Abstract
BACKGROUND
Previous small studies suggested reduced quality of life (QOL) for people with Marfan syndrome (MFS) compared to those without MFS. The national registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC) is a longitudinal observational cohort study of patients with conditions that predispose to thoracic aortic aneurysms and dissections, including MFS. At the time of registry enrollment, GenTAC participants are asked to complete questionnaires about demographics, medical history, health habits, and QOL.
OBJECTIVES
This study assessed QOL in GenTAC participants with MFS and identify associated factors using self-reported data.
METHODS
QOL was assessed using the 4 subscales of the Physical Component Summary (PCS) of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36): physical functioning (PF); role limitations due to physical health (RP); bodily pain (BP); and general health (GH). We studied the association of QOL with self-reported demographics, health behaviors, physical impairments, surgeries, co-morbid medical conditions, medications, and MFS severity.
RESULTS
In the GenTAC registry, 389 adults with MFS completed the SF-36. Mean age was 41, 51% were men, 92% were white, and 65% were college graduates. The mean PCS composite score was 42.3. In bivariate analysis, predictors of better QOL included college education, marital status, higher household income, private health insurance, full-time employment, moderate alcohol use, fewer prior surgeries, fewer comorbid conditions, absence of depression, and less severe MFS manifestations. In a multivariable analysis, insurance status and employment remained significant predictors of QOL.
CONCLUSIONS
In a large cohort of patients with MFS in the GenTAC registry, health-related QOL was below the population norm. Better QOL was independently associated with socioeconomic factors, not factors related to general health or MFS severity.
Keywords: GenTAC, Marfan syndrome, quality of life, SF-36
INTRODUCTION
Marfan syndrome (MFS) is a hereditary, autosomal dominant disorder due to mutations in the fibrillin 1 gene, that affects connective tissue in multiple organs, most notably the eyes, skeleton, and aorta, with increased risk for thoracic aortic aneurysm and dissection. With advances in aortic surgery over the past 40 years, survival for people with MFS has increased from the third or fourth decade to the eighth (1). However, there continues to be substantial morbidity associated with MFS, including the sequelae of multiple surgeries and lifelong medical therapy (2–4). Not surprisingly, a growing body of literature suggests impaired quality of life (QOL) in patients with MFS (5–11), with most studies using the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) to assess QOL (5–13). Prior studies, however, were limited by small sample sizes and were therefore not able to identify independent factors associated with better or worse quality of life.
The SF-36 is a widely used and extensively validated questionnaire that assesses health related QOL. The questionnaire is subdivided into the Physical Component Score (PCS) and Mental Component Score (MCS). Because previous studies found that MFS predominantly affected the PCS (8,10,12,13), we used the PCS of the SF-36 to assess health related QOL in patients with MFS, and we evaluated the association of QOL with self-reported demographic factors, health behaviors, physical impairments, clinical characteristics, and MFS severity.
METHODS
The development and design of the GenTAC (Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions) registry have been previously described (14,15). Briefly, GenTAC was created as a multicenter, longitudinal, observational cohort study of patients with aortic aneurysm and associated genetic conditions, including MFS. Patients were enrolled at 8 sites: Johns Hopkins University, Baylor College of Medicine, Oregon Health & Sciences University, University of Pennsylvania, University of Texas Health Science Center at Houston, Weill Cornell Medical College, National Institute of Aging-Harbor Hospital and Queen’s Medical Center. Each site obtained Institutional Review Board approval, and each participant patient provided informed consent. Standardized data collection included patient questionnaires, imaging studies, and information about prior surgical procedures. The Research Triangle Institute International in Rockville, Maryland, served as the data coordinating center and was responsible for data management and statistical design and analysis (14,15).
STUDY SUBJECTS
We included patients in the GenTAC database who had MFS diagnosed by Ghent or revised Ghent criteria and confirmed by confirmed by a core phenotyping laboratory at Johns Hopkins University (16, 17), were age 18 or older, and had completed the SF-36. We excluded patients <18 both to be consistent with the existing literature on QOL in MFS and because parents could complete questionnaires for pediatric patients in GenTAC. This study used de-identified survey data from the GenTAC registry. Patients were enrolled in GenTAC from 2006 through December 31, 2013. The most recent analyses of our data were performed in September 2016.
SF-36 SCALE SCORING
Our analyses focused on QOL, which was measured with the PCS of the SF-36 (18,19). The PCS is comprised of 4 subscales: physical functioning (PF); role limitations due to physical health (RP); bodily pain (BP); and general health (GH). Each score ranges from 0 to 100, and is standardized to the population norm of 50 with a standard deviation of 10; higher scores indicate better QOL (18–20). Each of the 4 SF-36 subscales was standardized using a z-score transformation by subtracting the mean and standard deviation from the 1998 general United States population. Composite PCS was computed using the score coefficients from the 1990 general US population per the standard SF-36 scoring. The composite score is transformed to the norm based scoring, where the norm is set as 50 with a standard deviation of 10 (20).
VARIABLES
Self-reported variables were extracted from the Clinical Evaluation Form and the Enrollment Patient Questionnaire. The Clinical Evaluation Form includes questions about enrollment diagnosis, age at diagnosis, number of prior surgeries, number of medications or use of specific medications. For the Enrollment Patient Questionnaire, patients provided their date of birth and answered multiple choice questions about sex, race/ethnicity (White, Black or African American, Asian, American Indian, Native Hawaiian, or Pacific Islander), education, marital status, household income, health insurance status (employer private health insurance plan, Medicare, Medicaid, other), employment (full time, part time, unable to work, student, homemaker, unemployed, and retired); health behaviors, including use of cigarettes, alcohol and illicit drugs; vision or hearing impairment. This form asks about 47 medical conditions, including the genetic conditions associated with thoracic aortic aneurysms (numbers 1 through 7); cardiovascular history including murmur, palpitation, angina, heart attack, cardiomyopathy and others (numbers 8 through 16); hypertension; stroke; aneurysms; cancer; diabetes; bleeding or clotting disease; gastrointestinal disease; arthritis; autoimmune diseases; joint dislocations; cognitive issues; and depression.
