Introduction
Cryptorchidism is one of the most common congenital anomalies in males, characterized by inability to palpate the testicle in the expected normal anatomical position (i.e., within its respective hemi-scrotum). It represents an abnormality of testicular descent and development associated with long-term concerns, including infertility, hypogonadism, and development of neoplasms.
Methodology
A search of MEDLINE, Cochrane, and EMBASE databases and conference proceedings (January 1988–December 2015) were included to evaluate data and select pertinent articles on the topic. Search terms included cryptorchidism or undescended testicle as the topic of interest with an English language limit. Retrospective and prospective study designs, case series, review articles, and consensus statements by relevant organizations were included.
Grading of evidence to base the presented summary guidelines followed the International Consultation on Urologic Disease (ICUD)/World Health Organization (WHO) modified Oxford Centre for Evidence-Based Medicine grading system (see inset).
Epidemiology
When assessed in the newborn period, incidence is somewhat variable, yet clearly dependent on gestational age at birth. Cryptorchidism is diagnosed in 1.0–4.6% of full-term and 1.1–45.3% of preterm male neonates.1 In up to one-third of cases, the condition may affect both gonads.2 Following spontaneous decent, often seen in the first 3–6 months of life, prevalence stabilizes at 0.7–1.0% of one-year-old boys.
Definition of grades and levels of evidence§.
Levels
Systematic review of randomized trials
Individual randomized trial
Controlled cohort
Case series or case control studies
Mechanism based reasoning
Grades
Consistent level 1
Consistent level 2 or extrapolation from level 1 or 3 studies
Level 4 studies or extrapolation from level 2 or 3 studies
Level 5 evidence or inconsistent/inconclusive studies of any level
Oxford Centre for Evidence-based Medicine Levels of Evidence (May 2009). Produced by Bob Phillips, Chris Ball, Dave Sackett, Doug Badenoch, Sharon Straus, Brian Haynes, Martin Dawes since November 1998. Updated by Jeremy Howick March 2009. http://www.cebm.net/index.aspx?o=1025. Accessed November 7, 2013.
When patients with a normal exam or retractile testicles are excluded, approximately 75% of undescended testes are palpable and unilateral.3 Acquired cryptorchidism (or ascending testicles) is found in approximately 1.5% of prepubertal boys, with up to 77% showing spontaneous descent at puberty.4 These statistics demonstrate that cryptorchidism represents a common healthcare problem that translates into an important burden to the healthcare system. In order to maximize efficiency and timely correction, it demands a structured approach with appropriate use of resources, avoiding redundancies, unnecessary tests, or delays in treatment.
Rationale for treatment
The goals of treatment are summarized in Table 1. Surgical correction is offered early after diagnosis in order to maximize fertility potential and adequate hormone production by preventing acquired damage to gonadal tissue from being in an extra-scrotal position. In addition, exploration and orchidopexy aim to relocate viable testicular tissue outside of the abdomen in a position amenable to regular self-exam later in life, which aids in early diagnosis of testicular cancer. Surgical correction also decreases the risk of future testicular torsion and addresses associated abnormalities (such as a patent processus vaginalis or clinically evident hernia). Orchidopexy may also aid in preventing direct testicular trauma against the pelvic bones during intercourse or sports and provides psychological benefits by attempting to recreate normal anatomy.
Table 1.
Goals of therapy
|
Important definitions and considerations.
Congenital vs. acquired cryptorchidism: Distinction based on findings documented during the neonatal exam. In congenital cases, the testicle is not palpable in the scrotum at birth, while an acquired cryptorchidism (or “ascending” testicle) is in a normal location at birth (or on subsequent well-child exams), but not later in life.
Retractile testis: Refers to a testicle that intermittently migrates to a higher location along the normal path of descent due to a brisk cremasteric reflex. On exam, the testicle can be manipulated to its normal location and remains there after releasing it.
“True” undescended vs. ectopic testes: Based on location (as determined during physical exam and/or surgery) within or outside the normal path of testicular descent, respectively. An ectopic testicle is unlikely to spontaneously descend or respond to hormonal stimulation.
