Summary
Viral vector delivery of RNA silencing constructs, when administered into vasculature, typically results in poor central nervous system (CNS) transduction due to the inability of the vector to cross the blood-brain-barrier (BBB). However, adeno-associated virus serotype 9 (AAV9) has the ability to cross the BBB and robustly transduce brain parenchyma and peripheral tissues at biologically meaningful levels when injected intravenously. Recent work by our lab has shown that this method can be used to deliver RNA silencing constructs, resulting in significant reductions in gene expression in multiple brain regions and in peripheral tissues. Here, we outline a method for delivery of AAV9 vectors expressing RNA interference (RNAi) constructs that lead to robust simultaneous transduction of mouse peripheral tissues and the CNS following a single injection into the jugular vein. Additionally, we outline methods for necropsy and immunofluorescence to detect AAV9 transduction patterns in the rodent CNS following a vascular delivery.
Keywords: AAV9, systemic, jugular vein, vascular, gene therapy, RNAi
1. Introduction
RNA interference (RNAi) is a powerful biological tool to query basic gene function or to silence diseased genes in therapeutic applications. Viral vector delivery of RNAi constructs is most frequently achieved with focal injections into particular peripheral organs or into specific sub-regions of the CNS using stereotaxic methods. For widespread and simultaneous delivery to peripheral tissues, a vascular delivery approach can be used and transduction is typically limited only by the tropism of the viral vector serotype that is selected. Many AAV serotypes confer promiscuous binding to peripheral tissues (serotypes 1, 7, 8, 9), while others are more selective in the tissue types that they transduce (serotypes 2, 3, 4, 5, 6) following vascular injection (10). However, widespread transduction from a single vector injection has not been a feasible strategy for the brain, as most viral vectors cannot cross the blood-brain-barrier (BBB) in appreciable levels. For diseases involving widespread CNS neuropathology (e.g. Alzheimer’s disease, Huntington’s disease, amyotrophic lateral sclerosis, lysosomal storage disorders, Rett Syndrome, etc), the inability to achieve widespread transduction has been a significant roadblock in therapeutic development.
Historically, in both pre-clinical and clinical studies assessing vector-mediated gene delivery, one or a few focal injections have been used to fill particular brain structures. While these studies successfully demonstrate the utility of viral vectors to confer long-term gene expression, or gene suppression in some cases, the widespread pathology observed in many CNS diseases makes a global delivery strategy an appealing approach. Faust and colleagues demonstrated that AAV serotype 9 (AAV9) crosses the BBB and transduces both neurons and astrocytes in the mouse CNS following a singular tail vein injection (4). Recent studies have shown that AAV9 can be used as a delivery tool to replace or modify diseased genes and successfully improve disease phenotypes in a variety of mouse models of human disease, including amyotrophic lateral sclerosis (5), mucopolysaccharidosis III (6), and Rett syndrome (7,8). Vascular delivery of AAV9-RNAi has been employed to reduce gene expression in mouse models of cardiac myopathy (3,9) and our laboratory was the first to demonstrate that a single jugular vein delivery of AAV9-RNAi crossed the BBB and significantly reduced expression of a disease-causing gene in multiple brain regions. Moreover, this global delivery strategy prevented both neuropathological and physiological manifestations of disease (2). As expected, this delivery method also resulted in significant reduction in disease-causing gene expression in multiple peripheral tissues (2).
Here, we describe, in detail, the methodology that our laboratory uses to target the mouse CNS and periphery using systemic AAV9-RNAi and to assess transduction patterns in the injected animals: 1) Intra-jugular vein injection to administer vector, 2) mouse necropsy, perfusion and tissue collection and 3) immunofluorescent processing of brain sections to evaluate the expression and transduction patterns of AAV9 in mice.