To evaluate QOL data in the presence of phenotypic variability, we created a clinical severity scale to differentiate mild, typical, and severe disease. Two scores have been created previously, but neither has been validated (21,22). For the score used in this paper, we included features of MFS that could be assessed based on the self-reported data included in the questionnaires completed at GenTAC enrollment. We assigned points for features of MFS falling into 4 broad groups: skeletal, ocular, vascular and “other”. Points (in parentheses) for skeletal features were: scoliosis (1), scoliosis repair (3), pectus excavatum (1), pectus carinatum (1), pectus repair (2), and kyphosis or lordosis (1). Points for ocular features were: lens dislocation (3), retinal detachment (2), early onset glaucoma (2), and early onset cataracts (2). Points for vascular features were: enlarged aorta (1), dissection (4), mitral valve repair (3), aortic root replacement or valve surgery (3), and descending/thoracolumbar aortic repair (3). Points for other features included pneumothorax (1), migraines (1), and joint pain (1). Scores were graded as mild (0 to 2), typical (3 to 8), and severe (≥9). Relative scoring is similar to prior MFS severity scales. Also, similar to other MFS severity scales, prior surgery related to MFS increased severity to greater than mild, and ectopia lentis and aortic dissection each increase severity to greater than mild (21,22).
DATA ANALYSIS
We used SAS software (SAS Institute, Inc., Cary, North Carolina) to extract data from the secure enterprise network database to create reports and summary tables and to perform statistical analyses. To examine between-group differences we used SAS PROC GLM to run solutions for Type III ANOVA models and least squares mean estimates. The Tukey-Kramer test was used for post hoc pairwise comparisons for significant factors with 3 or more level. For data security purposes, all analyses were performed and all data were stored in a password-protected remote workspace. We included variables that were significant in bivariate analysis (p ≤0.05) in a multifactor analysis of variance to identify those most strongly associated with QOL.
RESULTS
Of the 871 patients with MFS in the GenTAC registry 643 were >18 years, of whom 389 (60% of GenTAC MFS patients age 18 or older) completed the PCS of the SF-36 (Table 1) and 254 did not. There was no difference between these 2 groups in gender or race, but the group that completed the SF-36 was older and had more severe MFS (Table 2).
Table 1.
Patient Characteristics
| Age | 18–39 years | 192 (49.4%) |
| 40–69 years | 183 (47.0%) | |
| ≥70 years | 14 (3.6%) | |
|
| ||
| Gender | Male | 199 (51.2%) |
|
| ||
| Race | White | 356 (91.5%) |
| Black | 16 (4.1%) | |
| Asian | 10 (2.6%) | |
| Other | 7 (1.8%) | |
|
| ||
| Education | Post-college | 127 (34.1%) |
| College graduate | 116 (31.2%) | |
| Some college | 75 (20.2%) | |
| High school graduate/GED | 54 (14.5%) | |
|
| ||
| Marital status | Married/unmarried partners | 214 (56.9%) |
| Divorced or separated | 37 (9.8%) | |
| Widowed | 5 (1.3%) | |
| Never married | 120 (31.9%) | |
|
| ||
| Household income | ≤$25,000 | 63 (18.2%) |
| $25,001 – 50,000 | 51 (14.7%) | |
| $50,0001–$75,000 | 72 (20.8%) | |
| $75,0001–$100,000 | 48 (13.9%) | |
| >$100,000 | 112 (32.4%) | |
|
| ||
| Health insurance | Private coverage | 282 (78.3%) |
| Non-private | 78 (21.7%) | |
|
| ||
| Employment | Full-time | 178 (48%) |
| Part-time | 23 (6.2%) | |
| Student | 35 (9.4%) | |
| Self-employed | 21 (5.7%) | |
| Retired | 27 (7.3%) | |
| Unable to work/disabled | 58 (15.6%) | |
| Unemployed | 17 (4.6%) | |
| Homemaker | 12 (3.2%) | |
|
| ||
| Alcohol Use | Never or once monthly | 161 (42.1%) |
| >1 monthly but not daily | 192 (50.3%) | |
| Almost everyday | 29 (7.6%) | |
|
| ||
| Hearing impairment | Yes | 39 (10.3%) |
|
| ||
| Vision impairment | Yes | 317 (83.4%) |
|
| ||
| Smoking | Smoker (>100 cigarettes) | 132 (34.6%) |
| Non-smoker | 250 (65.4%) | |
|
| ||
| Recreational drug use | Never | 256 (65.8%) |
| Past | 72 (18.5%) | |
| Current | 61 (15.7%) | |
|
| ||
| Prior surgeries | 0 | 105 (27.0%) |
| 1–2 | 154 (39.6%) | |
| 3+ | 130 (33.4%) | |
|
| ||
| Co-morbid conditions | 0–2 | 113 (29%) |
| 3–6 | 199 (51.2%) | |
| 7+ | 77 (19.8%) | |
|
| ||
| Beta blocker use | Yes | 313 (80.5%) |
|
| ||
| ARB use | Yes | 142 (36.5%) |
|
| ||
| Age at diagnosis (years) | <5 | 52 (15.0%) |
| 5–17 | 122 (35.2%) | |
| 18–39 | 127 (36.6%) | |
| 40+ | 46 (13.3%) | |
|
| ||
| Depression (Ever) | Yes | 79 (24.8%) |
|
| ||
| MFS severity score | Mild (0–2) | 27 (6.9%) |
| Typical (3–8) | 200 (51.4) | |
| Severe (9+) | 162 (41.7%) | |
Table 2.