Palpable vs. non-palpable testicle: Based on ability to feel the testicle during physical exam. If non-palpable, diagnostic considerations expand to intra-abdominal testicle, inaccurate exam, and testicular absence or atrophy (so-called “nubbin”). The diagnosis is commonly confirmed during exam under anesthesia and surgical exploration.
Long-term implications
Reduced fertility
Any correlation of infertility with undescended testis (UDT) must be tempered by the fact that 15–20% of couples in the general Canadian population have difficulty conceiving,5 and there is often more than one factor involved. Paternity rates are largely unchanged for men with unilateral cryptorchidism compared to the general population (around 90%), but are significantly lower (33–65%) for those with bilateral UDT.6 Hence, only one-third to two-thirds of men with bilateral cryptorchidism will be able to father a child. In terms of histology, there is evidence that both location of the testicle and time correlate with Leydig and germ cell loss. Intra-abdominal/non-palpable testes depict severe germ cell loss, as do testes that remain undescended by the age of two years7 (Level 3 evidence, Grade C recommendation).
Risk of testicular cancer
Testicular cancer is rare, with an incidence around 4/100 000 in Canada.8 There has long been an association noted between UDT and testicular malignancy, with 11% of testicular cancers developing in men with a history of UDT. Recent studies have found the relative risk (RR) of developing testicular cancer in a boy with UDT is 2.75–8,9–11 corresponding to an absolute risk of 12–33 per 100 000.9 The risk is slightly increased also in the normally descended testis.12 Performing orchiopexy prior to puberty appears to decrease the RR of subsequent testicular cancer to 2.23 (confidence interval [CI] 1.58–3.06), but it still remains above that of the normal non-cryptorchid male (Level 3 evidence, Grade B recommendation).9 Thus, we recommend that for patients with unilateral intra-abdominal and inguinal hypotrophic testes identified after puberty, orchiectomy be offered as an option (not mandatory) (Level 4 evidence, Grade D recommendation).
Evaluation
Early diagnosis is instrumental for determining adequate followup and timely referral for specialized assessment and treatment. Genital exam by an experienced healthcare provider with good documentation of testicular position should be conducted in all newborn males. In addition, the presence of associated genitourinary abnormalities (such as hypospadias and inguinal hernia) and ipsilateral scrotal hypoplasia13 should be assessed. Features suggestive of a disorder of sexual development (DSD) should trigger appropriate evaluation by a multidisciplinary team with expertise in these conditions (see below). In particular, a virilized newborn with bilateral non-palpable gonads should be considered to be 46XX with congenital adrenal hyperplasia until proven otherwise. In these babies, laboratory evaluation to rule out a salt-wasting condition should be expedited (and completed prior to discharge) in order to avoid morbidity and potential mortality.14
Even though cryptorchidism is most commonly diagnosed in otherwise healthy children, it is important to remember that it is a component of almost 400 syndromes, many with important comorbidities that often raise concerns for surgical correction when anesthetic risks, life expectancy, and realistic future fertility interests are considered. These issues should be openly discussed with parents or other caretakers supported by healthcare providers with expertise in the particular condition (such as geneticists and a complex care pediatrics team), and anesthetists for counselling regarding anesthesia risk. In addition, syndromes associated with high likelihood of intra-abdominal gonads (and low likelihood of spontaneous descent), such as Prune Belly or Eagle Barrett syndrome, should be detected early and considered in the management plan.
Aside from newborn screening, a carefully documented genital exam should be part of well-child visits and during the assessment of children with suspected hernia or hydrocele and unexplained abdominal or inguinal pain. Healthcare providers should remember that a normal exam in the newborn period does not rule out the future development of cryptorchidism (i.e., testicular ascent).
Physical exam
Evaluation by an experienced healthcare provider remains the most important component of the assessment of children with suspected cryptorchidism, allowing the distinction between a normally located gonad, retractile testicle, palpable undescended/ectopic testicle, and non-palpable testicle. Associated conditions, such as an inguinal hernia, are concomitantly evaluated. The exam should be performed in a quiet, warm environment, assisted with lubrication if needed, and focus on the inguinal canal and scrotum, along with less common ectopic sites (perineal, femoral, prepubic). Ultrasound evaluation is not a substitute for a well-performed exam and it does not add diagnostic accuracy to an evaluation by a less experienced healthcare provider or a limited exam due to an uncooperative child.
