Abstract
Even in healthy individuals, renal function gradually declines during aging. However, an observed variation in the rate of this decline has raised the possibility of slowing or delaying age-related kidney disease. One of the most successful interventional measures that slows down and delays age-related kidney disease is caloric restriction. We undertook the present studies to search for potential factors that are regulated by caloric restriction and act as caloric restriction mimetics. Based on our prior studies with the bile acid–activated nuclear hormone receptor farnesoid X receptor (FXR) and G protein–coupled membrane receptor TGR5 that demonstrated beneficial effects of FXR and TGR5 activation in the kidney, we reasoned that FXR and TGR5 could be excellent candidates. We therefore determined the effects of aging and caloric restriction on the expression of FXR and TGR5 in the kidney. We found that FXR and TGR5 expression levels are decreased in the aging kidney and that caloric restriction prevents these age-related decreases. Interestingly, in long-lived Ames dwarf mice, renal FXR and TGR5 expression levels were also increased. A 2-month treatment of 22-month-old C57BL/6J mice with the FXR-TGR5 dual agonist INT-767 induced caloric restriction-like effects and reversed age-related increases in proteinuria, podocyte injury, fibronectin accumulation, TGF-β expression, and, most notably, age-related impairments in mitochondrial biogenesis and mitochondrial function. Furthermore, in podocytes cultured in serum obtained from old mice, INT-767 prevented the increases in the proinflammatory markers TNF-α, toll-like receptor 2 (TLR2), and TLR4. In summary, our results indicate that FXR and TGR5 may play an important role in modulation of age-related kidney disease.
Keywords: aging, bile acid, kidney, kidney metabolism, mitochondrial metabolism
Introduction
A gradual decline in renal function occurs even in healthy aging individuals (1–3). The fastest growing group of people in the United States with impaired kidney function is the oldest age group. The population older than 65 years in the United States is expected to double in the next 20 years. The number of elderly worldwide is expected to triple from 743 million in 2009 to 2 billion in 2050. This will result in a marked increase in the elderly population with chronic kidney disease. This increase may be further amplified by other age-related co-morbidities, including hypertension and metabolic syndrome that accelerate age-related decline in renal function (4–8). Hypertension, obesity, and insulin resistance can induce mitochondrial dysfunction, endoplasmic reticulum stress, oxidative stress, inflammation, altered lipid metabolism, and stimulation of profibrotic growth factors in the kidney, which collectively contribute to age-related kidney disease (1).
However, there is variation in the rate of decline given gender, race, and burden of co-morbid conditions (9–13). Although greater glomerular, vascular, and interstitial sclerosis is evident on renal tissue examination of healthy kidney donors with increasing age (14), closer examination of processes leading to sclerosis suggests a role for possible modifiable metabolic and hormonal factors that can decrease the rate of sclerosis. In this regard, the Baltimore Longitudinal Study of Aging revealed that nearly a third of older healthy adults have little change in renal function over time (15). These findings bring into question the inevitability of age-related decline in renal function and raise the possibility of slowing or delaying the process.
Similar findings have also been reported in rodent models of aging. Korstanje and co-workers at The Jackson Laboratory studied 30 inbred strains of mice, determining urine albumin/creatinine ratio and renal pathology at 12, 18, and 24 months of age (37). They found that although some strains of mice are more resistant to development of age-related albuminuria other strains of mice are more susceptible, suggesting a genetic role in age-related kidney disease.
One of the most successful interventional measures that have been shown to slow down and delay age-related kidney disease is caloric restriction (16–20). Studies in our laboratory have demonstrated that caloric restriction prevents age-related kidney disease in part by preventing the increased expression of the sterol regulatory element-binding protein-1 and -2 (SREBP-1 and SREBP-2)2, which are master regulators of fatty acid, triglyceride, and cholesterol synthesis (16, 17). Prevention of the age-related increases in SREBP-1 and SREBP-2 was associated with decreased renal triglyceride and cholesterol accumulation and decreased renal expression of the growth factors connective tissue growth factor and vascular endothelial growth factor (VEGF), matrix metalloproteinase inhibitor, and plasminogen activator inhibitor-1, resulting in prevention of mesangial expansion, podocyte injury, and proteinuria (16, 17).
We undertook the present studies to search for potential additional factors that may be regulated by caloric restriction and thus act as caloric restriction mimetics. Based on our earlier work with the bile acid–activated nuclear hormone receptor farnesoid X receptor (FXR) and G protein–coupled membrane receptor TGR5 (also known as GPBAR1 or GPR131) where we have shown highly beneficial effects of FXR and TGR5 in the kidney (21–24), we reasoned that FXR and TGR5 could be excellent candidates, and we therefore determined the effects of aging and caloric restriction on the expression of FXR and TGR5 in the kidney.