Comparison of Marfan patients in GenTAC who completed the SF-36 versus those who did not
| Completed SF-36 | No SF-36 | p-value | ||
|---|---|---|---|---|
| Age at enrollment | 18–39 years | 192 (49.4%) | 158(62.2%) | |
| 40–69 years | 183 (47.0%) | 92 (36.2%) | ||
| ≥70 years | 16 (4.2%) | 4 (1.6%) | 0.004 | |
|
| ||||
| Gender | Male | 199 (51.2%) | 142 (55.9%) | 0.2 |
|
| ||||
| Race | White | 356 (91.5%) | 217 (85.4%) | |
| Black | 16 (4.1%) | 19 (7.5%) | ||
| Asian | 10 (2.6%) | 10 (3.9%) | ||
| Other | 7 (1.8%) | 8 (3.1%) | 0.1 | |
|
| ||||
| MFS severity score | Mild (0–2) | 27 (6.9%) | 33 (13%) | |
| Typical (3–8) | 200 (51.4) | 146 (57.5%) | ||
| Severe (9+) | 162 (41.7%) | 75 (29.5%) | 0.001 | |
|
| ||||
| Age at diagnosis | mean (standard deviation) | 21 (15.8) | 18.1 (14.2) | 0.03 |
Of the respondents who completed the SF-36, mean age was 41, half were women, and most self-identified as white. Most had some college education, one-third earned more than $100,000 annually, and nearly three-fourths had private health insurance. Scores for each subscale were PF 72.6 (or 45.6 with norm-based scoring), RP 46.2 (41.0), BP 65.9 (48.2), and GH51.3 (41.2). Using norm-based scoring, the composite PCS was 42.3, within 1 standard deviation from the population norm of 50.
In bivariate analysis (Table 3), better QOL, as indicated by higher scores across all 4 subscales and a higher PCS composite score, was associated with college education (PCS composite score 38.9 for education less than college vs. 44.3 for college graduates; p =0.001), marital status (34.6 for divorced or separated, 42.0 for married or in a partnership, 44.3 for never married; p =0.001), higher household income (35.7 for ≤$25,000, 42.0 for $25,001–100,000, and 44.2 for >$100,000; p <0.0001), private insurance (33.7 for Medicare or Medicaid vs. 44.8 for private insurance; p <0.0001), working as opposed to unable to work, unemployed, or retired (p <0.0001), moderate alcohol use (38.5 for rare alcohol, 42.9 for near daily or daily, 45.1 for more than monthly but less than daily; p <0.0001), fewer comorbid medical conditions (46.7 for 0 to 2, 43.0 for 3 to 6, and 33.9 for ≥7; p <0.0001), less severe MFS based on our scale (48.7 for mild, 44.6 for typical, 38.3 for severe; p <0.0001), and absence of depression (44.0 if no depression vs. 36.8 if depressed; p <0.0001).
Table 3.
Bivariate analysis of PCS subscale scores and composite scores with GenTAC variables
| PF score | p-value | RP score | p-value | BP score | p-value | GH score | p-value | PCS composite | p-value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.0003 | 0.0085 | 0.06 | 0.054 | 0.0022 | ||||||
| 18–39 | 47.7 | 42.9 | 49.5 | 42.3 | 44.4 | ||||||
| ≥40 | 43.8 | 39.4 | 47.1 | 40 | 40.4 | ||||||
| Gender | NS | NS | NS | NS | NS | ||||||
| Male | 46.2 | 40.9 | 48.6 | 41.5 | 42.7 | ||||||
| Female | 45 | 41.2 | 47.8 | 40.9 | 41.8 | ||||||
| Race | NS | NS | NS | NS | NS | ||||||
| White | 45.6 | 40.8 | 48.4 | 41.4 | 42.3 | ||||||
| Non-White | 45.6 | 43 | 45.9 | 39 | 41.6 | ||||||
| Education | 0.0297 | 0.004 | <0.0001 | 0.004 | 0.001 | ||||||
| <College graduate | 44.2 | 38.5 | 44.2 | 39.2 | 38.9 | ||||||
| College graduate | 46.7 | 42.6 | 50.5 | 42.7 | 44.3 | ||||||
| Marital status | 0.019 | 0.018 | 0.001 | 0.022 | 0.001 | ||||||
| Married or partnership | 45 | 40.8 | 48.5 | 41.6 | 42 | ||||||
| Divorced or separated | 41.6 | 35 | 40.1 | 36.3 | 34.6 | ||||||
| Never married | 47.5 | 42.7 | 49.3 | 41.8 | 44.3 | ||||||
| Widowed | 48.6 | 43.5 | 54.6 | 48.9 | 48.3 | ||||||
| Household income | <0.0001 | 0.0004 | <0.0001 | 0.0084 | <0.0001 | ||||||
| ≤ 25 K | 41.6 | 35.8 | 42.7 | 37.5 | 35.7 | ||||||
| 25–100 K | 45 | 40.7 | 48 | 41.8 | 42 | ||||||
| >100K | 49.1 | 43.8 | 52.6 | 42.9 | 44.2 | ||||||
| Health insurance | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||
| Private | 47.5 | 42.9 | 50.3 | 42.6 | 44.8 | ||||||
| Non-private | 39.5 | 34.4 | 41.3 | 36.1 | 33.7 | ||||||
| Employment | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||
| Working/Student/Home maker | 48.9 | 43.8 | 51.4 | 43.6 | 46.1 | ||||||
| Unable to work due to disability | 33.4 | 29.5 | 35.7 | 32.1 | 26.4 | ||||||
| Retired | 40.4 | 40.2 | 49.7 | 40.7 | 40.7 | ||||||
| Unemployed | 48.5 | 39.4 | 43.1 | 38.5 | 40.4 | ||||||
| Alcohol use | 0.0001 | 0.0004 | 0.0003 | 0.0058 | <0.0001 | ||||||