In patients with unilateral cryptorchidism, evaluation of the contralateral gonad is important in order to detect potential problems with the normally located testicle (such as atrophy, varicocele, abnormal volume, or consistency for age). In addition, the presence of testicular hypertrophy (most often suspected when the axial length of the testicle is greater than 1.8–2 cm), is associated with a higher likelihood of an absent or atrophic non-palpable gonad. This information is valuable to provide preoperative counselling and can help decide on surgical approach and allocation of operating room time.
Imaging studies
When ordering imaging studies for evaluating suspected cryptorchidism, the healthcare provider should take into consideration the following issues:
– Imaging studies that require sedation or anesthesia (such as magnetic resonance imaging, [MRI]), regardless of the diagnostic performance of the test, do not have any therapeutic value. Thus, under most circumstances, surgical exploration is not avoided and a second anesthetic will be required for treatment.
– The use of imaging modalities that employ ionizing radiation (such as computed tomography [CT] scans) should be avoided, since the information obtained does not change management.15,16 The additive exposure over the child’s lifetime, along with delivery of radiation to gonadal tissue (which happens, by definition, as the goal is to localize it), adversely impacts any added value and supports discouraging this practice.
– None of the currently available imaging modalities have sufficient reproducible diagnostic accuracy to confidently rule out the presence of intra-abdominal viable gonadal tissue. Thus, ultimately, in most cases surgical exploration is not avoided. Imaging tests may have potential merit solely in directing the best initial approach (e.g. scrotal vs. inguinal vs. laparoscopic exploration).
– Inaccurate or incongruent diagnosis in comparison to physical exam adds uncertainty and may lead to suboptimal management. Clear examples include surgical intervention for retractile testicles diagnosed as cryptorchidism by ultrasound, foregoing surgical exploration and missing an intra-abdominal gonad based on lack of visualization in ultrasound, CT scan or MRI, and extensive surgical exploration in the setting of a false positive image suggesting the presence of an intra-abdominal gonad.
– The practice of systematically ordering imaging studies on children with cryptorchidism adds a significant burden on the healthcare system and increases healthcare expenditures with limited added value and may introduce delays in appropriate referral and timely treatment.
Based on the aforementioned points, imaging in cryptorchidism is not cost-effective, may delay referral and surgical treatment, and as such cannot be recommended as a standard adjunct to preoperative assessment of these children (Level 3 evidence, Grade B recommendation).
Need for investigation for DSD, karyotype, and other biochemical/genetic studies
The incidence of karyotype or other genetic abnormalities in boys with cryptorchidism is low (around 5% for those with persistent cryptorchidism after six months and 8% for boys with bilateral UDT).17 Hence, routine karyotype or genetic workup of patients with UDT is NOT recommended (Level 4 evidence, Grade D recommendation).
Patients with bilateral non-palpable gonads and a normal phallus with an orthotopic urethral meatus should undergo a karyotype (and further hormonal testing of 17-hydroxy progesterone levels if XX karyotype found) to rule out congenital adrenal hyperplasia.18 Although the yield of such practice is low, we recommend it due to the potential devastating issues (salt-wasting crisis, gender assignment discussions) associated with a missed diagnosis.
In patients with bilateral non-palpable testicles and a normal phallus bearing an XY karyotype, the diagnosis of bilateral vanishing testicles or testicular regression syndrome (TRS) should be considered. In such patients, the combination of high gonadotropins, low testosterone levels (even after stimulation), and very low or undetectable levels of anti-Mullerian hormone may preclude any surgical intervention.19,20 In this specific scenario, we recommend consultation with an endocrinologist to determine the best management on an individual basis since interpretation of these investigations is complex and sometimes inconclusive.
