Abstract
Background
The utility of alpha fetoprotein (AFP) for hepatocellular carcinoma (HCC) surveillance is controversial. We aimed to identify factors associated with elevated AFP and define the patients for whom AFP is effective for surveillance.
Methods
Data from the National Cancer Institute Early Detection Research Network Phase 2 HCC biomarker study (233 early stage HCC and 412 cirrhotic patients) were analyzed. We analyzed 110 early stage HCC and 362 cirrhotic HCV patients for external validation. Sensitivity, specificity and area under the ROC curve (AUC) for HCC were calculated.
Results
HCV etiology, Non-White race, and serum alanine transaminase (ALT) predicted elevated AFP in cirrhotics. Non-White race and ALT predicted elevated AFP in HCC patients. Higher AUC of AFP for HCC was noted in patients with HBV (0.85) and alcohol (0.84) while it was lower in patients with HCV (0.80) and Non-viral/Alcohol etiology (0.76). The AUC was higher in HCV patients with serum ALT ≤ 40 U/L than patients with serum ALT>40 U/L (0.91 vs 0.75, P<0.01). At 90% specificity, the sensitivity of AFP increased from 44% to 74% in Whites with HCV and from 50% to 85% in Non-Whites with HCV. There was a trend towards higher AUC in HCV patients with serum ALT≤40 U/L than those with serum ALT>40 U/L (0.79 vs 0.69, P=0.10) in the validation cohort.
Conclusions
The satisfactory performance of AFP in HCV patients with normal ALT should be further validated.
Impact
The AFP may serve as a valuable surveillance test in HCV patients with normal ALT.
Keywords: AFP, biomarker, liver cancer
Introduction
Patients with hepatocellular carcinoma (HCC) have an extremely poor prognosis as they frequently present with advanced stage.(1) The overall survival of patients with HCC has been improving over the past 3 decades, in part due to earlier detection of cancers that are amenable to potentially curative treatments.(2,3) As early diagnosis is a key determinant of clinical outcomes in patients with HCC, the major liver societies recommend semiannual HCC surveillance in high risk patients including patients with cirrhotic liver disease.(4–6)
Serum alpha fetoprotein (AFP) is the tumor marker that has been most widely used in clinical practice for HCC surveillance. However, the utility of serum AFP as a surveillance test has been the subject of active debate. The American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) currently recommend liver ultrasound (US) as the primary surveillance test and the use of serum AFP for surveillance only when high quality liver US is not available.(4,6) However, guidelines from the Asia Pacific Association for the Study of the Liver (APASL) recommend both liver US and serum AFP every 6 months for HCC surveillance.(5)
The AFP can be falsely elevated in cirrhosis patients with active hepatitis, particularly viral hepatitis.(7) However, the best evidence for the efficacy of surveillance in improving survival and decreasing mortality of patients with HCC is from studies that have used US and AFP in combination.(8) A recent study from Taiwan showed that the use of AFP in addition to US significantly improves the sensitivity of surveillance compared to US alone without significant loss of specificity.(9) Large population based studies have also shown a benefit for surveillance using AFP alone.(10,11)
In this study, we aimed to identify factors associated with elevated AFP in patients with cirrhosis and early stage HCC in order to define the patient subgroups for whom AFP is most effective as a surveillance test for HCC.
Materials and Methods
Patients and Database
Data from the National Cancer Institute Early Detection Research Network (EDRN) phase 2 biomarker study case-control study for HCC were obtained.(12) Briefly, the study included 233 consecutive early stage HCC patients and 412 cirrhotic patients without HCC seen between February 2005 and August 2007 at seven tertiary referral centers in the US. The study was performed in compliance with institutional review board approvals from the participating centers.
Clinical information
Clinical information, including patient demographics and clinical characteristics were extracted from the previous phase 2 biomarker study database.(12) Etiology of liver disease was determined based on the viral serology (positive anti-HCV Antibody and/or HCV RNA for HCV; positive HBV surface antigen for HBV) and history of excessive use of alcohol (alcohol abuse, alcohol dependence, or alcoholic liver disease). Non-Viral/Alcohol etiology included etiologies other than HCV, HBV or Alcohol. This group was not further subdivided due to the small number of subjects in each subgroup.
HCC was defined by histopathologic examination or by the specific radiologic characteristics endorsed by American Association for the Study of Liver Diseases (AASLD).3 HCC stage was determined using the Barcelona Clinic Liver Cancer (BCLC) staging system and only very early or early stage HCC were included in the current study.(13)
The presence of cirrhosis was defined by histology or clinical evidence of portal hypertension in subjects with chronic liver disease. Subjects in the control group had an US, CT, or MRI showing no evidence of a hepatic mass within 6 months prior to enrollment. Patients with an AFP ≥20 ng/mL at enrollment were also required to have a CT or MRI showing no mass suggestive of HCC within the 3 months prior to enrollment or up to 2 weeks after consent. All controls were assessed by AFP and imaging 6 months after enrollment to ensure that they did not have HCC. Serum AFP was measured by automated systems (Wako, Mountain View, CA) at the time of enrollment prior to HCC specific treatment.(12)
Validation cohort
We used a previously characterized cohort of HCV cirrhosis patients with and without HCC from Parkland Health and Hospital System as a validation set.(14) All HCC cases met diagnostic criteria per AASLD guidelines; we only included the subset of very early or early stage HCC cases for this analysis, Serum AFP was measured prior to any HCC-directed therapy as part of routine clinical care. Non-HCC cirrhosis controls had a clinical diagnosis of cirrhosis as documented by their clinic provider (based on histology, imaging with a cirrhotic appearing liver, or clinical signs of cirrhosis) and were required to have at least 6 months of follow-up after AFP assessment to confirm the absence of HCC.