2. Materials
2.1 Intra-jugular vein injection
PPE including scrubs, hair bonnets, water resistant gown, surgical masks, and gloves
Infusate (viral vector prep)
Ketamine (100mg/ml)
Xylazine (100mg/ml)
Ketamine/Xylazine Mix (1 ml of ketamine (100 mg/mL) + 0.1 ml of xylazine (100 mg/mL) + 8.9 mL of 0.9% sterile saline)
Hamilton glass luer tip (LT) syringes (25–500 μl or appropriate volume for infusion)
Hamilton Kel-F Hub removable surgical needles (30G, 20mm length, beveled tip style 4)
1cc syringes with 27G needles
Hair clippers
Surgical lamp
Sterile surgical chucks pads
Sterile gauze
Sterile surgical cotton swabs
Surgical instruments (sharp tipped surgical scissors, blunt tipped dissecting scissors, forceps)
Bead sterilizer
Laboratory tape
Betadine wipes
Alcohol wipes
Carprofen
Heating pad
Wound clip applicator
Wound clip removal tool
7mm wound clips
2.2 Necropsy, tissue collection and cutting for histology
PPE: Gown or lab coat, hair bonnet, nitrile gloves, facemask
10cc syringes
1cc syringe with 27G needle
BD Vacutainer Safety-Lok blood collection set (used for perfusion)
Dissection board, with needles to pin mouse
Surgical instruments (surgical scissors, fine tipped forceps, hemostats, small spatula, heavy duty scissors)
Spray bottle with 70% Ethanol
Sharps containers
Waste disposal bags
0.9% sterile saline
32% Paraformaldehyde (liquid stock)
Sodium Phosphate Monobasic Monohydrate NaH2PO4 (FW137.99)
Sodium Phosphate Dibasic Anhydrous Na2HPO4 (FW141.96)
0.1M Phosphate buffer solution (5.50g sodium phosphate dibasic, 1.56g sodium phosphate monobasic, 500 ml of dH2O).
4% paraformaldehyde (1:8 dilution of 32% liquid paraformaldehyde stock to 0.1M Phosphate buffer – i.e. 10 ml 32% paraformaldehyde + 70 ml 0.1M phosphate buffer)
Ice bucket with wet ice
Labeled tissue culture plates (6–24 well, size depends on tissue harvested)
For molecular analyses, the following materials will also be used: mouse brain matrix, sterile razor blades, micro scissors or tissue punchers for regional brain dissection, small fine tipped forceps, RNAse away, sterile petri dishes, 1.5 ml microcentrifuge tubes and/or DNAse/RNAse free foil packs, dry ice or liquid nitrogen.
2.3 Immunofluoresence staining of brain tissue to assess transduction
PPE: White lab coat, nitrile gloves
Sliding microtome and microtome blade
Paint brushes for tissue cutting
Dry ice
Trizma® pre-set crystals (pH 7.4, FW151.6)
NaCl (FW58.44)
Triton®X-100
Sodium Phosphate Dibasic Anhydrous Na2HPO4 (FW141.96)
Sodium Phosphate Monobasic Monohydrate NaH2PO4 (FW137.99)
Serum (goat or donkey)
Dilution media (7.46g Trizma, 8.77g NaCl, 0.5 ml TritonX-100, 1L dH2O)
TBS (7.46g Trizma, 8.77g NaCl, 1L dH2O)
PBS (5.47g Dibasic, 1.60 Monobasic, 9.26g NaCl, 1L dH2O)
Cryoprotectant solution (300g Sucrose, 300 ml ethylene glycol, 0.2g Sodium Azide, 500 ml PBS)
Netted staining dishes (with glass dishes to contain fluid)
Orbital shaker
Appropriate primary and secondary antibodies
24 well tissue culture plates
Hook tools or small paint brushes for mounting tissue
Clear shallow dish for mounting stained tissue
Vectashield mounting medium
Microscope slides
Cover slips
Clear nail polish
Microscope with fluorescent capabilities
3. Methods
3.1 Intra-jugular vein injection
Set up the surgical area. Tape down sterile chucks pads for the surgical space. Sterilize the surgical instruments (forceps, surgical scissors, blunt tipped dissecting scissors) using a bead sterilizer. Illuminate the area with a light source.
Put on the appropriate personal protective equipment (PPE), including a hair bonnet, face mask, sterile gown or lab coat, and gloves.
Restrain the mouse for anesthetic injection. First, place the mouse on the cage lid, gently press the body down against the lid, and scruff the skin on the back to create a gentle but firm grasp on the mouse.
Hold the mouse up, with its ventral surface placed upwards, and inject with ketamine/xylazine mix (Note 1) at approximately 0.01 cc per gram bodyweight (i.e. 0.20 cc for a 20 g mouse) into the intraperitoneal space (IP).
Place the mouse back into the home cage and wait 5 minutes to check the mouse’s anesthetic plane. To assess anesthetic plane, pinch the mouse’s tail and foot pad and look for an involuntary reflex. If the mouse does not show a reflex following tail and foot pinch, it is now in a surgical plane of anesthesia. If there is a reflex, give the animal a supplemental dose of ketamine/xylazine, approximately 0.05 – 0.10 cc, and wait another 5 minutes and reassess anesthetic plane. Repeat the pinch assessment and supplement with more ketamine/xylazine (stepwise, 0.05 – 0.10 cc at a time) until an anesthetic plane is reached.