In the current study, we determined the effects of the dual FXR-TGR5 agonist INT-767 (6α-ethyl-3α,7α,23-trihydroxy-24-nor-5β-cholan-23-sulfate sodium salt) in aging mice. INT-767 is the first compound described so far to potently and selectively activate both bile acid receptors. INT-767 is a semisynthetic analogue of the endogenous FXR agonist chenodeoxycholic acid (CDCA), containing one fewer carbon on the side chain and a sulfate ester and lacking the COOH group of CDCA. INT-767 has similar lipophilicity and detergency to CDCA but with a much lower pKa (<1), similar to taurine-conjugated CDCA (25). INT-767 is 300 times more potent than CDCA in FXR activation and about 5 times more potent than lithocholic acid, the endogenous TGR agonist, in TGR activation (26). INT-767 shows efficient intestinal absorption, and its biliary excretion is facilitated by 3-glucuronididation (25). Thus, INT-767 possesses a pharmacokinetic profile suitable for targeting both FXR and TGR5. Consistent with its dual agonist activities, INT-767 induces FXR-dependent lipid uptake by adipocytes, with the beneficial effect of shuttling lipids from central hepatic to peripheral fat storage, and promotes TGR5-dependent glucagon-like peptide-1 secretion by enteroendocrine cells, a validated target in the treatment of type 2 diabetes. Moreover, INT-767 treatment markedly decreases cholesterol and triglyceride levels in diabetic db/db mice and in mice rendered diabetic by streptozotocin administration (26). INT-767 also has marked anti-inflammatory (27), antiatherosclerotic (28), and anticholestatic effects (29).
Our results indicate that FXR and TGR5 expression levels are decreased in the aging kidney and that caloric restriction prevents the age-related decreases in FXR and TGR5 expression. 2-month treatment of 22-month-old aged C57BL/6J mice with the FXR-TGR5 dual agonist INT-767 has caloric restriction-like actions and reverses age-related increases in proteinuria and podocyte injury and, most notably, age-related mitochondrial dysfunction. In addition, in podocytes cultured in the presence of serum obtained from old mice, INT-767 prevents the increases in the inflammatory markers TNF-α, TLR2, and TLR4. Our results therefore indicate that FXR and TGR5 may play an important role in modulation of age-related kidney disease.
Results
Caloric restriction prevents age-related decreases in renal FXR and TGR5 expression
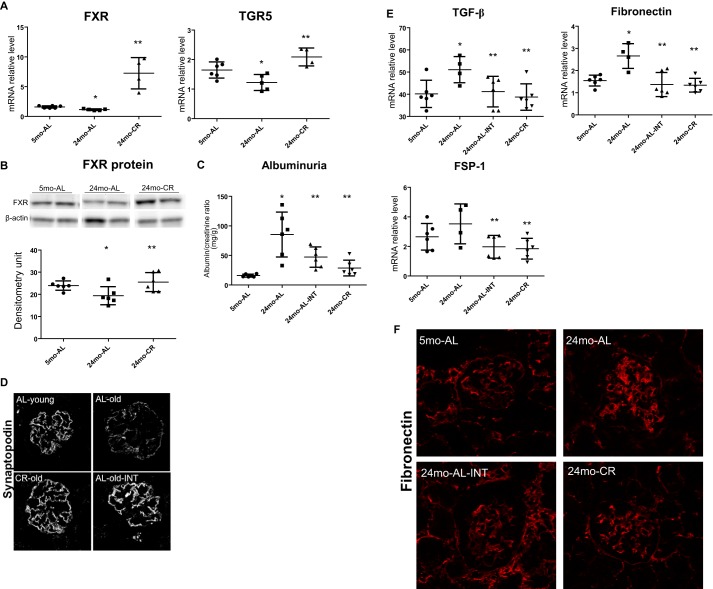
We found that, compared with 5-month-old ad libitum fed mice, 24-month-old ad libitum fed mice had significant decreases in FXR and TGR5 mRNA expression. These decreases were prevented in 24-month-old mice with lifelong caloric restriction (Fig. 1A). The effects of aging and caloric restriction on FXR were also documented at the protein level by Western blotting (Fig. 1B). We could not perform Western blotting for TGR5 because at present there are no specific antibodies available for mouse TGR5. Although a TGR5 antibody did reveal parallel changes in TGR5 protein, based on Western blotting performed with tissues from TGR5 KO mice, we do not believe that the signals are specific for TGR5.