Statistical analysis
The Chi-square test or Fisher’s Exact test were used to compare categorical variables and the nonparametric Kruskal-Wallis Test for continuous variables. Factors associated with elevated AFP were tested using logistic regression analysis. An AFP cutoff of 10.9 mg/mL was used based on the maximum sum of sensitivity and specificity for early detection of HCC from our previous study; alternatively a cutoff of 20 ng/mL was used based on other previous studies.(7,12) Backward elimination logistic regression was used to construct the best multivariate model.
The sensitivity and specificity of AFP and the corresponding 95% confidence intervals (CI) were calculated using Youden index method in the subgroup of patients with combinations of different etiologies, races, and serum ALT. The receiver operating characteristic (ROC) curves were plotted for each subgroup of patients. The area under the ROC curve (AUC) was calculated, and its 95% CI was determined via 1000 bootstrap samples. DeLong’s test was used for the comparison of different ROC curves. Cross-validation (between 2 to 10-fold) was performed to protect against overfitting in a predictive model, considering limited sample size in each subgroup. Statistical analysis was carried out using SAS 9.3 (SAS Institute, Cary NC) and R version 3.0.2 (R Foundation, Vienna, Austria).
Results
Clinical Characteristics
A total of 412 patients with cirrhosis without HCC and 233 patients with early stage HCC were included in the study. Clinical characteristics of the patients are summarized in Table 1. HCV was the leading etiology of both cirrhosis (57.2%) and HCC (52.4%). Non-Viral/Alcohol etiology was the second most common cause of both cirrhosis (25.5%) and HCC (17.6%). Cryptogenic cirrhosis was the most common cause in Non-Viral/Alcohol etiology (accounted for 40% of cirrhosis and 42% of HCC).
Table 1.
Clinical Characteristics
| Patients with cirrhosis without HCC (N=412) | |||||
|---|---|---|---|---|---|
| Alcohol (N= 48) | HBV (N=23) | HCV (N=240) | Non-Viral/Non-Alcohol (N=101) | P | |
| Age, Mean ± SD | 56.7 ± 7.5 | 50.1 ± 9.5 | 53.4 ± 7.0 | 58.5 ± 11.0 | <0.01 |
| Male, n, (%) | 41 (85.4%) | 21 (91.3%) | 178 (74.2%) | 47 (46.5%) | <0.01 |
| Race | <0.01 | ||||
| White | 43 (89.6%) | 9 (39.1%) | 189 (78.8%) | 83 (82.2%) | |
| Asian | 0 (0%) | 13(56.5%) | 10 (4.2%) | 7 (6.9%) | |
| Non-White/Asian | 5 (10.4%) | 1 (4.4%) | 41 (17.1%) | 11 (10.9%) | |
| Child-Pugh | 0.01 | ||||
| A, n, (%) | 25 (52.1%) | 19 (82.6%) | 133 (55.4%) | 46 (45.5%) | |
| B–C, n, (%) | 23 (47.9%) | 4 (17.4%) | 107 (44.6%) | 55 (54.5%) | |
| Serum ALT (10 units) | 3.7 ± 2.1 | 5.2 ± 4.9 | 8.1 ± 5.8 | 4.6 ± 2.9 | <0.01 |
| Serum ALT ≤ 40, n, (%) | 33 (68.8%) | 12 (52.2%) | 63 (26.3%) | 60 (59.4%) | <0.01 |
| Serum AFP ≥ 10.9, n, (%) | 3 (6.3%) | 2 (8.7%) | 83 (34.6%) | 5 (4.9%) | <0.01 |
| Serum AFP ≥ 20, n, (%) | 1 (2.1%) | 0 (0%) | 46 (19.2%) | 1 (1.0%) | <0.01 |
| Patients with cirrhosis and HCC (N=233) | |||||
| Alcohol (N= 24) | HBV (N=46) | HCV (N=122) | Non-Viral/Non-Alcohol (N=41) | P | |
| Age, Mean ± SD | 64.6 ± 10.1 | 60.8 ± 12.3 | 58.7 ± 8.5 | 65.0 ± 11.8 | <0.01 |
| Male, n, (%) | 21 (87.5%) | 35 (76.1%) | 92 (75.4%) | 24 (58.5%) | 0.07 |
| Race | |||||