Place the mouse in a recumbent position (lying on its back) on a pad (separate from the surgical pad to be used shortly), and shave the upper chest and neck of the mouse.
Move the mouse to the clean surgical chucks pad, and again place in a recumbent position. Tape the limbs of the mouse down to hold it in place for surgery.
Sterilize the shaved surgical site using betadine wipes. Wipe the betadine off using ethanol wipes – this will help to visualize the jugular vein through the skin.
To expose the jugular vein for injection, first pinch the skin on the upper chest (above the pectoral muscle) and make a small incision in the skin over the mouse’s right jugular vein (your left) using the sterile surgical scissors (Fig 1A). Pull the skin up from the mouse with the forceps and keep tension on it, then carefully slide the scissors into the small opening made from the initial incision and cut through the skin upwards towards the head (Fig. 1B). The final incision should be approximately 2.5 cm in length, and run parallel to the mouse’s midline, about 0.5 cm to the left of the midline (mouse’s right). If you can visualize the jugular vein through the skin prior to incision, the incision should run directly over the vein.
Expose the jugular vein by carefully cleaning off any connective or fat tissue and that may be in the way.
Prime the Hamilton needle/syringe with virus by filling and emptying the syringe 3 times (Note 2).
Fill the Hamilton syringe with the appropriate amount for virus for injection (Note 3).
Hold onto the pectoral muscle with forceps, and push needle tip through pectoral muscle and into the lumen of the vein (Fig. 1C and 1D).
Visually confirm that the needle tip is in the lumen of the jugular vein, then slowly infuse virus (approximately 100 μl per minute). (Notes 4 and 5).
Slowly pull the needle out from the jugular vein and pectoral muscle. Keep pressure on the pectoral muscle with the forceps while pulling the needle out. Gently press a cotton tipped swab on the injection site to reduce backflow of blood or virus.
Gently pull the skin back together.
Pinch the incision closed with forceps and pull the closed skin away from the jugular vein with the forceps. Close skin incision with 7 mm wound clips (Fig. 1E and 1F). Make sure that the skin is pulled away from the body when the wound clip is placed, to avoid damaging the muscle or veins under the skin.
Inject the mouse with analgesic – 5 mg/kg carprofen once daily, for two days. The first injection should be immediately after surgery with the second at 24 hours post-surgery.
Place the recovering mouse in a clean cage, with half of the cage placed on a heating pad, on low heat, until the mouse awakens.
Return mouse to the home cage only after it has awoken to avoid manipulation from cage mates. Wound clips can be removed 1 week after surgery.
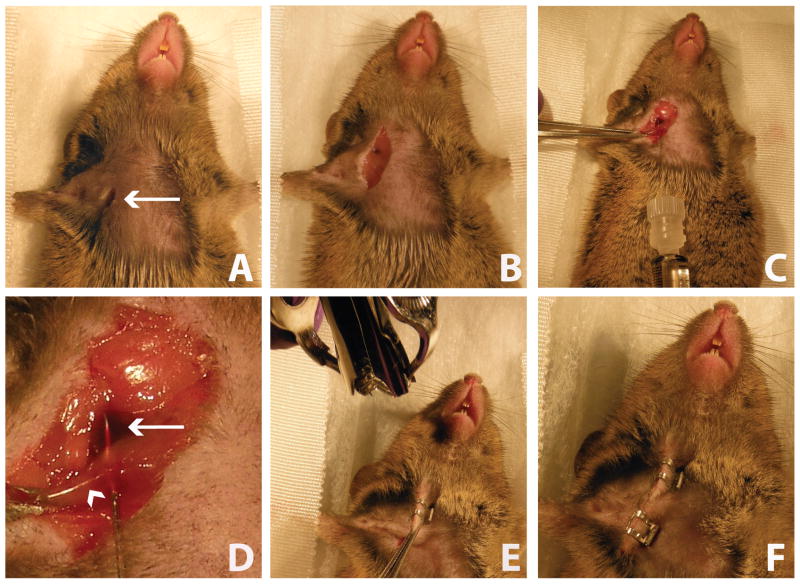
Figure 1.