Figure 1.
Short-term treatment with INT-767 reverses age-related kidney disease. A and B, caloric restriction prevents age-related decreases in renal FXR and TGR5 expression. C, INT-767 induces a significant decrease in urinary albumin like lifelong caloric restriction. D, INT-767 prevents the age-related decrease in synaptopodin expression as determined by immunofluorescence microscopy. E, INT-767 prevents the age-related increases in TGF-β, fibroblast specific protein-1 (FSP-1), and fibronectin mRNA. F, age-related increase in fibronectin protein expression as determined by immunofluorescence microscopy is prevented by INT-767 treatment. Error bars represent S.D. n = 6 mice. *, p < 0.05 versus 5-month-old ad libitum fed (5mo-AL); **, p < 0.05 versus 24-month-old ad libitum fed (24mo-AL). INT, INT-767-treated; CR, calorie-restricted; pAMPK, phospho-AMPK.
Treatment of 22-month-old aging ad libitum fed mice for 2 months with the FXR and TGR5 dual agonist reverses the age-related increase in urinary albumin excretion
We then treated 22-month-old ad libitum fed mice with the dual FXR and TGR5 agonist INT-767 to determine whether a 2-month treatment would be able to reverse the age-related increase in urinary albumin excretion. Our results indicate that treatment of 22-month-old ad libitum fed mice with INT-767 induced a significant decrease in urinary albumin like the beneficial effects achieved with lifelong caloric restriction (Fig. 1C). In addition, INT-767 prevented the age-related decrease in synaptopodin expression as determined by immunofluorescence microscopy (Fig. 1D). The beneficial effects of INT-767 were associated with prevention of the age-related increases in transforming growth factor-β (TGF-β), fibroblast specific protein-1, and fibronectin mRNA (Fig. 1E) and protein (Fig. 1F) as determined by immunofluorescence microscopy.
Treatment of 22-month-old aging ad libitum fed mice for 2 months with the FXR and TGR5 dual agonist reverses the age-related decreases in mitochondrial biogenesis and function
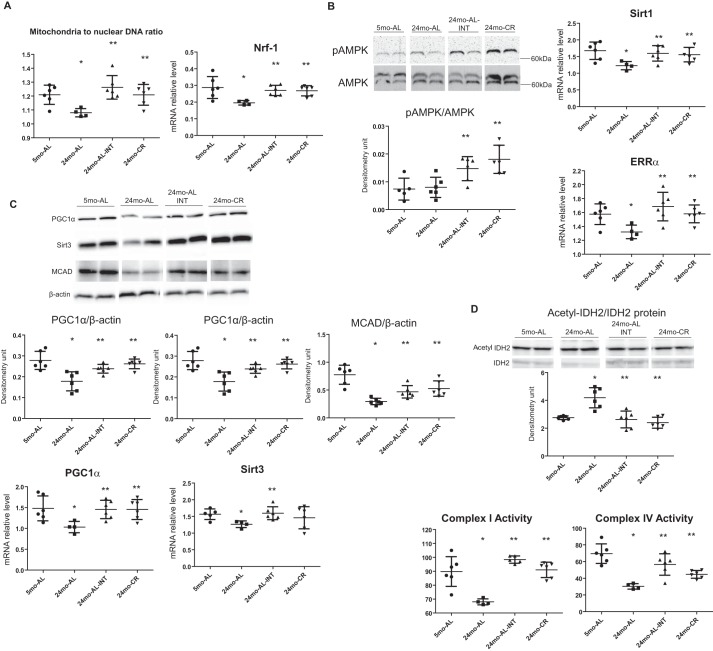
Because mitochondria have been known to play a crucial role in the process of aging (30–33), we examined whether the treatment with INT-767 could modulate mitochondrial biogenesis and function in the aging kidney. We found that the mitochondrial to nuclear DNA ratio, a hallmark of mitochondrial biogenesis, and the master regulator of mitochondrial biogenesis, nuclear respiratory factor-1 (NRF1), were significantly decreased in the kidneys of 24-month-old ad libitum fed mice and reversed by the treatment with INT-767 (Fig. 2A). We also found that the INT-767 treatment concomitantly increased the activated AMPK level and reversed the age-related decreases in SIRT1 mRNA and the nuclear hormone receptor estrogen-related receptor-α (ERR-α) mRNA expression (Fig. 2B). Furthermore, treatment of 22-month-old ad libitum fed mice with INT-767 for 2 months reversed the age-related decrease in PGC-1α mRNA and protein and mitochondrial SIRT3 mRNA and protein (Fig. 2C). The increase in SIRT3 was accompanied by increases in medium-chain acyl-CoA dehydrogenase (MCAD) protein, an important mediator of mitochondrial fatty acid β-oxidation (Fig. 2C). At the same time, INT-767 also reversed the age-related increase in acetylated form of mitochondrial isocitrate dehydrogenase (acetyl-IDH2/IDH2), another target of SIRT3 activity (Fig. 2D). This is ultimately associated with reversal of the decreased mitochondrial complex I and complex IV activity in aged kidneys by 2-month INT-767 treatment, achieving levels identical to lifelong caloric restriction (Fig. 2D).