Approximately one-third of patients with proximal hypospadias and at least one undescended testicle (particularly if non-palpable) have a DSD.21 DSD has not been observed in patients with the association of distal hypospadias and UDT.22 Therefore, we recommend performing a karyotype in patients with at least one undescended testicle and proximal hypospadias, especially in the setting of non-palpable gonads (Level 4 evidence, Grade D recommendation).
WT1 mutations have been identified in a single series of patients with the association of proximal hypospadias and at least one UDT in 6/80 (7.5%) boys who were tested.23 Further development of renal disease and/or Wilms’ tumour was documented in those patients. Hence, we recommend that consideration be given to include targeted WT1 genetic testing in patients that fit that profile (Level 3 evidence, Grade C recommendation).
Persistent Mullerian Duct Syndrome (PMDS) is suggested by the presence of Mullerian structures (uterus, fallopian tube) attached to an undescended testicle (more commonly intra-abdominal) and is usually an intraoperative finding. It is caused by a mutation on the gene that encodes either AMH or its receptor; such mutation is transmitted following an autosomal recessive trait. Rarely, PMDS can lead to both testicles occupying the same side of the abdomen (transverse testicular ectopia). Surgical removal of the Mullerian structures seems logical since malignancies have been reported and at times their attachments can hinder the performance of a tension-free orchidopexy. Removal can be achieved through open surgery or laparoscopically with care being taken not to damage the vas deferens, which can be quite adherent to the Mullerian structures.24,25 When Mullerian remnants are found incidentally during an inguinal orchidopexy, the proximal aspect of the fallopian tube can be transected and removed with the uterus, leaving its distal component attached to the vas deferens, allowing the testis to be brought to a scrotal position (Level 4 evidence, Grade D recommendation). Such maneuver avoids separation of the tube from the cord structures, protecting the deferential and testicular blood supply. Patients should have AMH levels checked and be referred to endocrinology/genetics for investigation.
If a DSD is discovered, patients should be followed in a multidisciplinary clinic specific to these complex diagnoses.
Management options (Fig. 1)
Fig. 1.
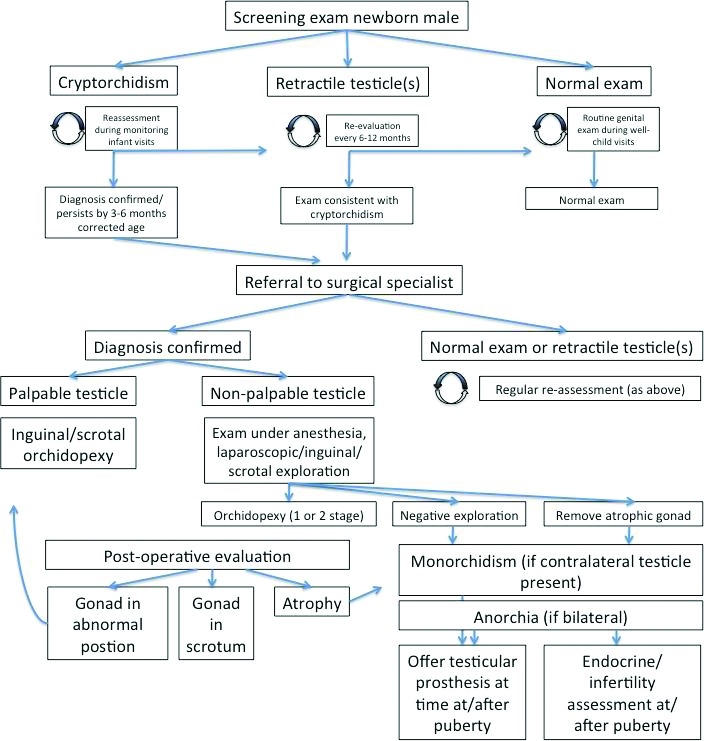
Basic management algorithm for cryptorchidism diagnosed in infancy/early childhood.
