunction. Int J Mol Sci. 2013;14(7):15074–15091. doi: 10.3390/ijms140715074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, OK Homocysteine stimulates the expression of monocyte chemoattractant protein-1 receptor (CCR2) in human monocytes: possible involvement of oxygen free radicals. Biochem J. 2001;357(1):233–240. doi: 10.1042/bj3570233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Siow YL, OK Homocysteine stimulates nuclear factor kappaB activity and monocyte chemoattractant protein-1 expression in vascular smooth-muscle cells: a possible role for protein kinase C. Biochem J. 2000;352(3):817–826. doi: 10.1042/0264-6021:3520817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen S, Yao T, Li D, Wang Y, Li Y, et al. Homocysteine as a risk factor for hypertension: a 2-year follow-up study. PLoS One. 2014;9(10):e108223. doi: 10.1371/journal.pone.0108223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel U, Turner JE, Krebs C, Kurts C, Harrison DG, Ehmke H. Immune mechanisms in arterial hypertension. J Am Soc Nephrol. 2016;27:677–686. doi: 10.1681/ASN.2015050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester LJ, Veeranki S, Givvimani S, Tyagi SC. Homocysteine elicits an M1 phenotype in murine macrophages through an EMMPRIN-mediated pathway. Can J Physiol Pharm. 2015;93(7):577–584. doi: 10.1139/cjpp-2014-0520. [DOI] [PubMed] [Google Scholar]
- Yang Q, Xue HM, Underwood MJ, Yu CM. Mechanistic studies of AVE3085 against homocysteine in endothelial protection. Cardiovasc Drugs Ther. 2013;27(6):511–520. doi: 10.1007/s10557-013-6478-5. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zeng X, Guo J, Wang X. Oxidant stress mechanism of homocysteine potentiating Con A-induced proliferation in murine splenic T lymphocytes. Cardiovasc Res. 2002;53(4):1035–1042. doi: 10.1016/S0008-6363(01)00541-7. [DOI] [PubMed] [Google Scholar]
- Zhu S, Li J, Bing Y, Yan W, Zhu Y, Xia B, Chen M. Diet-induced hyperhomocysteinaemia increases intestinal inflammation in an animal model of colitis. J Crohns Colitis. 2015a;9(9):708–719. doi: 10.1093/ecco-jcc/jjv094. [DOI] [PubMed] [Google Scholar]