Figure 2.
INT-767 treatment increases mitochondrial biogenesis and function. A, INT-767 increases the mitochondrial to nuclear DNA ratio and mRNA level of NRF1 in aging kidneys. B, INT-767 treatment increases the activated AMPK level and prevents the age-related decreases in SIRT1 mRNA and nuclear hormone receptor ERR-α mRNA. C, INT-767 prevents the age-related decrease in PGC-1α mRNA and protein; mitochondrial SIRT3 mRNA and protein; and its target, MCAD protein. D, INT-767 reverses the age-related increase in the acetylated form of mitochondrial isocitrate dehydrogenase as shown in the Western blot. 2-month INT-767 treatment also reverses the decreased mitochondrial complex I and complex IV activity in aged kidneys. Error bars represent S.D. n = 6 mice. *, p < 0.05 versus 5-month-old ad libitum fed (5mo-AL); **, p < 0.05 versus 24-month-old ad libitum fed (24mo-AL). INT, INT-767-treated; CR, calorie-restricted.
INT-767 decreases inflammation in human podocytes conditioned with aging serum
To determine whether INT-767 has direct effects in aging podocytes, we treated human podocytes with serum from young (4-month-old) or old (28-month-old) mice. Aging serum increased the expression of TNF-α, TLR2, and TLR4 (Fig. 3). Treatment with INT-767 prevented these changes (Fig. 3), indicating a direct effect of FXR and TGR5 activation on the cultured podocytes.
Figure 3.
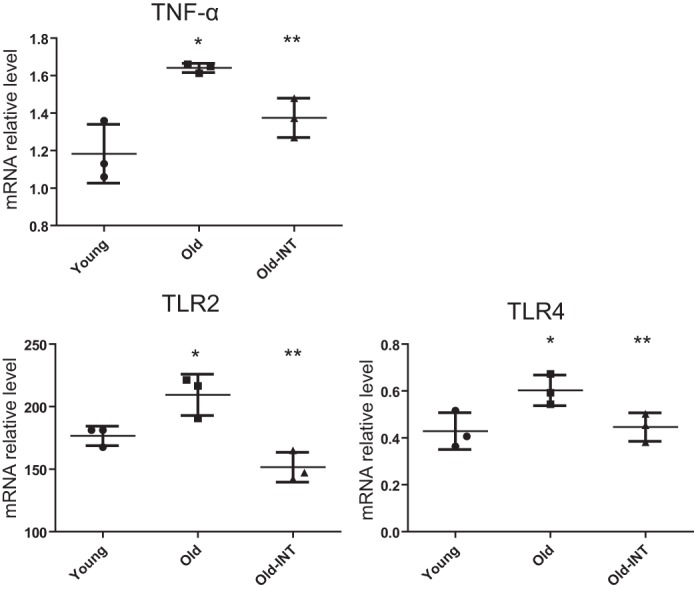
INT-767 has direct effects in cultured human podocytes. The expression of TNF-α, TLR2, and TLR4 is increased in the aging serum-conditioned human podocytes, but this effect is abolished by treatment with INT-767. Error bars represent S.D. n = 3. *, p < 0.05 versus old; **, p < 0.05 versus old. INT, INT-767-treated.
FXR and TGR5 expression is increased in long-lived Ames dwarf mice
Because caloric restriction is associated with prevention of age-related pathologies and life and health span extension, we studied the Ames dwarf mice, which exhibit delayed aging and extended longevity, to determine whether FXR and TGR5 expression was regulated like caloric restriction. We found that, like caloric restriction, FXR and TGR5 mRNA levels are increased in the kidneys of Ames dwarf mice (supplemental Fig. S1A). In addition, we found that the genes involved in the mitochondrial biogenesis and function, including NRF1, SIRT1, PGC-1α, ERRα, SIRT3, COX4, and long-chain acyl-CoA dehydrogenase, were also increased in the kidneys of Ames dwarf mice, consistent with the findings with INT-767 treatment of aging C57BL/6 mice (supplemental Fig. S1B).