| Never or once/month | 43 | 37.8 | 45.1 | 39 | 38.5 | ||||||
| >1/month but not daily | 47.7 | 43.2 | 50.4 | 42.8 | 45.1 | ||||||
| Almost daily | 44.6 | 42.4 | 49 | 43 | 42.9 | ||||||
| Vision impairment | 0.65 | 0.69 | 0.19 | 0.36 | 0.68 | ||||||
| No | 46.3 | 40.4 | 46.4 | 42.4 | 41.7 | ||||||
| Yes | 45.6 | 41.1 | 48.7 | 41 | 42.5 | ||||||
| Hearing impairment | NS | 0.015 | 0.0001 | 0.05 | 0.0052 | ||||||
| No | 46 | 41.6 | 49 | 41.8 | 43 | ||||||
| Yes | 43.2 | 36.2 | 41.8 | 38.1 | 36.8 | ||||||
| Smoking | 0.044 | 0.06 | 0.09 | 0.65 | 0.046 | ||||||
| Smoker (>100 cigarettes) | 44.1 | 39.3 | 46.6 | 40.9 | 40.4 | ||||||
| Non-smoker | 46.4 | 41.8 | 48.9 | 41.4 | 43.2 | ||||||
| Recreational drug use | 0.18 | 0.58 | 0.26 | 0.066 | 0.18 | ||||||
| Never | 45.2 | 41.1 | 47.9 | 41.7 | 42.1 | ||||||
| Past | 47.7 | 41.9 | 50.1 | 42.2 | 44.5 | ||||||
| Current | 45.1 | 39.6 | 46.8 | 38.1 | 40.3 | ||||||
| Number of prior MFS-related surgeries | 0.0012 | 0.0084 | 0.04 | 0.06 | 0.002 | ||||||
| 0 | 47.4 | 44.1 | 49.3 | 42.4 | 44.8 | ||||||
| 1–2 | 46.7 | 40.7 | 49.3 | 42.1 | 43.1 | ||||||
| 3 or more | 42.9 | 38.9 | 45.9 | 39.3 | 39.1 | ||||||
| Number of comorbid conditions | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||
| 0–2 | 49.1 | 44.4 | 51.5 | 44.2 | 46.7 | ||||||
| 3–6 | 46.1 | 41.5 | 48.5 | 41.9 | 43 | ||||||
| 7 or more | 39.4 | 34.9 | 42.5 | 35.1 | 33.9 | ||||||
| Beta blocker use | 0.68 | 0.95 | 0.43 | 0.72 | 0.99 | ||||||
| No | 46.1 | 40.9 | 47.1 | 40.8 | 42.2 | ||||||
| Yes | 45.5 | 41 | 48.4 | 41.3 | 42.3 | ||||||
| Angiotensin receptor blocker use | 0.13 | 0.13 | 0.62 | 0.69 | 0.37 | ||||||
| No | 46.3 | 41.8 | 47.9 | 41.4 | 42.7 | ||||||
| Yes | 44.6 | 39.7 | 48.6 | 40.9 | 41.5 | ||||||
| Age at MFS diagnosis | NS | 0.0256 | NS | NS | 0.10 | ||||||
| <5 | 45.4 | 40.8 | 47.2 | 38.8 | 40.9 | ||||||
| 5–17 | 47.4 | 43.7 | 49.6 | 42.1 | 44.6 | ||||||
| 18–39 | 45 | 38.9 | 46.9 | 41.1 | 40.8 | ||||||
| 40+ | 45.8 | 39.6 | 49.5 | 42.8 | 42.6 | ||||||
| MFS Severity score | <0.0001 | <0.0001 | 0.0085 | 0.0003 | <0.0001 | ||||||
| Mild (0–2) | 49.7 | 48.9 | 50.5 | 46.0 | 48.7 | ||||||
| Typical (3–8) | 47.3 | 43.2 | 49.7 | 42.6 | 44.6 | ||||||
| Severe (9+) | 42.9 | 37.1 | 45.9 | 38.7 | 38.3 | ||||||
| Depression | <0.0001 | 0.0211 | <0.0001 | <0.0001 | <0.0001 | ||||||
| No | 47.1 | 41.9 | 49.7 | 42.1 | 44 | ||||||
| Yes | 42.3 | 38 | 43.1 | 35.7 | 36.8 |
PF = Physical functioning, RF = Role physical BP = Bodily pain, GH = General health, NS = not significant
Those unable to work due to disability had significantly lower scores on the PCS composite and each subscale, as compared to those who were retired, unemployed, or working. The working group scored highest on the composite and all subscales. Those unable to work due to disability also scored higher on the MFS severity score, had more MFS related surgeries, and had more comorbid conditions (Table 4).
Table 4.
Association of Unable to work due to disability and other variables
| Unable to work due to disability | ||||
|---|---|---|---|---|
| No | Yes | p-value | ||
| MFS Severity score | Mild (0–2) | 27 (8.6%) | 1 (1.7%) | 0.0002 |
| Typical (3–8) | 193 (61.7%) | 24 (41.4%) | ||
| Severe (9+) | 93 (29.7%) | 33 (56.9%) | ||
| Number of MFS-related surgeries | 0 | 89 (28.4%) | 7 (12.1%) | 0.002 |
| 1–2 | 126 (40.3%) | 20 (34.5%) | ||
| 3 or more | 98 (31.3%) | 31 (53.5%) | ||
| Number of comorbid conditions | 0–2 | 102 (35.6%) | 9 (15.5%) | <0.0001 |
| 3–6 | 168 (53.7%) | 18 (31.0%) | ||
| 7 or more | 43 (13.7%) | 31 (53.5%) | ||
Four variables were associated with better QOL in the PCS composite, but not across all 4 subscales: younger age (score of 44.4 for ages 18 to 39 vs. 40.4 for >40; p =0.0022), with 2 subscales significant (PF and RP) and 2 subscales trending toward significant (BP with p =0.0594 and GH, p =0.054); unimpaired hearing (43.0 vs. 36.8; p =0.0052); non-smokers (43.2 vs. 40.4; p =0.046); and fewer prior surgeries (no surgeries 44.8, 1 or 2 surgeries 43.1, ≥3 surgeries 39.1; p =0.002), with 3 subscales significant (PF, p =0.0012; BP, p =0.0084; and RP, p =0.04) and GH not significant (p =0.06).
Gender, race, recreational drug use, vision impairment, use of beta-blockers, and use of angiotensin receptor blockers were not significantly different across the composite or any of the subscales.