Intra-jugular injection of AAV9-RNAi. A) An incision is made in the upper chest and neck of the mouse. Arrow indicates the incision. B) The jugular vein and pectoral muscle are exposed. C) The pectoral muscle is stabilized using forceps and a 30G Hamilton syringe is inserted through the pectoral muscle and into the jugular vein lumen. AAV9 vector is infused into the vein. D) Close-up image of jugular vein injection with needle tip inside of the vein lumen (arrow = jugular vein; arrowhead = pectoral muscle). E) The skin is pinched and pulled away from the body and closed with wound clip. F) The incision is completely closed with wound clips.
3.2 Necropsy, tissue collection and sectioning for histology
Prepare a space for the necropsy – set up with sterile pads, dissection board, light source, and clean surgical instruments. You will also need ice cold sterile 0.9% saline and 4% paraformaldehyde (keep both on ice, until ready for injection).
Prepare tissue culture plates (6-well to 24-well, use the plate with the appropriate size wells for the tissues you are planning to collect) by labeling and filling them with 4% paraformaldehyde.
Fill two 10cc syringes - one with 10cc of 0.9% saline and the other with 4% paraformaldehyde. Keep both syringes on ice until ready for use. Connect each syringe to BD Vacutainer Safety-Lok blood collection set (this set consists of a needle, with a piece of plastic used to grip the needle, and thin tubing that connects to the syringe).
Modifications to this procedure can be made for collecting tissues for molecular analysis (i.e. qPCR or western blot to assess gene expression). See Note 6 for modifications.
Anesthetize the mouse in its home cage by injecting IP with the ketamine/xylazine mix at approximately 0.01 cc per gram bodyweight (i.e. 0.20 cc for a 20 gram mouse). The mouse should be in a surgical/deep plane of anesthesia before proceeding with the necropsy - there should be no response to toe pinch. If there is still a response then supplement with additional ketamine/xylazine (0.05 – 0.10 cc at a time), and wait for suppression of response to toe pinch.
Once a deep plane of anesthesia is achieved, secure the animal to the dissection board in a recumbent position by placing 27G needles through all four paws (Fig. 2A). Spray the mouse’s body with 70% ethanol, to prevent hair from spreading over the surgical openings and tissues during necropsy.
Using forceps, grab the skin on the lower abdomen at midline and make an incision using surgical scissors to open the abdominal cavity (Fig. 2B).
Carefully cut up each side of the mouse’s body until you reach the diaphragm (near the point when you reach the ribs with the lateral cuts).
Carefully cut through the diaphragm using surgical scissors (Fig. 2C). Cut along the outer ventral rim of the diaphragm to help avoid cutting organs (particularly the heart and lungs) with the scissors.
Continue cutting up the lateral sides of the mouse, through the ribs, to fully expose the heart (Fig. 2D).
Use hemostats to grip the lower sternum, clamp and drape hemostats over the rostral end of the mouse. Hemostats can be held in place by resting them on the needle holding the mouse’s forepaw to the dissection board. This keeps the chest out of the way so that the heart is easily accessible during perfusion.
Gently grip the mouse’s right atrium (see Fig. 2F) with forceps and cut it using surgical scissors. This creates an exit for the mouse’s blood that will be pushed out during the perfusion.
Perfuse the mouse with 10 cc of 0.9% saline. To do this, gently insert the needle tip approximately 2–3 mm into the mouse’s left ventricle (Fig. 2E and 2F). Make sure not to breach the wall between the left and right ventricle. Slowly infuse the saline over 30 seconds (Note 6). When done, remove the needle from the right ventricle.
Perfuse the mouse with 4% paraformaldehyde. First, place the needle into the same opening in the left ventricle. Slowly perfuse with 10 cc of paraformaldehyde over 30 seconds. If the needle placement is correct, the animal will move slightly as the paraformaldehyde fixes proteins in skeletal muscle.
To harvest the brain, first cut off the head of the mouse using a large pair of scissors. Next, cut the skin on the dorsal surface of the head from the cervical cut down to the nose. Pull the skin down on the sides. Cover the loose skin with a Kimwipe, and use the skin to hold the head firmly in place while you open the skull and remove the brain.
To open the skull for brain removal, first use surgical scissors to cut the bone between the two eyes (Fig. 2G). Next, carefully make a 5mm cut up the posterior skull (region covering the back of the cerebellum). Make sure not to insert the scissor blade into the tissue – it should slide in directly touching the inner surface of the skull. Gently pull off the pieces of skull adjacent to the cervical cut using forceps. Next make another cut up the midline of the skull using the same technique, approximately 1cm. Again, remove the skull covering each hemisphere of brain using forceps (Fig. 2H). Finally, make one final cut upwards, to connect the midline cut to the cut between the eye-sockets. Again, remove the skull covering the final rostral portion of the brain.