Discussion
Our studies have identified the nuclear hormone receptor FXR and the G protein–coupled receptor TGR5 as two important modulators of age-related kidney disease that are regulated by lifelong caloric restriction. FXR and TGR5 expression levels are decreased in the aging kidney, and caloric restriction results in increases in expression of both FXR and TGR5 in the kidney.
Remarkably, only a 2-month treatment of 22-month-old mice with the dual FXR-TGR5 agonist INT-767 reverses the age-related increase in urinary albumin excretion and the significant decrease of the podocyte marker synaptopodin. These effects were comparable with those achieved with lifelong caloric restriction, which is known to protect against age-related co-morbidities, including loss of renal function (16, 17).
These improvements in renal function were associated with reversal of the age-related impairments in mitochondrial biogenesis and function. INT-767, like caloric restriction, increased the mitochondrial DNA content and complex I and complex IV activities. These improvements were associated with significant increases in phospho-AMPK, PGC-1α, and Sirtuin 3 protein expression in the kidney. Increased Sirtuin 3 expression was paralleled by increased protein expression of MCAD, an important mediator of mitochondrial fatty acid β-oxidation, and decreased acetyl-IDH2, an important mediator of mitochondrial oxidative phosphorylation. INT-767 also increased the expression of Sirtuin 1 mRNA as well as expression of the mitochondrial transcription factor NRF1 and estrogen-related receptor-α. Once again, these effects were identical to those achieved by lifelong caloric restriction.
Interestingly, the long-lived Ames mice display changes in the kidney like those induced by FXR-TGR5 activation and caloric restriction, including increases in FXR and TGR5 and the mitochondrially related NRF1, Sirtuin 1, PGC-1α, Sirtuin 3, ERR-α, and Sirtuin 3 target long-chain acyl-CoA dehydrogenase, an important mediator of mitochondrial fatty acid β-oxidation. In addition, in human podocytes cultured in the presence of serum from young versus old mice, INT-767 prevented increases in TNF-α, TLR2, and TLR4 induced by serum from aged mice, indicating a direct beneficial effect of FXR and TGR5 activation on cultured podocytes.
In summary, our results indicate that FXR and TGR5 are up-regulated by caloric restriction and may act as caloric restriction mimetics. Indeed, 2-month treatment of aged mice with the FXR-TGR5 dual agonist INT-767 could reverse age-related kidney disease, including age-related impairments in mitochondrial biogenesis and mitochondrial function. Our studies therefore indicate that FXR and TGR5 agonists may play an important role in prevention and/or reversal of age-related decline in renal function.
Experimental procedures
Animal models
Aging mice
3- and 22-month-old C57BL/6 mice fed ad libitum and 22-month-old C57BL/6 mice with caloric restriction were obtained from an aging colony through the National Institute on Aging. The mice continued to be fed ad libitum with NIH31 diet or calorie-restricted NIH31 diet according to the instructions of the National Institute on Aging. A group of 22-month-old ad libitum fed mice was also fed with diet containing INT-767 (6α-ethyl-3α,7α,23-trihydroxy-24-nor-5β-cholan-23-sulfate sodium salt), the dual FXR-TGR5 agonist (30 mg/kg of body weight/day) (26).
Ames mice
Ames dwarf mice and their controls were acquired from The Jackson Laboratory (Bar Harbor, ME) and studied at 21 months old. Animal studies and related protocols were approved by the Animal Care and Use Committees at the Veterans Affairs Medical Center and University of Colorado Anschutz Medical Campus.
Urine chemistry
Urine albumin and creatinine concentrations were determined with kits (Exocell, Philadelphia, PA).
RNA extraction, quantitative real-time PCR, and Western blotting
Quantitative real-time PCR was performed as described previously (21–24). Primer sequences are listed in supplemental Table 1.