In the multivariate model (Table 5), only private insurance status (p =0.013) and employment (p <0.0001) were associated with better QOL as assessed by the composite PCS. In addition to the PCS score remaining significant, employment status also remained significant across all 4 subscales whereas insurance status was only significant across the PF and RP subscales. Marital status (p =0.057) and alcohol use (p =0.053) were no longer significant.
Table 5.
Adjusted model of PCS composite and subscale scores
| PF | p-value | RP (n = 249) | p-value | BP | p-value | GH | p-value | PCS Composite | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.38 | 0.7 | 0.44 | 0.47 | 0.65 | |||||
| 18 – 39 | 45.8 | 39.0 | 46.3 | 38.0 | 40.1 | |||||
| ≥ 40 | 44.7 | 38.4 | 45.2 | 39.0 | 39.5 | |||||
| Education | 0.67 | 0.89 | 0.043 | 0.67 | 0.37 | |||||
| Not a college graduate | 45.5 | 38.6 | 44.3 | 38.2 | 39.3 | |||||
| College graduate | 45.0 | 38.8 | 47.4 | 38.8 | 40.4 | |||||
| Marital Status | 0.13 | 0.14 | 0.08 | 0.43 | 0.057 | |||||
| Married or living with a partner | 43.2 | 38.2 | 44.1 | 37.0 | 37.9 | |||||
| Never married | 46.2 | 40.9 | 46.8 | 37.1 | 40.9 | |||||
| Widowed | 47.1 | 40.9 | 50.7 | 44.6 | 44.4 | |||||
| Divorced or separated | 44.3 | 34.8 | 41.6 | 35.3 | 36.0 | |||||
| Household income, annual | 0.022 | 0.59 | 0.07 | 0.97 | 0.10 | |||||
| ≤ $25K | 44.5 | 37.8 | 44.8 | 38.1 | 38.6 | |||||
| $>25–100K | 43.8 | 38.2 | 44.4 | 38.7 | 38.8 | |||||
| >$100K | 47.4 | 40.1 | 48.2 | 38.6 | 42.1 | |||||
| Health insurance | 0.033 | 0.042 | 0.07 | 0.18 | 0.013 | |||||
| Private coverage | 47.0 | 41.1 | 47.7 | 39.9 | 42.3 | |||||
| Non-Private | 43.5 | 36.3 | 43.9 | 37.1 | 37.4 | |||||
| Employment | <0.0001 | 0.0007 | 0.0001 | 0.012 | <0.0001 | |||||
| Working/Student/H omemaker | 48.1 | 40.5 | 47.7 | 39.7 | 42.4 | |||||
| Unable to work due to disability | 37.0 | 31.2 | 38.5 | 33.6 | 30.4 | |||||
| Retired | 45.3 | 43.8 | 51.3 | 43.0 | 44.3 | |||||
| Unemployed | 50.5 | 39.3 | 45.7 | 37.6 | 42.1 | |||||
| Alcohol Use in last 12 months | 0.045 | 0.24 | 0.19 | 0.41 | 0.053 | |||||
| 0 or <1 per month | 43.3 | 37.0 | 43.9 | 38.6 | 37.8 | |||||
| >1 month to 4 per week | 45.8 | 39.6 | 46.1 | 39.9 | 40.9 | |||||
| Almost every day or everyday | 46.6 | 39.4 | 47.4 | 36.9 | 40.8 | |||||
| Hearing impairment | 0.99 | 0.10 | 0.09 | 0.16 | 0.12 | |||||
| Yes | 45.2 | 42.5 | 43.9 | 37.0 | 38.2 | |||||
| No | 45.2 | 45.3 | 47.7 | 40.0 | 41.4 | |||||
| Number of prior MFS related surgeries | 0.32 | 0.56 | 0.55 | 0.50 | 0.69 | |||||
| None | 44.2 | 38.1 | 45.5 | 38.7 | 39.1 | |||||
| 1–2 surgeries | 46.3 | 38.0 | 46.8 | 39.3 | 40.5 | |||||
| 3+ surgeries | 45.2 | 40.0 | 45.1 | 37.4 | 39.8 | |||||
| Number of comorbid conditions | 0.18 | 0.37 | 0.54 | 0.25 | 0.12 | |||||
| 0–2 | 47.0 | 40.6 | 47.0 | 39.9 | 42.1 | |||||
| 3–6 | 45.0 | 38.3 | 45.3 | 39.3 | 39.7 | |||||
| 7+ | 43.7 | 37.2 | 45.2 | 36.3 | 37.7 | |||||
| Depression | 0.29 | 0.65 | 0.14 | 0.07 | 0.21 | |||||
| Yes | 44.5 | 39.1 | 44.5 | 36.9 | 38.8 | |||||
| No | 46.0 | 38.3 | 47.1 | 40.0 | 40.8 | |||||
| MFS severity score | 0.39 | 0.035 | 0.86 | 0.52 | 0.13 | |||||
| Mild (0–2) | 45.7 | 41.7 | 45.3 | 40.1 | 41.4 | |||||
| Typical (3–8) | 45.8 | 39.2 | 46.4 | 38.2 | 40.4 | |||||
| Severe (9+) | 44.2 | 35.2 | 45.7 | 37.1 | 38.7 |
NS = not significant
Tukey-Kramer post-hoc tests were performed on variables with ≥3 levels that were significant in the multivariate model. While the post hoc test for income showed significant differences in the PF subscale between the 2 higher income groups ($25,000 to $100,000 and >$100,000, p =0.02), the lower income group did not differ significantly from the other 2 categories. In the case of employment, QOL was significantly lower for those unable to work compare to the other employment categories but there was no significant difference between the other groups.