| White | 21 (87.5%) | 6 (13.0%) | 63 (51.6%) | 35 (85.4%) | <0.01 |
| Asian | 0 (0%) | 35 (76.1%) | 15 (12.3%) | 2 (4.9%) | |
| Non-White/Asian | 3 (12.5%) | 5 (10.9%) | 44 (36.1%) | 4 (9.8%) | |
| CTP | 0.35 | ||||
| A, n, (%) | 17 (70.8%) | 40 (87.0%) | 93 (76.2%) | 33 (80.5%) | |
| B, n, (%) | 7 (29.2%) | 6 (13.0%) | 29 (23.8%) | 8 (19.5%) | |
| Largest tumor size (cm), Mean ± SD | 3.8 ± 2.8 | 4.1 ± 2.7 | 3.4 ± 1.9 | 5.3 ± 3.6 | 0.01 |
| Number of lesions, Mean±SD | 1.3 ± 0.7 | 1.2 ± 0.5 | 1.3 ± 0.6 | 1.2 ± 0.6 | 0.90 |
| Serum ALT (10 units) | 4.9 ± 2.9 | 4.4 ± 2.6 | 8.9 ± 7.3 | 5.1 ± 4.7 | <0.01 |
| Serum ALT ≤ 40, n, (%) | 12 (50.0%) | 24 (52.2%) | 32 (26.2%) | 22 (53.7%) | <0.01 |
| Serum AFP ≥ 10.9, n, (%) | 14 (58.3%) | 30 (65.2%) | 99 (81.2%) | 22 (53.7%) | <0.01 |
| Serum AFP ≥ 20, n, (%) | 12 (50.0%) | 28 (60.9%) | 78 (63.9%) | 19 (46.3%) | 0.19 |
BCLC: Barcelona clinic liver cancer; CTP: Child-Turcotte-Pugh
In the cirrhosis group, a higher proportion of HCV patients had serum ALTs >40 IU/L, AFP ≥10.9 ng/mL or ≥20 ng/mL than in the HBV, Alcohol, or Non-Viral/Alcohol groups of patients. Similarly, a higher proportion of HCV patients had serum ALT>40 IU/L or AFP ≥10.9 ng/mL than the rest of the patients in the HCC group.
Factors associated with elevated AFP in patients with cirrhosis and early stage HCC groups
The distribution of serum AFP in each of the race, etiology and serum ALT subgroups is shown in supplementary Figure 1. Table 2 summarizes factors associated with elevated AFP in the cirrhosis group. For serum AFP ≥10.9 ng/mL or 20 ng/mL, Non-White race, HCV etiology and serum ALT were independent predictors of elevated AFP. Factors associated with elevated AFP in the HCC group are summarized in Table 3. For serum AFP ≥10.9 ng/mL, serum ALT was independently associated with elevation of AFP. When the cut-off for serum AFP was increased to ≥20 ng/mL, serum ALT, male gender and Non-White race were independently associated with elevation of AFP.
Table 2.
Factors associated with elevated AFP (AFP ≥10.9 or 20) in patients with cirrhosis
| Serum AFP≥10.9 ng/mL | Serum AFP≥20 ng/mL | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| OR | P | AOR | P | OR | P | AOR | P | |
| Age | 0.98 (0.95–1.00) | 0.06 | 0.98 (0.95–1.02) | 0.25 | ||||
| Gender (Male) | 0.92 (0.56–1.53) | 0.76 | 1.05 (0.55–2.01) | 0.88 | ||||
| Race | 0.10 | 0.03 | 0.04 | <0.01 | ||||
| White (reference) | - | |||||||
| Asian | 1.42 (0.61–3,34) | 3.44 (1.09–10.8) | 1.89 (0.68–5.29) | 0.23 | 6.06 (1.69–21.7) | |||
| Non-White/Asian | 1.90 (1.03–3.51) | 1.91 (0.95–3.83) | 2.47 (1.18–5.14) | 0.02 | 2.48 (1.12–5.50) | |||
| CTP | 0.88 | 0.22 | ||||||
| A (reference) | - | |||||||
| B | 0.96 (0.61–1.53) | 0.68 (0.37–1.26) | ||||||
| Etiology | <0.01 | <0.01 | <0.01 | <0.01 | ||||
| Non-Viral/Alcohol (reference) | - | |||||||
| Alcohol | 1.28 (0.29–5.59) | 1.82 (0.40–8.27) | 2.13 (0.13–34.8) | 3.10 (0.18–52.1) | ||||
| HBV | 1.83 (0.33–10.07) | 0.68 (0.09–5.42) | - | - | ||||
| HCV | 10.2 (3.97–25.9) | 6.75 (2.53–18.0) | 23.7 (3.22–174) | 19.0 (2.50–144) | ||||
| ALT (per 10 units) | 1.22 (1.16–1.29) | <0.01 | 1.17 (1.11–1.24) | <0.01 | 1.13 (1.08–1.18) | <0.01 | 1.10 (1.04–1.16) | <0.01 |
ALT: Alanine transaminase; CTP: Child-Turcotte-Pugh;
Table 3.