Western blotting
Cortical homogenate protein content was measured by BCA assay (Thermo Fisher Scientific, Waltham, MA). Equal amounts of total protein were separated by SDS-PAGE and transferred onto PVDF membranes. The antibodies against phospho-AMPK/AMPK (catalogue numbers 4184 and 2795, Cell Signaling Technology, Danvers, MA), PGC-1α (catalogue number AB3242, Millipore, Billerica, MA), and SIRT3 (catalogue number 5490, Cell Signaling Technology) were used for Western blotting. After incubation with HRP-conjugated secondary antibodies, the immune complexes were detected by chemiluminescence captured on a UVP Biospectrum 500 Imaging System (Upland, CA), and densitometry was performed with ImageJ software. β-Actin (catalogue number A5316, Sigma) was used as a loading control, and all signals were normalized to the β-actin signal.
Immunofluorescence microscopy
Frozen sections (4 μm thick) were used for immunostaining for synaptopodin (catalogue number S9442, Sigma) and fibronectin (catalogue number F3648, Sigma) and imaged with a laser-scanning confocal microscope (LSM 780, Zeiss, Germany).
Mitochondrial complex activity assay
The mitochondrial fraction was isolated from the kidney as described previously (24) and used for the measurement of complex I (NADH dehydrogenase) and complex IV (cytochrome c oxidase) activity with kits from Abcam (Cambridge, UK).
Podocyte cell culture
Human podocytes obtained from Dr. Moin Saleem were maintained in RPMI 1640 medium, 1% insulin-transferrin-selenium, 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin at 33 °C as described previously (34–36). Podocyte differentiation was induced by thermoshifting the cells from 33 to 37 °C for 7 days. The differentiated podocytes were then cultured in the presence of 5% 4-month-old C57/BL6 mouse serum or 5% 28-month-old mouse serum obtained from the National Institute on Aging to replace fetal bovine serum for 72 h. In the last 24 h, 10 μm INT-767 was added to the treatment group.
Statistical analysis
Results are presented as the means ± S.E. for at least three independent experiments. Data were analyzed by analysis of variance and Student-Newman-Keuls tests for multiple comparisons or by Student's t test for unpaired data between two groups. Statistical significance was accepted at the p < 0.05 level.
Author contributions
X. X. W. and M. L. conceived and designed the whole study. X. X. W., Y. L., D. W., and E. D. performed the study and analyzed the data. X. X. W., L. A., M. P., and M. L. wrote the paper. All authors analyzed the results and approved the final version of the manuscript.
Supplementary Material
This work was supported by National Institutes of Health Grants NIA R01 AG049493 and NIDDK R01 DK098336 and an Intercept medical school Investigator Initiated Study grant (to M. L.). Luciano Adorini and Mark Pruzanski are employees of Intercept. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supplemental Fig. S1 and Table 1.
- SREBP
- sterol regulatory element-binding protein
- FXR
- farnesoid X receptor
- CDCA
- chenodeoxycholic acid
- TGR5
- G protein–coupled bile acid receptor 1 (GPBAR1), also known G protein–coupled receptor 19 (GPCR19), membrane-type receptor for bile acids (M-BAR)
- TLR
- toll-like receptor
- NRF1
- nuclear respiratory factor-1
- AMPK
- AMP kinase
- ERR-α
- estrogen-related receptor-α
- MCAD
- medium-chain acyl-CoA dehydrogenase
- IDH
- isocitrate dehydrogenase.
References
- 1. Choudhury D., and Levi M. (2011) Kidney aging—inevitable or preventable? Nat. Rev. Nephrol. 7, 706–717 [DOI] [PubMed] [Google Scholar]
- 2. Bitzer M., and Wiggins J. (2016) Aging biology in the kidney. Adv. Chronic Kidney Dis. 23, 12–18 [DOI] [PubMed] [Google Scholar]
- 3. Hodgin J. B., Bitzer M., Wickman L., Afshinnia F., Wang S. Q., O'Connor C., Yang Y., Meadowbrooke C., Chowdhury M., Kikuchi M., Wiggins J. E., and Wiggins R. C. (2015) Glomerular aging and focal global glomerulosclerosis: a podometric perspective. J. Am. Soc. Nephrol. 26, 3162–3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tonelli M., and Riella M. (2014) Chronic kidney disease and the aging population. Am. J. Physiol. Renal Physiol. 306, F469–F472 [DOI] [PubMed] [Google Scholar]
- 5. Tonelli M., and Riella M. (2014) Chronic kidney disease and the aging population. Nephrol. Dial. Transplant. 29, 221–224 [DOI] [PubMed] [Google Scholar]