Slide a surgical spatula under the brain at the rostral end and continue to slide under the ventral surface of the brain. Tip the skull/brain upside down (at a 45 to 90 degree angle), and use the surgical spatula or dissecting scissors to gently cut the large trigeminal and other cranial nerves that hold the brain in place (Fig. 2I). Gently remove the brain and place it into a tissue culture plate or other collection vessel filled with 4% paraformaldehyde overnight (approximately 12 – 16 hours) to post-fix.
Harvest all peripheral tissues of interest. Antal et al. (1) provides a good overview for mouse organ dissection. Store tissues in a tissue culture plate filled with 4% paraformaldehyde overnight (approximately 12 – 16 hours) to post-fix.
Dispose of mouse carcass.
After an overnight post-fix, move all harvested tissue into a new tissue culture plate filled with 30% sucrose. Once the 30% sucrose fully permeates the tissues and they sink to the bottom of the wells they are ready for sectioning.
Cut brains using a frozen, sliding microtome at a thickness of 40 μm and collect tissues in 24-well plates filled with cryoprotectant solution.
Cut peripheral tissues using a cryostat, at an appropriate temperature and thickness for the tissue being cut.
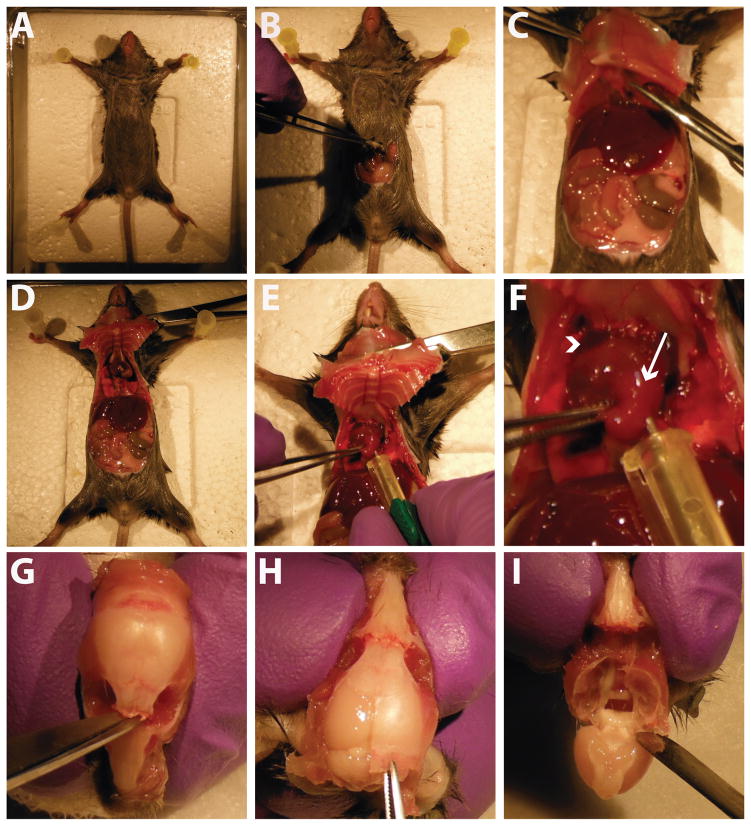
Figure 2.
Mouse necropsy and tissue collection. A) The mouse is pinned down to the surgical board in all four paws. B) An incision is made into the ventral abdomen. C) The incision is extended up to the diaphragm, which is carefully cut open. D) The incision is extended on both sides to open the chest cavity. Hemostats are used to hold the cavity open. E) A needle is placed into the mouse’s left ventricle for perfusion of saline and paraformaldehyde. F) Close-up image of heart at perfusion. Arrowhead indicates the mouse’s right atrium, which is cut at the start of the perfusion. Arrow indicates the left ventricle, where the needle is placed for perfusion. G) After removing the mouse’s head and exposing the skull, a small incision is made through the skull between the eye sockets. H) A cut is made up the midline of the skull, starting at the cervical wound, and the cut skull is peeled off using forceps. I) After full removal of the dorsal skull, the brain is gently removed using a spatula.
3.3 Immunofluoresence staining of brain to assess transduction (Note 8)
Day 1
Wash free-floating tissue sections in dilution media in netted staining dishes (5 X 5 minutes) (Note 9).
Block tissue in 5% of the appropriate serum (goat, donkey, etc) in netted staining dishes (5 ml serum per 100 ml dilution media).