Hormonal stimulation/hormonal therapy
Treatment of UDT with either human chorionic gonadotropin (hCG) or luteinizing hormone-releasing hormone (LHRH) does not seem to cause harm and may be effective; however, reported success rates are inconsistent (9–62%), with no single agent standing out.26 Bilateral cases seem to harbour the best response (25–30%).27
There have been reports suggesting that administration of gonadotropin-releasing hormone (GnRH) either pre or postorchidopexy may improve fertility based on improved fertility indices (ascertained by proxy with the ratio of adult spermatogonia per tubule on testicular biopsies taken at the time of orchidopexy).28–30 This is a highly controversial topic with conflicting recommendations having been published28,31 and should therefore be regarded as experimental.
There is paucity of data on long-term outcomes of hormone therapy, such as fertility and cancer development.26
Our recommendation is that hormone therapy has a limited role in the management of cryptorchidism and should not be recommended as first-line therapy (Level 2 evidence, Grade B recommendation).
Surgical exploration
Timing
There seems to be a general consensus regarding the ideal age for orchidopexy, although an evidence-based guideline is still lacking. According to the 1996 American Academy of Pediatrics recommendation32 and the recently published American Urological Associaton (AUA) guidelines33 on the topic, orchidopexy should be performed before one year of age based on changes in the number of germ cells in the UDT that start to occur beyond that age.34
Results from a randomized, controlled trial comparing testicular growth after surgery performed at nine months vs. at three years of age indicated that early orchidopexy was followed by a partial catch-up testicular growth, which was not seen after late operation.35 These findings, as well as the fact that testicular descent is unlikely to occur in full-term babies after six months of age,4 support our current recommendation of performing orchidopexy between six and 18 months of age (Level 2 evidence, Grade B recommendation).
Orchidopexy techniques
Surgical approach to the palpable testicle
Inguinal orchidopexy
Palpable testicles are approached most commonly through an inguinal incision. High (proximal) ligation of the processus vaginalis is an essential surgical step to allow placement of the testis in a sub-dartos pouch within the hemi-scrotum, without tension. Fixation sutures through the tunica albuginea can be used. The weighted success rate for primary inguinal orchidopexy was 96.4% based on a systematic review.26
Scrotal orchidopexy
The scrotal approach for management of cryptorchidism was first described by Bianchi in 1989,36 and has since gained wide acceptance.37–41 Evidence suggests that most palpable testicles can be successfully managed through this incision.37,38 According to a recent review that analyzed 1558 scrotal orchidopexies, recurrence was observed in only nine cases, testicular hypo/atrophy in five, and surgical site infections in 13. A secondary inguinal incision was needed in 3.5% of the boys to facilitate high (proximal) testicular dissection. Overall, success rates ranged from 88–100%.42
In comparison to standard inguinal orchidopexy, recent evidence from observational studies has suggested that the scrotal approach has equivalent success rates and complications, with advantage of a significantly shorter operative time.38,39,43 At least two randomized, controlled trials comparing the two techniques (inguinal vs. scrotal) have been attempted and essentially confirmed those findings;44,45 however, in one of the studies the authors also report mean length of stay above two days for both procedures, which questions the generalizability of the conclusions to our environment, where these procedures are almost universally undertaken on an outpatient basis.44 Furthermore, none of these randomized, controlled trials prespecified the minimal clinically important difference in operative time to justify sample size calculation; therefore, their conclusions should be interpreted with caution.
Our recommendation is that for palpable UDT undergoing surgery, both the inguinal and the prescrotal techniques are acceptable based on the surgeon’s preference and experience (Level 2 evidence, Grade B recommendation).
Surgical approach for the non-palpable testicle
If the testicle is not palpable preoperatively, as it may occur in up to 20% of UDT cases, examination under anesthesia (EUA) can sometimes allow identification of the testicle. Otherwise, diagnostic laparoscopy is the procedure of choice in most centres.46 In certain non-palpable testicle (NPT) cases, confident palpation of an ipsilateral scrotal nubbin and identification of contralateral compensatory testicular hypertrophy may preclude diagnostic laparoscopy by means of initially performing a scrotal incision, which allows for testicular nubbin removal and confirmation of the vanishing testicle diagnosis.47 Inguinal exploration and/or laparoscopy can then be reserved for cases in which the initial scrotal approach is non-diagnostic.