- 6. Glassock R. J., and Rule A. D. (2012) The implications of anatomical and functional changes of the aging kidney: with an emphasis on the glomeruli. Kidney Int. 82, 270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Musso C. G., and Oreopoulos D. G. (2011) Aging and physiological changes of the kidneys including changes in glomerular filtration rate. Nephron. Physiol. 119, Suppl. 1, p1–p5 [DOI] [PubMed] [Google Scholar]
- 8. Abdelhafiz A. H., Brown S. H., Bello A., and El Nahas M. (2010) Chronic kidney disease in older people: physiology, pathology or both? Nephron. Clin. Pract. 116, c19–24 [DOI] [PubMed] [Google Scholar]
- 9. Berg U. B. (2006) Differences in decline in GFR with age between males and females. Reference data on clearances of inulin and PAH in potential kidney donors. Nephrol. Dial. Transplant. 21, 2577–2582 [DOI] [PubMed] [Google Scholar]
- 10. Tauchi H., Tsuboi K., and Okutomi J. (1971) Age changes in the human kidney of the different races. Gerontologia 17, 87–97 [DOI] [PubMed] [Google Scholar]
- 11. Luft F. C., Fineberg N. S., Miller J. Z., Rankin L. I., Grim C. E., and Weinberger M. H. (1980) The effects of age, race and heredity on glomerular filtration rate following volume expansion and contraction in normal man. Am. J. Med. Sci. 279, 15–24 [DOI] [PubMed] [Google Scholar]
- 12. Fliser D., Franek E., Joest M., Block S., Mutschler E., and Ritz E. (1997) Renal function in the elderly: impact of hypertension and cardiac function. Kidney Int. 51, 1196–1204 [DOI] [PubMed] [Google Scholar]
- 13. Ribstein J., Du Cailar G., and Mimran A. (2001) Glucose tolerance and age-associated decline in renal function of hypertensive patients. J. Hypertens. 19, 2257–2264 [DOI] [PubMed] [Google Scholar]
- 14. Rule A. D., Amer H., Cornell L. D., Taler S. J., Cosio F. G., Kremers W. K., Textor S. C., and Stegall M. D. (2010) The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann. Intern. Med. 152, 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindeman R. D., Tobin J., and Shock N. W. (1985) Longitudinal studies on the rate of decline in renal function with age. J. Am. Geriatr. Soc. 33, 278–285 [DOI] [PubMed] [Google Scholar]
- 16. Jiang T., Liebman S. E., Lucia M. S., Phillips C. L., and Levi M. (2005) Calorie restriction modulates renal expression of sterol regulatory element binding proteins, lipid accumulation, and age-related renal disease. J. Am. Soc. Nephrol. 16, 2385–2394 [DOI] [PubMed] [Google Scholar]
- 17. Jiang T., Liebman S. E., Lucia M. S., Li J., and Levi M. (2005) Role of altered renal lipid metabolism and the sterol regulatory element binding proteins in the pathogenesis of age-related renal disease. Kidney Int. 68, 2608–2620 [DOI] [PubMed] [Google Scholar]
- 18. Zha Y., Taguchi T., Nazneen A., Shimokawa I., Higami Y., and Razzaque M. S. (2008) Genetic suppression of GH-IGF-1 activity, combined with lifelong caloric restriction, prevents age-related renal damage and prolongs the life span in rats. Am. J. Nephrol. 28, 755–764 [DOI] [PubMed] [Google Scholar]
- 19. Kanasaki K., Kitada M., and Koya D. (2012) Pathophysiology of the aging kidney and therapeutic interventions. Hypertens. Res. 35, 1121–1128 [DOI] [PubMed] [Google Scholar]
- 20. Kume S., Uzu T., Horiike K., Chin-Kanasaki M., Isshiki K., Araki S., Sugimoto T., Haneda M., Kashiwagi A., and Koya D. (2010) Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J. Clin. Investig. 120, 1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang T., Wang X. X., Scherzer P., Wilson P., Tallman J., Takahashi H., Li J., Iwahashi M., Sutherland E., Arend L., and Levi M. (2007) Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes 56, 2485–2493 [DOI] [PubMed] [Google Scholar]