Incubate tissue in primary antibody solution (primary antibody solution: 3 ml serum and 400 μl triton-X per 100 ml PBS in 24 well tissue culture plates (either shaking at room temp overnight or at 4 degrees for 48 hours). Each primary antibody should be used at its own concentration.
Day 2
Wash tissue in dilution media in netted staining dishes (5 X 5 minutes).
Incubate tissue in the appropriate fluorophore-conjugated secondary antibody solution in 24 well tissue culture plates. Secondary antibody solution: 3 ml normal serum per 100 ml dilution media. Each secondary antibody should be added to this solution at its own required concentration, which is typically 1:500. Incubation time will depend on each primary and secondary antibody, which can range from 15 minutes to 4 hours (Notes 10, 11).
Wash tissue in TBS in netted staining dishes (3 X 5 minutes).
Incubate tissue in Hoechst 333258 pentahydrate solution (diluted to a concentration of 1:10,000 in water) for 1 minute. Hoechst stains DNA fluorescent blue which is used to visualize nuclei.
Wash tissue again in TBS in netted staining dishes (3 × 5 minutes)
Store tissue at 4 degrees in PBS either in the netted staining dishes, or in a new storage container, covered in foil to protect from light, until able to mount onto slides.
Mount tissue onto either subbed (gelatin coated) or charged microscope slides. Allow for tissue to partially dry (approximately 5 minutes) so that they are well adhered to the slide, yet not completely dried out.
Apply approximately 75 μl of wet mounting medium to the slide (Note 12), and place coverslip. Allow to dry for 30 – 60 min. Seal the outside of the slide (around the coverslip) using clear nail polish, to prevent the wet-mount and tissue from drying out.
Store slides at 4 degrees in an opaque container.
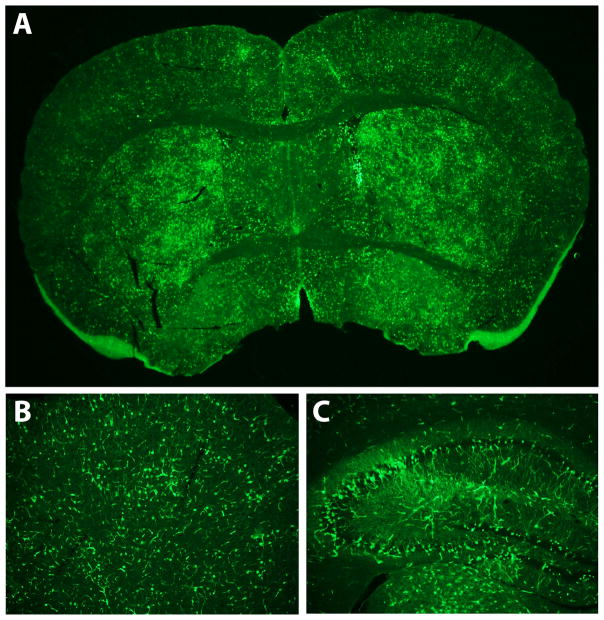
Figure 3.
Immunofluorescence detection of expressed transgene. A) Immunofluorescence-stained coronal brain section of an AAV9-GFP injected mouse, showing widespread GFP expression. Examples of regional brain expression are shown: B) GFP expression in frontal cortex and C) hippocampus.
Acknowledgments
This research was supported by a research grant from the Hereditary Disease Foundation (J.L.M.), ONPRC Core Grants RR000163 (J.L.M.) and P51OD011092 (J.L.M.), and a T32 Neuroscience training grant NS7466-14 (B.D.D.). Confocal microscopy was supported by grants S10RR024585 and P30-NS061800.
Footnotes
Some mouse strains (general background and/or particular transgenic lines) can be sensitive to anesthesia. Exercise caution when injecting with anesthesia for the first time. The dosing can be altered slightly to accommodate the required dose for the particular strain utilized in a study.
As there is a significant amount of dead space in regular syringes and needles, we use a glass Hamilton luer tip syringe, which dramatically reduces the amount of virus lost during each injection (roughly 50 μl vs 5 μl). A small gauge needle is also desirable for jugular injection as to cause a minimal amount of tissue damage and to reduce the amount of blood and virus that backs out of the opening. 700 series Hamilton syringes, which fit Hamilton 30G Kel-F hub needles, work particularly well.