Factors associated with elevated AFP (AFP≥10.9 or 20) in patients with HCC
| Serum AFP≥10.9 ng/mL | Serum AFP≥20 ng/mL | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| OR | P | AOR | P | OR | P | AOR | P | |
| Age | 0.98 (0.95–1.00) | 0.10 | 0.99 (0.96–1.01) | 0.34 | ||||
| Gender (Male) | 1.37 (0.70–2.66) | 0.36 | 1.62 (0.88–3.00) | 0.12 | 1.97(1.03–3.76) | 0.04 | ||
| Race | 0.04 | <0.01 | <0.01 | |||||
| White (reference) | ||||||||
| Asian | 1.34 (0.66–2.71) | 1.92 (0.98–3.75) | 2.02 (1.02–4.01) | |||||
| Non-White/Asian | 2.84 (1.27–6.33) | 2.78 (1.40–5.52) | 2.68 (1.33–5.43) | |||||
| CTP | 0.84 | 0.65 | ||||||
| A (reference) | ||||||||
| B | 1.08 (0.54–2.16) | 0.86 (0.46–1.63) | ||||||
| Etiology | <0.01 | 0.19 | ||||||
| Non-Viral/Alcohol (reference) | - | |||||||
| Alcohol | 1.21 (0.44–3.35) | 1.16 (0.42–3.17) | ||||||
| HBV | 1.62 (0.68–3.84) | 1.80 (0.77–4.23) | ||||||
| HCV | 3.72 (1.73–7.80) | 2.05 (1.00–4.20) | ||||||
| ALT (per 10 units) | 1.14 (1.05–1.24) | <0.01 | 1.14 (1.05–1.24) | <0.01 | 1.07 (1.01–1.13) | 0.03 | 1.07(1.01–1.14) | 0.02 |
ALT: Alanine transaminase; CTP: Child-Turcotte-Pugh
Etiology and race specific performance of serum AFP for the diagnosis of early stage HCC
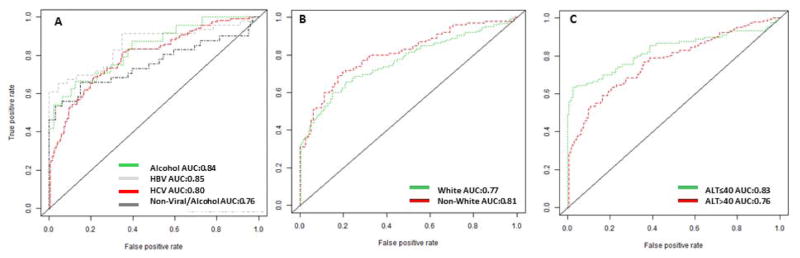
As elevation of serum AFP was associated with etiology of liver disease, race and serum ALT, we generated ROC curves for each subgroup of patients based on etiology, race and serum ALT (Figure 1 A–C). The best test performance for AFP was achieved in the subgroup with HBV etiology, with an overall AUC of 0.85, compared with AUCs of 0.84 for alcohol etiology, 0.80 for HCV etiology and 0.76 for Non-Viral/Alcohol etiology (0.85 vs. 0.76, p=0.17) (Figure 1A).
Figure 1.
The ROC of AFP for HCC diagnosis in each subgroup.
X-axis: 1-specificity; Y-axis: Sensitivity
1A-The ROC of AFP for HCC diagnosis per etiology
1B-The ROC of AFP for HCC diagnosis per race
1C-The ROC of AFP for HCC diagnosis per ALT
The AUC for AFP was marginally higher but not significantly different in the Non-White group compared to the White group (P=0.31) (Figure 1B). There was a trend towards higher AUC for AFP in the serum ALT≤40 U/L group compared to the group with serum ALT>40 U/L (P=0.14) (Figure 1C).
Table 4 shows the best cutoffs for serum AFP determined by the point in the ROC curve that maximizes sensitivity and specificity. The sensitivity of serum AFP with specificity set at 90% and the specificity when sensitivity was set at 70% are reported in Supplementary Table 1.
Table 4.
Cutoffs for AFP at the Maximum Sensitivity and Specificity in the Receiver Operating Characteristic Curve
| Whole group (N=645) | ||||
|---|---|---|---|---|
| Subgroups | AFP Cut off | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) |
| Alcohol | 8.3 | 0.67 (0.45–0.84) | 0.88 (0.75–0.95) | 0.84 (0.74–0.94) |
| Alcohol in White | 8.3 | 0.71 (0.48–0.89) | 0.88 (0.75–0.96) | 0.86 (0.76–0.96) |
| Alcohol in Non-White | 4.4 | 1.00 (0.16–1.00) | 0.60 (0.15–0.95) | 0.80 (0.34–1.00) |