The phenomenon of contralateral compensatory testicular hypertrophy has been well-described in the literature and shown to correlate with the laparoscopic finding of an absent testicle (monorchism) in children with unilateral NPT.4–10,14 Boys with monorchism were found to have a mean contralateral testicular length >2 cm5 or >1.8 cm.6,10 Based on these findings, it could be debated that boys with NPT and contralateral compensatory hypertrophy should be initially approached by a scrotal incision to look for a testicular nubbin, reserving diagnostic laparoscopy only for cases with a patent processus vaginalis or lack of compensatory hypertrophy.47 It is critical to highlight the importance of confidently identifying atrophic testicular tissue with associated vas deferens and gonadal vessels if a scrotal or inguinal approach is chosen, as any doubt should trigger further exploration. Presence of a looping vas or incorrectly identifying non-gonadal tissue as a nubbin may lead to misdiagnosis, potentially leaving viable testicular tissue in the abdomen. In uncertain cases or when tissue analysis is not consistent with atrophic testicular tissue, laparoscopic exploration should be strongly considered (Level 4 evidence, Grade C recommendation).
If laparoscopy is unavailable, a lengthy inguinal incision extending to the abdominal cavity is sometimes necessary to rule out the presence of an intra-abdominal testicle. When a laparoscopic approach is chosen, up to three ports may be needed: a 3 or 5 mm umbilical trocar for the camera and two 3 mm ports for the working instruments. Single-port laparoscopic management for the intra-abdominal testicle has been described and constitutes an alternative option.
Diagnostic laparoscopy is the most useful modality for assessing NPT, as it permits identification of three surgical scenarios that will lead to different courses of action:
Blind-ending vas and vessels indicate a vanishing intra-abdominal testicle (IAT), and no further exploration is necessary (10–30% of cases).
Testicular vessels and vas entering the inguinal canal through the internal inguinal ring. Inguinal exploration may find a healthy palpable UDT amenable to standard orchidopexy, or a testicular nubbin either in the inguinal region or, most commonly, in the scrotum. Remnant cord structures are usually removed to confirm the diagnosis and because viable residual testicular elements are present in up to 14% of the cases.48 It should be noted that to date, no cases of intratubular germ cell neoplasia have been reported within these specimens.
Peeping or IAT (50%), which will require either an open or a laparoscopic orchidopexy in one or two stages.
Laparoscopy allows for accurate diagnosis of any of the three scenarios at the time of surgery, followed by the appropriate definitive management, i.e., orchidopexy in one or two stages or removal of nubbin/non-viable testis.
Inguinal approach for the high inguinal canalicular, or IAT
Bringing a high testicle down to the scrotum while preserving its blood supply can sometimes be a surgical challenge. Helpful maneuvers include division of the lateral fibrous attachments of the cord at the internal inguinal ring, blunt dissection of the retroperitoneal spermatic vessels (which are usually the limiting factor) up to the lower pole of the kidney, and mobilization of the cord medial to the inferior epigastric vessels (Prentiss maneuver). Despite these steps, if the testicle still does not reach the scrotum, a Fowler-Stephens (FS) orchidopexy may be performed.49
Fowler-Stephens orchidopexy
The FS technique was originally described as a single-stage open inguinal approach for the IAT in which the testicular artery and veins were too short to allow adequate testicular mobilization into the scrotum through standard orchidopexy.50 It involves ligating and dividing the testicular vessels while maintaining the normal pathway of testicular descent through the inguinal canal. The distal gubernacular attachments and the collateral vessels on the floor of the inguinal canal are left undisturbed, preserving the cremasteric blood supply.50
The laparoscopic FS orchidopexy entails division of the gonadal vessels and cremasteric collaterals during advancement of the IAT medial to the inferior epigastric vessels or obliterated umbilical artery. Despite its widespread use,51–54 either as a one- or two-stage procedure, atrophy rates can be as high as 33%, probably due to failure of developing adequate collateral blood supply through the deferential artery. The presence of a long looping vas deferens may increase this risk, especially when the procedure is done laparoscopically.55 In contrast, preservation of the gubernaculum during laparoscopic FS orchidopexy, mimicking one of the surgical steps of the open FS technique, may help decrease the likelihood of testicular atrophy.56–59