From our own studies, we have discovered that the amount of AAV9 used is critical for successful silencing using RNAi. The degree of silencing will depend on various factors including the promoter used, the potency of the silencing sequence, the total number of cells transduced, total viral genomes injected, and bodyweight of the animals injected. We found that when we injected approximately 7.5e10 viral genomes per gram bodyweight (i.e. 7.5e11 tvg for a 10 gram mouse) that we achieved significant 12%-33% reductions of our target gene within various regions of the CNS. Theoretically, increased dosing (increased viral genomes per gram body weight) should increase the number of cells transduced and the degree of silencing achieved. In a previous study in which we injected mice in the range of 2e10 to 3e10 vg/gram bodyweight we did not achieve significant reductions in our target gene in the CNS using RNAi. However, at both doses, significant reductions of our target gene in the periphery were achieved. For further information, see Dufour et al (2).
To further confirm correct placement in the jugular vein, you can pull back on the plunger of the Hamilton and backfill the syringe with a small amount of blood from the vein. When infusing, the viral solution visibly mixes directly with the blood, providing further confirmation of correct placement. If the needle tip is not accurately placed in the jugular vein lumen, virus will typically collect under the connective tissue that covers the jugular and create a bubble there. If this occurs, remove the needle from the pectoral muscle and jugular vein and replace the needle adjacent to the first site.
Fu et al. (6) showed that vascular pretreatment with 25% mannitol, prior to tail-vein injection of AAV9, increased transduction of the CNS. In our studies, we found that pre-treatment with 25% mannitol, when compared to saline pretreatment, did not increase AAV9 transduction following intra-jugular administration (2).
The protocol listed here is easily modifiable for collecting tissues for molecular analyses instead of histology. The main modification is that paraformaldehyde is not used with tissues that are used for molecular analyses. Step 14 (perfuse with 10 cc of 4% paraformaldehyde) should be omitted entirely. To account for reduced solution clearing the blood from the circulatory system, step 13 should be modified so that 15 cc of saline is used for the perfusion instead of 10 cc. For steps 17 and 18, do not post-fix tissues in paraformaldehyde for molecular analyses - instead, tissues are harvested/dissected, placed in RNAse/DNAse free microcentrifuge tubes, frozen on dry ice, and stored at -80°C until use. For region specific analyses of the CNS – immediately after removal, keep the brain cold in a saline-filled petri dish sitting on wet ice, then cut the brain into 1 mm thick coronal slabs using an ice-cold mouse brain matrix and sterile razor blades. Regions of interest can be dissected using micro-scissors and small forceps, placed into microcentrifuge tubes, frozen on dry ice, and stored at -80°C until use. If both molecular and histological analyses are desired from the same animal, collect and store specimens for molecular analysis first; next, post-fix any tissues for histological analysis in 4% paraformaldehyde for 24 hours, then sink and store in 30% sucrose until ready to section.
If the saline is pushing through the vasculature appropriately, the mouse’s blood should drain from the right atria, visible blood vessels should clear, and the liver should lighten in color from a deep red-brown to a light pink-tan color. If the needle breaches the wall between the ventricles, then the lungs fill with saline and saline will pushing out through the mouth and/or nose of the mouse. When this occurs, the blood does not clear from the circulatory system well, which will create artifacts for histological analysis. If this occurs, you can push more saline and/or paraformaldehyde through (approximately an extra 5ml) and do so at increased pressure/rate. Sometimes pulling back on the needle can also help.
In our experience, reporter gene expression delivered with AAV9 is visualized more completely when the tissue is stained using immunofluorescence methods as opposed to simply visualizing the native reporter gene expression (i.e. GFP autofluorescence) or staining using standard DAB immunohistochemistry. For this reason, immunofluorescence staining methodology is included here.
For staining sections that are not free-floating (i.e. paraffin embedded or cryostat cut sections that are immediately placed on slides), the immunofluorescence protocol outlined here will work by making minor modifications. The sections should be stained directly on the slide. First, bring slides/sections to room temperature, by leaving them on a lab bench for 20 minutes. Next, use a pap-pen to outline the tissue on the slide, which creates a wax barrier to hold solutions onto the slide for staining. Follow the same incubation times and sequence of reagents included in the free-floating protocol outlined in this chapter.
With slight modifications to this protocol, double label immunofluorescence can also be used to identify specific cell types that are transduced by AAV9. First, the tissue should be simultaneously incubated in both primary antibodies of interest, in Day 1 – Step 3, ensuring that the primary antibodies are made in different animals. The other modification is for Day 2 – Step 2: the tissue should be incubated in the appropriate fluorophore-conjugated secondary antibodies in sequence (i.e. incubate in secondary antibody #1, followed by 3 × 5 minute washes in dilution media, then incubate in secondary antibody #2). After this modification, the rest of the procedure can proceed as outlined, continuing again with Day 2 – Step 3.