| HBV | 13.6 | 0.65 (0.50–0.79) | 0.96 (0.78–1.00) | 0.85 (0.77–0.94) |
| HBV in White | 9.9 | 0.67 (0.22–0.96) | 1.00 (0.66–1.00) | 0.87 (0.67–1.00) |
| HBV in Non-White | 21.5 | 0.63 (0.46–0.77) | 1.00 (0.77–1.00) | 0.84 (0.73–0.95) |
| HCV | 17.6 | 0.69 (0.60–0.77) | 0.79 (0.73–0.84) | 0.80 (0.75–0.85) |
| HCV in White | 14.1 | 0.67 (0.54–0.78) | 0.75 (0.68–0.81) | 0.76 (0.69–0.83) |
| HCV in Non-White | 21.9 | 0.74 (0.60–0.85) | 0.72 (0.57–0.84) | 0.79 (0.70–0.88) |
| Non-Viral/Alcohol | 7.4 | 0.66 (0.49–0.80) | 0.85 (0.77–0.91) | 0.76 (0.65–0.86) |
| Non-Viral/Alcohol in White | 10.7 | 0.54 (0.37–0.71) | 0.95 (0.88–0.99) | 0.74 (0.62–0.85) |
| Non-Viral/Alcohol in Non-White | 29 | 0.75 (0.19–0.99) | 1.00 (0.79–1.00) | 0.91 (0.74–1.00) |
| Patients with ALT≤40 (N=258) | ||||
| Subgroups | AFP Cut off | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) |
| Alcohol | 14 | 0.50 (0.21–0.79) | 1.00 (0.89–1.00) | 0.75 (0.58–0.93) |
| Alcohol in White | 14 | 0.50 (0.19,0.81) | 1.00 (0.89–1.00) | 0.75 (0.56–0.94) |
| Alcohol in Non-White | 4.4 | 1.00 (0.16–1.0) | 0.50 (0.01–0.99) | 0.75 (0.58–1.00) |
| HBV | 4.7 | 0.92 (0.73–0.99) | 0.83 (0.52–0.98) | 0.88 (0.77–1.00) |
| HBV in White | 5.3 | 1.00 (0.40–1.00) | 0.75 (0.35–0.97) | 0.94 (0.79–1.00) |
| HBV in Non-White | 4.7 | 0.90 (0.68–0.99) | 1.00 (0.040–1.00) | 0.90 (0.76–1.00) |
| HCV | 10.9 | 0.78 (0.60–0.91) | 0.95 (0.87–0.99) | 0.91 (0.85–0.98) |
| HCV in White | 10.9 | 0.74 (0.49–0.91) | 0.96 (0.85–0.99) | 0.91 (0.83–.099) |
| HCV in Non-White | 15.4 | 0.85 (0.55–0.98) | 0.93 (0.66–1.00) | 0.89 (0.76–1.00) |
| Non-Viral/Alcohol | 15.3 | 0.59 (0.36–0.79) | 0.98 (0.91–1.00) | 0.74 (0.57–0.90) |
| Non-Viral/Alcohol in White | 15.3 | 0.56 (0.31–.07.8) | 1.00 (0.93–1.00) | 0.69 (0.50–0.88) |
| Non-Viral/Alcohol in Non-White | 29 | 1.00 (0.16–1.00) | 1.00 (0.63–1.00) | 1.00 (1.00–1.00) |
Serum ALT and performance of serum AFP for the diagnosis of early stage HCC
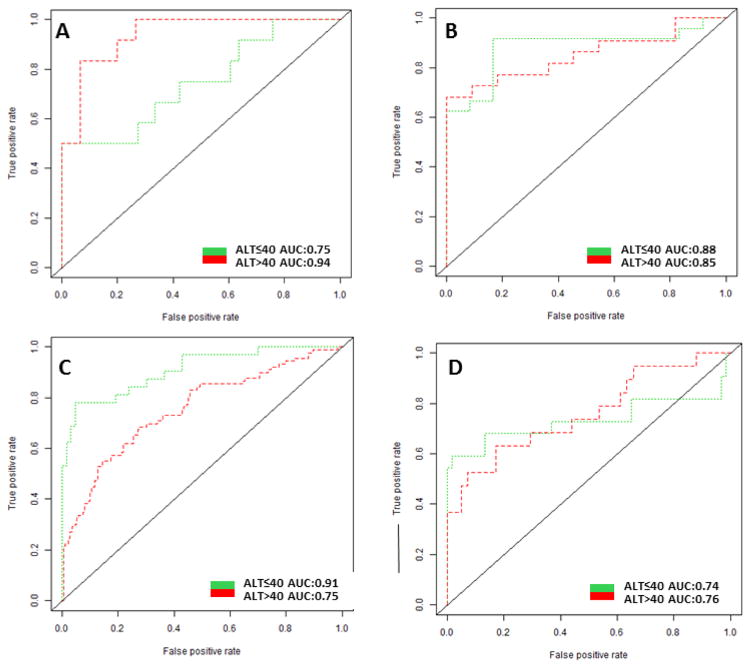
Figure 2 shows AUCs in patients with serum ALT≤40 compared to patients with serum ALT>40 in each etiology subgroups. The AUC was significantly higher in HCV patients with serum ALT≤40 compared to patients with serum ALT>40 (P<0.01) (Figure 2C). The AUC increased from 0.76 to 0.91 in Whites with HCV and from 0.79 to 0.89 in Non-Whites with HCV (Table 4). At 90% specificity, the sensitivity increased from 44% to 74% in Whites with HCV and from 58% to 85% in Non-Whites with HCV (Supplementary Table 1). At a fixed sensitivity of 70%, the specificity increased from 69% to 96% in Whites with HCV and from 74% to 93% in Non-Whites with HCV (Supplementary Table 1). In the alcohol etiology subgroup, there was a trend towards higher AUC in patients with serum ALT>40 compared to patients with serum ALT≤40 (0.94 vs 0.75, P=0.06) (Figure 2A). A test for cross-validation showed similar AUCs of AFP for HCC detection in each subgroup (Supplementary Table 2).
Figure 2.