Orchidopexy success rates of testicular descent
Success rates of testicular descent are directly related to the anatomic position of the testicle. These rates range from 92% for standard inguinal open orchidopexy for testicles located below the external inguinal ring to 67% for one-stage laparoscopic FS orchidopexy for non-palpable testicles.60
A recent systematic review has compared the success rates of testicular descent for primary orchidopexy (palpable testes), one-stage, and two-stage FS procedures (non-palpable testes). According to this review, the weighted success rates for all three approaches exceeded 75%. Independently, the overall success rates were 78.7%, 86%, and 96.4% for one-stage FS, two-stage FS, and primary orchiopexy, respectively.26
Open vs. laparoscopic orchidopexy for NPT
Laparoscopic orchidopexy outcomes are comparable to those of open surgery.61,62 Based on a randomized, controlled trial that compared outcomes after two-stage laparoscopic FS orchidopexy vs. open orchidopexy for NPT, patients who underwent the laparoscopic approach were noticed to have statistically significantly shorter operative time and return to normal activities. Although all testicles in both groups were noted to have satisfactory scrotal position after surgery, two (10%) of the 20 testes in the laparoscopic arm and three (19%) of the 16 testes in the open group had atrophied after one year of followup.63
Complications
The most alarming complication of inguinal orchidopexy is testicular atrophy, which occurs when the testicular vessels are damaged. According to a recent systematic review on this topic,26 pooled atrophy rates were 1.83% for primary orchidopexy (range 0–4%), 28.1% for one-stage FS (range 22–67%), and 8.2% for two-stage FS (range 0–12%). Similarly, another study has shown that surgical outcomes for IAT were better with a one-stage orchidopexy preserving the testicular vessels as opposed to the one-stage FS technique.64
Rare complications include testicular ascent, where the testicle gets pulled to the entrance of the scrotum, and vas deferens injury. Other orchidopexy related complications might include those associated with any surgical procedure, such as wound infection, dehiscence, and hematoma.
Prophylatic contralateral orchidopexy
Preventive orchidopexy of the normally descended contralateral testicle in the setting of blind-ending spermatic vessels found upon exploration of a non-palpable testis has been advocated by some authors. This is based on the reported risk of bell-clapper deformity and abnormal testicular fixation found in the remaining solitary testis (Bellinger 1985, Savage 2001). The risk of torsion is admittedly low, conceptually not different from the general population. Thus, any potential benefit must be weighed against the risk of damage to the solitary gonad during surgery. In the absence of literature strongly supporting or discouraging prophylactic orchidopexy, the decision should be made based on informed discussion of options with the patient parents or legal guardian (Level 5 evidence, Grade D recommendation).
Testicular biopsy
Testicular biopsy is not indicated at the time of orchidopexy. Recent evidence has shown that total germ cell histopathology at the time of orchiopexy was not predictive of significant changes in hormone levels or semen analysis results in adulthood.49 According to these authors, it may be clinically useful in predicting fertility potential for those with bilateral undescended testicles, but this approach remains investigational.49
Orchiectomy
Orchiectomy remains the treatment of choice for the majority of postpubertal males presenting with unilateral cryptorchidism, especially when these testicles are small in size (hypotrophic/atrophic). Histological analysis of cryptorchid testicles in postpubertal patients has shown that most of these testes have significant malignant potential and cannot contribute to fertility (Sertoli only syndrome).65
Conservative management
UDT is associated with a multitude of syndromes, some of which can lead to limited life expectancy and/or severe developmental delay (e.g., Down’s, Prader-Willi, and Noonan’s syndromes). Furthermore, there is evidence that in many of these patients, testicular function suffers progressive deterioration over time.66 Nonetheless, given the reports of testicular cancer (sometimes at an early age67) in these patients, we recommend orchidopexy when they are clinically fit for anesthesia for the purpose of surveillance (Level 4 evidence, Grade D recommendation).