- 22. Wang X. X., Jiang T., Shen Y., Caldas Y., Miyazaki-Anzai S., Santamaria H., Urbanek C., Solis N., Scherzer P., Lewis L., Gonzalez F. J., Adorini L., Pruzanski M., Kopp J. B., Verlander J. W., et al. (2010) Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes 59, 2916–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang X. X., Jiang T., Shen Y., Adorini L., Pruzanski M., Gonzalez F. J., Scherzer P., Lewis L., Miyazaki-Anzai S., and Levi M. (2009) The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am. J. Physiol. Renal Physiol. 297, F1587–F1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang X. X., Edelstein M. H., Gafter U., Qiu L., Luo Y., Dobrinskikh E., Lucia S., Adorini L., D'Agati V. D., Levi J., Rosenberg A., Kopp J. B., Gius D. R., Saleem M. A., and Levi M. (2016) G protein-coupled bile acid receptor TGR5 activation inhibits kidney disease in obesity and diabetes. J. Am. Soc. Nephrol. 27, 1362–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roda A., Pellicciari R., Gioiello A., Neri F., Camborata C., Passeri D., De Franco F., Spinozzi S., Colliva C., Adorini L., Montagnani M., and Aldini R. (2014) Semisynthetic bile acid FXR and TGR5 agonists: physicochemical properties, pharmacokinetics, and metabolism in the rat. J. Pharmacol. Exp. Ther. 350, 56–68 [DOI] [PubMed] [Google Scholar]
- 26. Rizzo G., Passeri D., De Franco F., Ciaccioli G., Donadio L., Rizzo G., Orlandi S., Sadeghpour B., Wang X. X., Jiang T., Levi M., Pruzanski M., and Adorini L. (2010) Functional characterization of the semisynthetic bile acid derivative INT-767, a dual farnesoid X receptor and TGR5 agonist. Mol. Pharmacol. 78, 617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McMahan R. H., Wang X. X., Cheng L. L., Krisko T., Smith M., El Kasmi K., Pruzanski M., Adorini L., Golden-Mason L., Levi M., and Rosen H. R. (2013) Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease. J. Biol. Chem. 288, 11761–11770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miyazaki-Anzai S., Masuda M., Levi M., Keenan A. L., and Miyazaki M. (2014) Dual activation of the bile acid nuclear receptor FXR and G-protein-coupled receptor TGR5 protects mice against atherosclerosis. PLoS One 9, e108270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baghdasaryan A., Claudel T., Gumhold J., Silbert D., Adorini L., Roda A., Vecchiotti S., Gonzalez F. J., Schoonjans K., Strazzabosco M., Fickert P., and Trauner M. (2011) Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2−/− (Abcb4−/−) mouse cholangiopathy model by promoting biliary HCO−3 output. Hepatology 54, 1303–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perico N., Remuzzi G., and Benigni A. (2011) Aging and the kidney. Curr. Opin. Nephrol. Hypertens. 20, 312–317 [DOI] [PubMed] [Google Scholar]
- 31. Choksi K. B., Nuss J. E., DeFord J. H., and Papaconstantinou J. (2011) Mitochondrial electron transport chain functions in long-lived Ames dwarf mice. Aging 3, 754–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jankauskas S. S., Pevzner I. B., Andrianova N. V., Zorova L. D., Popkov V. A., Silachev D. N., Kolosova N. G., Plotnikov E. Y., and Zorov D. B. (2017) The age-associated loss of ischemic preconditioning in the kidney is accompanied by mitochondrial dysfunction, increased protein acetylation and decreased autophagy. Sci. Rep. 7, 44430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mouchiroud L., Houtkooper R. H., Moullan N., Katsyuba E., Ryu D., Cantó C., Mottis A., Jo Y. S., Viswanathan M., Schoonjans K., Guarente L., and Auwerx J. (2013) The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154, 430–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Herman-Edelstein M., Thomas M. C., Thallas-Bonke V., Saleem M., Cooper M. E., and Kantharidis P. (2011) Dedifferentiation of immortalized human podocytes in response to transforming growth factor-β: a model for diabetic podocytopathy. Diabetes 60, 1779–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saleem M. A., O'Hare M. J., Reiser J., Coward R. J., Inward C. D., Farren T., Xing C. Y., Ni L., Mathieson P. W., and Mundel P. (2002) A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 13, 630–638 [DOI] [PubMed] [Google Scholar]
- 36. Welsh G. I., Hale L. J., Eremina V., Jeansson M., Maezawa Y., Lennon R., Pons D. A., Owen R. J., Satchell S. C., Miles M. J., Caunt C. J., McArdle C. A., Pavenstädt H., Tavaré J. M., Herzenberg A. M., et al. (2010) Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 12, 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsaih S. W., Pezzolesi M. G., Yuan R., Warram J. H., Krolewski A. S., and Korstanje R. (2010) Genetic analysis of albuminuria in aging mice and concordance with loci for human diabetic nephropathy found in a genome-wide association scan. Kidney Int. 77, 201–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.