DISCUSSION
In this largest study to date of QOL in adults with MFS, using the extensively validated SF-36, health-related QOL was 42.3, below the population norm of 50 but within 1 standard deviation of the mean (18–20). The PCS composite score of 42.3 is better than scores seen in previous smaller studies of MFS patents (Table 6), including scores of 34.7 in Foran (22 patients with MFS) (8) and 36 in Rand-Hendriksen (84 patients with MFS) (12), but lower than the composite score of 45.5 in Schoormans’s study (121 patients with MFS) (10). Despite small sample sizes, nearly all of the prior studies of MFS patients found a reduction in the PCS composite or in the 4 component subscales (5,8–10,12,13) Three of these studies used control groups derived from national datasets as comparators (8,10,12). Fusar-Poli et al. found a reduction in MCS but not PCS, but their study was limited by a low response rate, with only 36 MFS patients enrolled out of 380 families who were approached (9). When Lane et al. looked at QOL in adults with congenital heart disease, only 6 of 276 patients had MFS, and so the authors could not comment about QOL in MFS (6). In a study of 174 MFS patients that assessed QOL with a different scale, the Ferrans and Powers Quality of Life Index, Cardiac Version III (QLI-Cardiac III) (7,23), QOL scores were low but comparable to adults with cardiovascular disease, while scores on the psychological/spiritual subscale were significantly lower than for cardiovascular disease (23).
Table 6.
Comparison of SF-36 PCS scores in MFS patients across different studies
| Study first author and year | Number of patients | PF | RP | BP | GH | PCS composite |
|---|---|---|---|---|---|---|
| Verbraecken J, 2001(5) | 15 MFS | 71 | 60 | 71 | 57 | Not reported |
| 24 healthy controls | 97 | 96 | 92 | 84 | Not reported | |
| Foran JR, 2005(8) | 22 MFS | Not reported | 34.7 | |||
| Normal controls | Not reported | 50 (population norm) | ||||
| Fusar-Poli P, 2008(9) | 36 MFS | 77.9 | 70.8 | 67.5 | 51.6 | 50.4 |
| No control group | 50 (population norm) | |||||
| Rand-Hendriksen S, 2010(12) | 84 MFS | 70 | 43 | 55 | 47 | 36 |
| Control, n = 420 (dataset derived) | 90 | 83 | 77 | 79 | 51 | |
| Schoormans, 2012(10) | 121 MFS | 79.2 | 68.8 | 70.6 | 57.0 | 45.5 |
| Control, n = 1742 (Dutch population)(37) | 83 | 76 | 75 | 71 | 50 (population norm) | |
| Current study | 389 MFS | 72.6 | 46.2 | 65.9 | 51.3 | 42.3 |
In the current study, variables associated with worse QOL in the bivariate model were less education, being divorced (as opposed to married, in a partnership, or never married), lower household income, public health insurance (as opposed to private), and inability to work due to disability (as opposed to working, unemployed, or retired). Interestingly, MFS severity, number of MFS-related surgeries, and number of comorbid conditions did not impact QOL as independent variables. However, people who were unable to work due to disability were more likely to score in the severe range on the MFS severity score. Of that group, 56.9% had a score of severe MFS, whereas only 29.7% of those in other employment categories had severe MFS, p =0.0002), to have had >3 surgeries (53.5% vs. 31.3%, p =0.002), and to have >7 comorbid conditions (53.5% vs. 13.7%, p <0.0001). This suggests that MFS patients in this study who were unable to work due to disability represent a category of patients with more severe disease.
Bathen et al. looked at fatigue in 73 adults with MFS, and were surprised to find no correlation between fatigue and MFS-related health problems, like aortic dissection or vision impairment. Instead, chronic pain and being unable to work (vs. employed or in school) were associated with increased fatigue (24). It is possible that those with chronic pain who were unable to work may also fall into this category of disability preventing employment.
In Rand-Hendriksen’s study of 84 MFS patients, a low PCS composite and low subscale scores were not associated with any of the variables assessed, which included gender, body mass index, ascending aortic surgery, use of beta-blockers, visual acuity, joint hypermobility, or number of Ghent criteria fulfilled (12). In a study of 121 MFS patients, a low PCS and low subscale scores did not correlate with disease severity (10). Similarly, in a study of 857 MFS patients that used a nonvalidated questionnaire, 26.5% of the 857 respondents thought they were severely affected by MFS, which did not correlate with MFS severity (22). In semi-structured interviews with 17 MFS patients, childhood teasing, concerns about physical appearance, and, for women, concerns about childbearing impacted QOL (25). These features were unfortunately not captured by the GenTAC registry.
In the multivariate model, registry participants with private health insurance, compared to public health insurance, and those who were working or retired, compared to unable to work due to disability, had better quality of life.
There was borderline significance to better quality of life with more frequent alcohol use (p =0.053) and with marital status (p =0.057). The divorced or separated group had the lowest PCS composite score, and the lowest scores for 3 of the 4 subscales. The widowed group had the highest scores, but it is premature to draw any conclusions about the widowed group, as it included only 5 patients (1.3% of study participants). While being married would seem to be a surrogate marker for presence of social support, studies in patients with acute coronary syndromes (26), congestive heart failure (27), and colorectal cancer (28) show that being married or in a partnership does not necessarily connote social support, and the relationship between social support and QOL is not linear.
Having private health insurance has correlated with improved QOL in patient groups as diverse as pediatric patients with sickle cell disease (29), >5.7 million adults with arrhythmias (30), adult survivors of colorectal cancer (31), a predominantly Black and Latino group of stroke survivors (32), and men with prostate cancer (33). Higher household income was not significantly associated with better QOL in our study, despite being associated with better QOL in a broad range of studies in non-MFS patients, including a nationwide sample of 2,700 American children (34), Chinese survivors of stroke (35), and lung cancer survivors (36).
STUDY LIMITATIONS
Limitations of the current study include the use of registry data. There is the possibility of selection bias as only 60% of MFS patients in GenTAC who were 18 or older completed the questionnaire, although prior studies of QOL in MFS also had low response rates (9,11,22–24). Those who completed the SF-36 tended to be older than those who did not, and had more severe MFS, as 41.7% of the completers had severe MFS, compared to 29.5% of those who did not complete the SF-36. As with all GenTAC studies, there may be differences between MFS patients that enroll in GenTAC and those who do not. GenTAC enrollees are seen at 1 of 8 major medical centers, which are referral centers for patients with genetic diseases that predispose to aortic aneurysms. While patients seen at these centers may not reflect MFS patients seen at local centers, each site strives to recruit all eligible patients to GenTAC. There may be limits to generalizability, as this population was predominantly white and well educated. Also, all variables were self-reported, although this has been previously validated.