When staining for GFP expression, we were able to best detect our signal when using our secondary antibody (Alexafluor 488 Goat anti Rabbit, Life technologies, catalog #A11034) at a concentration of 1:500 and incubating the tissue in this antibody solution for approximately 3 to 4 hours (Fig 3). When we increased the concentration of secondary antibody, there was too much background, and when we decreased the concentration we lost the signal. By extending the incubation time from our 1 hour standard, we were able to have a really good signal to noise ratio. This secondary was used in conjunction with a rabbit anti-GFP antibody (Life Technologies, catalog # A6455) used at a 1:1000 concentration in Day 1 – Step 3.
Various companies offer mounting media that slows the degradation of the fluorophore used for immunofluorescence (e.g. Vectashield mounting medium, Vector laboratories), which is helpful for maintaining the life of the tissue as well as reducing photobleaching during examination under the microscope.
5.0 References
- 1.Antal C, Teletin M, Wendling O, Dgheem M, Auwerx J, Mark M. Tissue collection for systematic phenotyping in the mouse. Curr Protoc Mol Biol. 2007;Chapter 29(Unit) doi: 10.1002/0471142727.mb29a04s80. [DOI] [PubMed] [Google Scholar]
- 2.Dufour BD, Smith CA, Clark RL, Walker TR, McBride JL. Intrajugular vein delivery of AAV9-RNAi prevents neuropathological changes and weight loss in Huntington’s disease mice. Mol Ther. 2014;22:797–810. doi: 10.1038/mt.2013.289. mt2013289 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fechner H, Sipo I, Westermann D, Pinkert S, Wang X, Suckau L, Kurreck J, Zeichhardt H, Muller O, Vetter R, Erdmann V, Tschope C, Poller W. Cardiac-targeted RNA interference mediated by an AAV9 vector improves cardiac function in coxsackievirus B3 cardiomyopathy. J Mol Med(Berl) 2008;86:987–997. doi: 10.1007/s00109-008-0363-x. [DOI] [PubMed] [Google Scholar]
- 4.Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. nbt.1515 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foust KD, Salazar DL, Likhite S, Ferraiuolo L, Ditsworth D, Ilieva H, Meyer K, Schmelzer L, Braun L, Cleveland DW, Kaspar BK. Therapeutic AAV9-mediated Suppression of Mutant SOD1 Slows Disease Progression and Extends Survival in Models of Inherited ALS. Mol Ther. 2013 doi: 10.1038/mt.2013.211. mt2013211 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu H, Dirosario J, Killedar S, Zaraspe K, McCarty DM. Correction of neurological disease of mucopolysaccharidosis IIIB in adult mice by rAAV9 trans-blood-brain barrier gene delivery. Mol Ther. 2011;19:1025–1033. doi: 10.1038/mt.2011.34. mt201134 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadalla KK, Bailey ME, Spike RC, Ross PD, Woodard KT, Kalburgi SN, Bachaboina L, Deng JV, West AE, Samulski RJ, Gray SJ, Cobb SR. Improved survival and reduced phenotypic severity following AAV9/MECP2 gene transfer to neonatal and juvenile male Mecp2 knockout mice. Mol Ther. 2013;21:18–30. doi: 10.1038/mt.2012.200. mt2012200 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg SK, Lioy DT, Cheval H, McGann JC, Bissonnette JM, Murtha MJ, Foust KD, Kaspar BK, Bird A, Mandel G. Systemic Delivery of MeCP2 Rescues Behavioral and Cellular Deficits in Female Mouse Models of Rett Syndrome. J Neurosci. 2013;33:13612–13620. doi: 10.1523/JNEUROSCI.1854-13.2013. 33/34/13612 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suckau L, Fechner H, Chemaly E, Krohn S, Hadri L, Kockskamper J, Westermann D, Bisping E, Ly H, Wang X, Kawase Y, Chen J, Liang L, Sipo I, Vetter R, Weger S, Kurreck J, Erdmann V, Tschope C, Pieske B, Lebeche D, Schultheiss HP, Hajjar RJ, Poller WC. Long-term cardiac-targeted RNA interference for the treatment of heart failure restores cardiac function and reduces pathological hypertrophy. Circulation. 2009;119:1241–1252. doi: 10.1161/CIRCULATIONAHA.108.783852. CIRCULATIONAHA.108.783852 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. mt200876 [pii] [DOI] [PubMed] [Google Scholar]