The ROC of AFP for HCC diagnosis in patients with ALT≤40 vs. ALT>40
X-axis: 1-specificity; Y-axis: sensitivity
2A-The ROC of AFP for HCC diagnosis per ALT in patients with Alcohol etiology
2B-The ROC of AFP for HCC diagnosis per ALT in patients with HBV etiology
2C-The ROC of AFP for HCC diagnosis per ALT in patients with HCV etiology
2D-The ROC of AFP for HCC diagnosis per ALT in patients with Non-Viral/Alcohol etiology
Performance of serum AFP for the diagnosis of early stage HCC in HCV patients with serum ALT≤40 in the validation cohort
Baseline characteristics of the validation cohort are summarized in Supplementary Table 3. Among the 110 HCV patients with HCC, 31 (29%) had ALT ≤40. Among 362 HCV patients with cirrhosis, 140 (39%) had ALT ≤40. Overall, an AFP cut-off of 9.9 ng/mL yielded a sensitivity of 65% and specificity of 66%, with an AUC of 0.73. There was a trend towards higher AUC in HCV patients with serum ALT≤40 U/L than those with serum ALT>40 U/L (0.79 vs 0.69, P=0.10) (Supplementary Figure 2). When the analysis was repeated among HCV patients with normal ALT, an AFP cut-off of 8 ng/mL (cut-off that maximized sensitivity and specificity) yielded a sensitivity of 71% and specificity of 75%. The same AFP cut off of 8 ng/mL yielded a sensitivity of 78% and specificity of 84% in the discovery set.
Discussion
In the current study, we identified factors associated with elevated AFP in patients with cirrhosis and early stage HCC, with the goal of defining the specific subgroups in which AFP shows the greatest utility for HCC screening. HCV etiology, Non-White race and serum ALT were independently associated with elevated AFP in patients with cirrhosis, while Non-White race and ALT were independent predictors of elevated AFP in patients with early stage HCC. The performance of serum AFP was heavily influenced by serum ALT in HCV patients. After excluding HCV patients with ALT>40 U/L, the AUC of AFP for HCC increased from 0.76 to 0.91 in Whites with HCV and from 0.79 to 0.89 in Non-Whites with HCV (Table 4). At 90% specificity, the sensitivity of AFP for HCC increased from 44% to 74% in Whites with HCV and from 58% to 85% in Non-Whites with HCV (Supplementary Table 1). At a fixed sensitivity of 70%, the specificity of AFP for HCC increased from 69% to 96% in Whites with HCV and from 74% to 93% in Non-Whites with HCV (Supplementary Table 1). The cross-validation test confirmed stable AUCs of AFP for HCC detection in each subgroup and limited external validation again showed a trend towards improved performance of the AFP for HCC detection in HCV patients with normal ALT (Supplementary Figure 2).
The utility of serum AFP as a screening test for use in HCC surveillance has been controversial, due to its reported low sensitivity for detection of early stage HCC.(8,10,11,15,16) Cost effectiveness analysis showed that semi-annual AFP and US is the most effective strategy in reducing HCC mortality with 46% reduction in HCC mortality.(17) A single center prospective cohort study of 446 cirrhosis patients showed that US and AFP had sensitivities of 44% and 66% and specificities of 92% and 91%, respectively, for the detection of HCC. The sensitivity significantly improved to 90%, with a minimal decrease in specificity to 83% when AFP and US were used in combination, suggesting a benefit of the combination of US and AFP as HCC surveillance tests.(18) A more recent study from Taiwan clearly demonstrated the benefit of AFP in HCC surveillance.(19) In this large retrospective cohort study of 1597 Taiwanese cirrhosis patients, US had a sensitivity and specificity of 92.0% and 74.2%, respectively. Using an AFP cut-off of 20 ng/mL or an AFP level increase of ≥2-fold from its nadir during the previous 1 year as a trigger for cross-sectional imaging, the combination of US and AFP improved the overall sensitivity of surveillance from 92% to 99.2%, with a minimal decrease in specificity from 74.2% to 71.5%, supporting the use of AFP as an adjunct to US for HCC surveillance. When stratified by etiology, the area under the ROC curve was highest in patients with HBV-induced HCC, suggesting that AFP performs best in patients with HBV. A large population-based study from Alaska also showed that serial AFP testing in patients with chronic HBV infection was associated with earlier diagnosis of HCC, a higher likelihood of resection, and improved overall survival.(11)
Prior studies have also shown worse performance of AFP as a screening test for HCC in patients with HCV. A multicenter retrospective study showed that approximately 20% of individuals with HCV cirrhosis had an AFP ≥20 ng/mL in the absence of HCC.(20) In the prospective Hepatitis C Antiviral Long-Term Treatment against Cirrhosis (HALT-C) study of HCV patients with advanced fibrosis or cirrhosis, a significant proportion of the participants had falsely elevated AFP in the absence of HCC; 27% of subject with HCV cirrhosis had a serum AFP ≥20ng/dL at enrollment.(21) Interestingly, the HALT-C study results showed that as the HCV RNA level declined with antiviral treatment, the serum AFP concentration also declined, suggesting that active replication of HCV virus and inflammation of the liver contribute to the false positive elevation of AFP levels.(21) The satisfactory performance of serum AFP in the subset of HCV patients with normal ALT in the current study is in line with the evidences reported in the literature.(22,23) As more and more patients with HCV cirrhosis achieve sustained virologic response in the era of highly potent directly acting antiviral (DAA) therapy, serum AFP may prove to be an excellent surveillance test in this increasing subgroup of patients who remained at high risk of developing HCC.(24,25)
The association between race and the performance of AFP in surveillance has not been evaluated rigorously in the literature. Several studies have reported higher frequencies of falsely elevated AFP levels in African American patients with cirrhosis, primarily patients with HCV cirrhosis.(14,20,21) Whether there is an independent association between African American race and false positive elevation of AFP levels or the apparent association was confounded by other factors such as virologic features or activity of hepatitis remains to be determined. The small number of African American subjects (7%) in our study precluded us from evaluating the performance of AFP in African Americans.