CONCLUSIONS
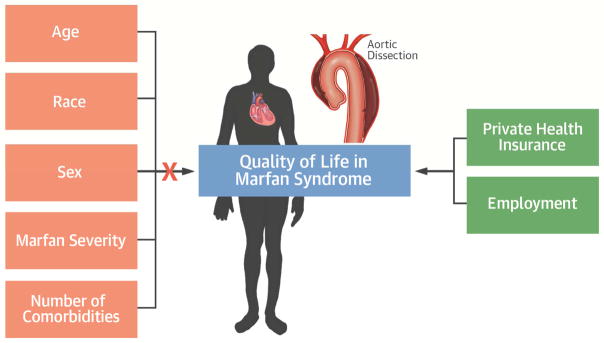
In a large cohort of adults with MFS, health-related QOL was 42.3, below the population norm of 50 but within 1 standard deviation of the mean. Registry participants with private health insurance, as opposed to public health insurance, and those who were working or retired, compared to unable to work due to disability, had better QOL. Notably, factors related to health and MFS severity did not correlate with better or worse QOL.
FIGURE 1.
Central Illustration. Factors that Impact Quality of Life in Marfan Syndrome
CLINICAL PERSPECTIVES.
COMPETENCY IN SYSTEMS-BASED PRACTICE
Health-related quality of life in patients with Marfan syndrome is below the population norm and more closely associated with socioeconomic factors, specifically employment and medical insurance, than with disease severity or general health status.
TRANSLATIONAL OUTLOOK
Factors that influence the quality of life should be considered in the design of clinical trials and systems of care to improve clinical outcomes for patients with Marfan syndrome.
Acknowledgments
Grants/Contracts/financial support: The GenTAC Registry has been supported by US Federal Government contracts HHSN268200648199C and HHSN268201000048C from the National Heart Lung and Blood Institute and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (Bethesda, MD).
Abbreviations
- BP
Bodily Pain (subscale of PCS)
- GenTAC
Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions
- FH
General Health (subscale of PCS)
- MFS
Marfan syndrome
- PCS
Physical Component Summary (PCS) of the MOS 36-Item Short-Form Health Survey
- PF
Physical functioning (subscale of PCS)
- RP
Role limitations due to physical health (subscale of PCS)
- QOL
Quality of life
- SF-36
Medical Outcomes Study 36-Item Short-Form Health Survey
Footnotes
Disclosures: None of the authors have relationships with industry relevant to the contents of the paper to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silverman DI, Burton KJ, Gray J, et al. Life expectancy in the Marfan syndrome. Am J Cardiol. 1995;75:157–60. doi: 10.1016/s0002-9149(00)80066-1. [DOI] [PubMed] [Google Scholar]
- 2.Song HK, Bavaria JE, Kindem MW, et al. Surgical treatment of patients enrolled in the national registry of genetically triggered thoracic aortic conditions. Ann Thorac Surg. 2009;88:781, 7. doi: 10.1016/j.athoracsur.2009.04.034. discussion 787–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes KW, Maslen CL, Kindem M, et al. GenTAC registry report: Gender differences among individuals with genetically triggered thoracic aortic aneurysm and dissection. Am J Med Genet A. 2013;161A:779–86. doi: 10.1002/ajmg.a.35836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinsaft JW, Devereux RB, Preiss LR, et al. Aortic dissection in patients with genetically mediated aneurysms: incidence and predictors in the GenTAC registry. J Am Coll Cardiol. 2016;67:2744–54. doi: 10.1016/j.jacc.2016.03.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verbraecken J, Declerck A, Van de Heyning P, De Backer W, Wouters EF. Evaluation for sleep apnea in patients with Ehlers-Danlos syndrome and Marfan: A questionnaire study. Clin Genet. 2001;60:360–5. doi: 10.1034/j.1399-0004.2001.600507.x. [DOI] [PubMed] [Google Scholar]
- 6.Lane DA, Lip GY, Millane TA. Quality of life in adults with congenital heart disease. Heart. 2002;88:71–5. doi: 10.1136/heart.88.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters KF, Kong F, Hanslo M, Biesecker BB. Living with Marfan syndrome III. Quality of life and reproductive planning. Clin Genet. 2002;62:110–20. doi: 10.1034/j.1399-0004.2002.620203.x. [DOI] [PubMed] [Google Scholar]
- 8.Foran JR, Pyeritz RE, Dietz HC, Sponseller PD. Characterization of the symptoms associated with dural ectasia in the Marfan patient. Am J Med Genet A. 2005;134A:58–65. doi: 10.1002/ajmg.a.30525. [DOI] [PubMed] [Google Scholar]
- 9.Fusar-Poli P, Klersy C, Stramesi F, Callegari A, Arbustini E, Politi P. Determinants of quality of life in Marfan syndrome. Psychosomatics. 2008;49:243–8. doi: 10.1176/appi.psy.49.3.243. [DOI] [PubMed] [Google Scholar]
- 10.Schoormans D, Radonic T, de Witte P, et al. Mental quality of life is related to a cytokine genetic pathway. PLoS One. 2012;7:e45126. doi: 10.1371/journal.pone.0045126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velvin G, Bathen T, Rand-Hendriksen S, Geirdal AO. Systematic review of the psychosocial aspects of living with Marfan syndrome. Clin Genet. 2015;87:109–16. doi: 10.1111/cge.12422. [DOI] [PubMed] [Google Scholar]
- 12.Rand-Hendriksen S, Johansen H, Semb SO, Geiran O, Stanghelle JK, Finset A. Health-related quality of life in Marfan syndrome: A cross-sectional study of short form 36 in 84 adults with a verified diagnosis. Genet Med. 2010;12:517–24. doi: 10.1097/GIM.0b013e3181ea4c1c. [DOI] [PubMed] [Google Scholar]