The association between elevation of AFP and severity of hepatic inflammation has been well reported but the underlying mechanism of such association is poorly understood.(26) The association between elevated ALT and AFP in the absence of HCC suggests that AFP production increase in the presence of enhanced hepatocyte destruction and regeneration of liver progenitor cells with a less differentiated phenotype.(27) However this association seems to be etiology and race specific given that both race and etiology are important determinants of elevated AFP even after controlling for serum ALT. Underlying mechanisms connecting severity of hepatic inflammation, Non White race, viral etiology of liver disease and elevated AFP levels should be further investigated in future studies.
Unique features of our study are the focus on identifying factors associated with elevated AFP in patients with cirrhosis versus early stage HCC. We also specifically evaluated the performance of AFP as a screening test in different etiologic and racial subgroups, allowing comparison between the subgroups. On the other hand, our data strongly suggest that AFP is a good test for early detection of HCC in patients with HCV cirrhosis with minimal hepatic inflammation.
Our study has several limitations. This is a retrospective phase 2 biomarker case control study that could have been affected by unmeasured potential biases. Evaluating the performance of AFP for HCC in the presence of known HCC may not be the same as evaluating the performance of AFP for HCC detection in a prospective cohort of patients with cirrhosis. A larger phase three prospective multicenter cohort biomarker study is now underway to validate the current findings.(27,28) This ongoing biomarker study will be able to address whether baseline serum AFP can predict subsequent development of HCC on imaging. Due to sample size limitations, subgroup analysis results might not be as reliable, particularly in non HCV subgroups. Sample sizes became smaller when further broken down by race or ALT, which has limited the power of statistical analysis. In the same line, it was hard to interpret data on the lack of improvement of ROC in non HCV subgroup patients with normal ALT and should be further evaluated in the future study. It is important to note that the HCV cohort in the current study was established before highly potent DAA treatments became available. Therefore, the proportion of patients with normal ALT was lowest in the HCV subgroup. More recently, a large proportion of HCV cirrhosis patients are recipients of DAA treatment. Thus, it is likely that the proportion of HCV cirrhosis patients with normal ALT will further increase with time and the good performance of AFP for HCC detection in HCV patients with normal ALT will be more generalizable.
While normal serum ALT cut off is somewhat controversial, normal ALT cut off was set at 40 U/L in the current study due to small sample size as setting up normal ALT cut off at 20 or 30 U/L did not provide enough sample sizes for subgroup analysis. Our study was also not able to assess the performance of longitudinal trends in the serum AFP. As shown in previous studies and applied in clinical practice, we anticipate that assessing longitudinal trends in AFP levels would further improve the sensitivity and specificity of AFP compared to the levels reported in the current study.(19,29–31) Lastly, our study does not have information on the performance of US in HCC screening. Thus the results of the current study cannot address whether AFP is of additional benefit to US in HCC surveillance. However our study shows that AFP may perform better than the historically reported performance of US for the diagnosis of HCC particularly in subjects with HCV cirrhosis and normal serum ALT.
In conclusion, our study suggests that race, etiology of HCC, and severity of hepatic inflammation are associated with the elevation of AFP in patients with cirrhosis with/without HCC. The good performance of serum AFP for early stage HCC diagnosis in HCV patients with normal ALT is clinically relevant in the era of highly-potent directly acting antiviral (DAA) therapy. Given the relatively small sample size of the HCV patients with normal ALT and the retrospective study design, the performance of AFP in HCV with normal ALT should be further validated in large prospective cohort studies.
Supplementary Material
Acknowledgments
Fundings: This publication was supported by Grant Number T32 DK07198 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (to J. Yang) and CA165076 from the National Cancer Institute (NCI)(to L.R. Roberts). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
We appreciate generous support from American Liver Foundation: 2016 Hans Popper Memorial Postdoctoral Research Fellowship award (to J.Yang)
Footnotes
Conflict of Interest:
Nothing to disclose except for Hiroyuki Yamada and Amit G. Singal. Mr. Yamada works for Wako Diagnostics that provided test kits for the measurement of AFP.
Dr. Singal receives speakers bureau honoraria from Bayer and Consultant/Advisory Board fee from Bayer and Wako Diagnostics
References
- 1.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nature reviews Gastroenterology & hepatology. 2010;7:448–58. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittal S, Kanwal F, Ying J, Chung R, Sada YH, Temple S, et al. Effectiveness of Surveillance for Hepatocellular Carcinoma in Clinical Practice: A United States cohort. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan CH, Low SC, Thng CH. APASL and AASLD Consensus Guidelines on Imaging Diagnosis of Hepatocellular Carcinoma: A Review. International journal of hepatology. 2011;2011:519783. doi: 10.4061/2011/519783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Journal of hepatology. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Yang JD, Kim WR. Surveillance for hepatocellular carcinoma in patients with cirrhosis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10:16–21. doi: 10.1016/j.cgh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. Journal of cancer research and clinical oncology. 2004;130:417–22. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 9.Chang TS, Wu YC, Tung SY, Wei KL, Hsieh YY, Huang HC, et al. Alpha-Fetoprotein Measurement Benefits Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis. The American journal of gastroenterology. 2015;110:836–44. doi: 10.1038/ajg.2015.100. [DOI] [PubMed] [Google Scholar]
- 10.Marrero JA, El-Serag HB. Alpha-fetoprotein should be included in the hepatocellular carcinoma surveillance guidelines of the American Association for the Study of Liver Diseases. Hepatology. 2011;53:1060–1. doi: 10.1002/hep.24033. [DOI] [PubMed] [Google Scholar]
- 11.McMahon BJ, Bulkow L, Harpster A, Snowball M, Lanier A, Sacco F, et al. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology. 2000;32:842–6. doi: 10.1053/jhep.2000.17914. [DOI] [PubMed] [Google Scholar]
- 12.Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–8. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Seminars in liver disease. 1999;19:329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 14.Gopal P, Yopp AC, Waljee AK, Chiang J, Nehra M, Kandunoori P, et al. Factors that affect accuracy of alpha-fetoprotein test in detection of hepatocellular carcinoma in patients with cirrhosis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:870–7. doi: 10.1016/j.cgh.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherman M. Serological surveillance for hepatocellular carcinoma: time to quit. Journal of hepatology. 2010;52:614–5. doi: 10.1016/j.jhep.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–41. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Jackson S, et al. Surveillance of cirrhosis for hepatocellular carcinoma: a cost-utility analysis. British journal of cancer. 2008;98:1166–75. doi: 10.1038/sj.bjc.6604301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singal AG, Conjeevaram HS, Volk ML, Fu S, Fontana RJ, Askari F, et al. Effectiveness of hepatocellular carcinoma surveillance in patients with cirrhosis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:793–9. doi: 10.1158/1055-9965.EPI-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang TS, Wu YC, Tung SY, Wei KL, Hsieh YY, Huang HC, et al. Alpha-Fetoprotein Measurement Benefits Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis. The American journal of gastroenterology. 2015;110:836–44. doi: 10.1038/ajg.2015.100. quiz 45. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen MH, Garcia RT, Simpson PW, Wright TL, Keeffe EB. Racial differences in effectiveness of alpha-fetoprotein for diagnosis of hepatocellular carcinoma in hepatitis C virus cirrhosis. Hepatology. 2002;36:410–7. doi: 10.1053/jhep.2002.34744. [DOI] [PubMed] [Google Scholar]
- 21.Di Bisceglie AM, Sterling RK, Chung RT, Everhart JE, Dienstag JL, Bonkovsky HL, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. Journal of hepatology. 2005;43:434–41. doi: 10.1016/j.jhep.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 22.El-Serag HB, Kanwal F, Davila JA, Kramer J, Richardson P. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology. 2014;146:1249–55. e1. doi: 10.1053/j.gastro.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson P, Duan Z, Kramer J, Davila JA, Tyson GL, El-Serag HB. Determinants of serum alpha-fetoprotein levels in hepatitis C-infected patients. Clin Gastroenterol Hepatol. 2012;10:428–33. doi: 10.1016/j.cgh.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology. 2016;64:130–7. doi: 10.1002/hep.28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182–8. e1. doi: 10.1053/j.gastro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdel-Razik A, Mousa N, Abdel-Aziz M, Elhelaly R, Elzehery R, Zalata K, et al. Elevated serum alpha-fetoprotein levels in patients with chronic hepatitis C virus genotype 4: not the end of the story. European journal of gastroenterology & hepatology. 2016;28:313–22. doi: 10.1097/MEG.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 27.Kakisaka K, Kataoka K, Onodera M, Suzuki A, Endo K, Tatemichi Y, et al. Alpha-fetoprotein: A biomarker for the recruitment of progenitor cells in the liver in patients with acute liver injury or failure. Hepatology research : the official journal of the Japan Society of Hepatology. 2015;45:E12–20. doi: 10.1111/hepr.12448. [DOI] [PubMed] [Google Scholar]
- 28.Marrero JA Early Detection Research Network N. Hepatocellular carcinoma Early Detection Strategy study (HEDS) Available from: http://edrn.nci.nih.gov/protocols/316-hepatocellular-carcinoma-early-detection-strategy.
- 29.Lee E, Edward S, Singal AG, Lavieri MS, Volk M. Improving screening for hepatocellular carcinoma by incorporating data on levels of alpha-fetoprotein, over time. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:437–40. doi: 10.1016/j.cgh.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 30.Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biselli M, Conti F, Gramenzi A, Frigerio M, Cucchetti A, Fatti G, et al. A new approach to the use of alpha-fetoprotein as surveillance test for hepatocellular carcinoma in patients with cirrhosis. British journal of cancer. 2015;112:69–76. doi: 10.1038/bjc.2014.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.