- 13.Winter MM, Reisma C, Kedde H, et al. Sexuality in adult patients with congenital heart disease and their partners. Am J Cardiol. 2010;106:1163, 8, 1168.e1–8. doi: 10.1016/j.amjcard.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Eagle KA GenTAC Consortium. Rationale and design of the national registry of genetically triggered thoracic aortic aneurysms and cardiovascular conditions (GenTAC) Am Heart J. 2009;157:319–26. doi: 10.1016/j.ahj.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroner BL, Tolunay HE, Basson CT, et al. The national registry of genetically triggered thoracic aortic aneurysms and cardiovascular conditions (GenTAC): results from phase I and scientific opportunities in phase II. Am Heart J. 2011;162:627–32. e1. doi: 10.1016/j.ahj.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476–85. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 17.De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996;62:417–26. doi: 10.1002/(SICI)1096-8628(19960424)62:4<417::AID-AJMG15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 19.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Ware JEKM. SF-36 physical and mental health summary scales: A manual for users of version 1. 2. Lincoln, RI: QualityMetric Incorporated; 2001. [Google Scholar]
- 21.Gray JR, Davies SJ. A clinical severity grading scale for Marfan syndrome. J Med Genet. 1996;33:758–9. doi: 10.1136/jmg.33.9.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Bie S, De Paepe A, Delvaux I, Davies S, Hennekam RC. Marfan syndrome in Europe. Community Genet. 2004;7:216–25. doi: 10.1159/000082265. [DOI] [PubMed] [Google Scholar]
- 23.Peters K, Apse K, Blackford A, McHugh B, Michalic D, Biesecker B. Living with Marfan syndrome: coping with stigma. Clin Genet. 2005;68:6–14. doi: 10.1111/j.1399-0004.2005.00446.x. [DOI] [PubMed] [Google Scholar]
- 24.Bathen T, Velvin G, Rand-Hendriksen S, Robinson HS. Fatigue in adults with Marfan syndrome, occurrence and associations to pain and other factors. Am J Med Genet A. 2014;164A:1931–9. doi: 10.1002/ajmg.a.36574. [DOI] [PubMed] [Google Scholar]
- 25.Van Tongerloo A, De Paepe A. Psychosocial adaptation in adolescents and young adults with Marfan syndrome: an exploratory study. J Med Genet. 1998;35:405–9. doi: 10.1136/jmg.35.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lett HS, Blumenthal JA, Babyak MA, et al. Social support and prognosis in patients at increased psychosocial risk recovering from myocardial infarction. Health Psychol. 2007;26:418–27. doi: 10.1037/0278-6133.26.4.418. [DOI] [PubMed] [Google Scholar]
- 27.Heo S, Lennie TA, Moser DK, Kennedy RL. Types of social support and their relationships to physical and depressive symptoms and health-related quality of life in patients with heart failure. Heart Lung. 2014;43:299–305. doi: 10.1016/j.hrtlng.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Sultan S, Fisher DA, Voils CI, Kinney AY, Sandler RS, Provenzale D. Impact of functional support on health-related quality of life in patients with colorectal cancer. Cancer. 2004;101:2737–43. doi: 10.1002/cncr.20699. [DOI] [PubMed] [Google Scholar]
- 29.Robinson MR, Daniel LC, O’Hara EA, Szabo MM, Barakat LP. Insurance status as a sociodemographic risk factor for functional outcomes and health-related quality of life among youth with sickle cell disease. J Pediatr Hematol Oncol. 2014;36:51–6. doi: 10.1097/MPH.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang DH, Gilligan AM, Romero K. Association of patient demographics on quality of life in a sample of adult patients with cardiac arrhythmias. Qual Life Res. 2014;23:129–34. doi: 10.1007/s11136-013-0445-2. [DOI] [PubMed] [Google Scholar]
- 31.Chambers SK, Meng X, Youl P, Aitken J, Dunn J, Baade P. A five-year prospective study of quality of life after colorectal cancer. Qual Life Res. 2012;21:1551–64. doi: 10.1007/s11136-011-0067-5. [DOI] [PubMed] [Google Scholar]
- 32.Dhamoon MS, Moon YP, Paik MC, et al. Quality of life declines after first ischemic stroke. The northern Manhattan study. Neurology. 2010;75:328–34. doi: 10.1212/WNL.0b013e3181ea9f03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadetsky N, Lubeck DP, Pasta DJ, Latini DM, DuChane J, Carroll PR. Insurance and quality of life in men with prostate cancer: data from the cancer of the prostate strategic urological research endeavor. BJU Int. 2008;101:691–7. doi: 10.1111/j.1464-410X.2007.07353.x. [DOI] [PubMed] [Google Scholar]
- 34.Vella SA, Magee CA, Cliff DP. Trajectories and predictors of health-related quality of life during childhood. J Pediatr. 2015;167:422–7. doi: 10.1016/j.jpeds.2015.04.079. [DOI] [PubMed] [Google Scholar]
- 35.Delcourt C, Hackett M, Wu Y, et al. Determinants of quality of life after stroke in china: The ChinaQUEST (QUality evaluation of stroke care and treatment) study. Stroke. 2011;42:433–8. doi: 10.1161/STROKEAHA.110.596627. [DOI] [PubMed] [Google Scholar]
- 36.Kenzik KM, Martin MY, Fouad MN, Pisu M. Health-related quality of life in lung cancer survivors: latent class and latent transition analysis. Cancer. 2015;121:1520–8. doi: 10.1002/cncr.29232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aaronson NK, Muller M, Cohen PD, et al. Translation, validation, and norming of the Dutch language version of the SF-36 health survey in community and chronic disease populations. J Clin Epidemiol. 1998;51:1055–68. doi: 10.1016/s0895-4356(98)00097-3. [DOI] [PubMed] [Google Scholar]