Previously failed orchidopexy
Overall orchidopexy failure rates are low (around 10%),68 especially when only pediatric referral centre results are considered (1–2%).69 When faced with a testicle in an inadequate (high) position after orchidopexy, redo surgery offers high success rates in terms of bringing the testicle to a scrotal position.68,69 Data on long-term (functional) outcomes of such testes are non-existent. We recommend offering redo orchidopexy for cases where inadequate position is detected postoperatively (Level 5 evidence, Grade D recommendation).
Followup
Although UDT are unquestionably associated with a higher risk for development of testicular cancer, the incidence rates of this type of cancer are small and hence no screening policy is justified. There is no need for formal long-term urological followup of patients with UDT. Nonetheless, periodic self-exam after puberty is recommended with prompt referral to an urologist if an abnormality is noted.
Age at which orchiectomy is advisable over orchidopexy
Data suggest that the risk of malignancy within a postpubertal UDT is higher compared to those that underwent prepubertal orchidopexy.70 Additionally, testicular cancer is exceedingly rare in older adults (i.e., after 50 years of age).9,71 Hence, we recommend considering orchiectomy for postpubertal patients with hypotrophic/atrophic undescended testicles up to the age of 50. After that age, observation is likely appropriate (Level 4 evidence, Grade D recommendation).
Acquired cryptorchidism
Acquired UDT are diagnosed at an average age of 8–11 years. The reasons for this late diagnosis remain unknown. Careful serial physical examination is recommended to accurately determine testicular position and identify cases of acquired cryptorchidism in boys with retractile testes. Some authors believe that acquired UDT represent a milder subtype of congenital cryptorchidism that has escaped detection in infancy.72 The percentage of retractile testicles that ascends and requires orchidopexy is difficult to estimate, ranging from 3–30% in prepubertal children.73 Based on that, it is difficult to set a specific age for correction of these cases because it may vary from child to child.
Appendix 1.
Summary of recommendations
| Physical exam is the cornerstone of cryptorchidism evaluation, and should be conducted by an experienced healthcare provider in a warm, relaxed environment |
| Documentation in patients with cryptorchidism should include history of prematurity, scrotal asymmetry, if the gonad(s) is palpable or not, and associated genitourinary abnormalities (such as hypospadias) |
| Phenotypic males with bilateral non-palpable gonads should raise the index of suspicion of congenital adrenal hyperplasia with a 46XX karyotype (along with other disorders of sexual development), and appropriate workup should be conducted prior to discharge to rule out a salt-wasting condition |
| If cryptorchidism is documented on newborn exam, regular monitoring is warranted to assess for spontaneous descent, and appropriate referral for specialized evaluation should be secured at or before six months of corrected age |
| Imaging studies, such as ultrasound, computed tomography scan or magnetic resonance imaging, are unnecessary, expensive, potentially misleading, and not warranted. They can be selectively ordered after specialist evaluation, including patients with suspected disorder of sexual development, and prior to surgical intervention at the discretion of the specialist |
| Unless the child has important comorbidities or high anesthetic risk, there is no role for conservative (i.e., expectant) management in children diagnosed with cryptorchidism past six months corrected age |
| Children with retractile testicle(s) should be regularly examined and the location of the gonad in the absence of an active cremasteric reflex clearly documented. If noted to ascend into an ectopic/undescended location, specialist referral is warranted |
| Acute abdominal/inguinal pain in a child with cryptorchidism should be considered a possible torsion and trigger appropriate urgent surgical assessment. A genital exam indicating the presence and location of the testicles should be documented in all boys with abdominal/inguinal pain |
| Hormonal therapy has an unknown impact on subsequent gonadal function and has no advantage over timely surgical correction |
| There is no role for medical (hormonal) or surgical intervention(s) for children with retractile testicle(s) |
| Palpable undescended testicles can be addressed through a prescrotal or inguinal approach, based on location of the gonad and the ability to manipulate into the scrotum, as well as surgeon preference and expertise |
| If the testicle is not palpable on preoperative physical evaluation, an exam under-anesthesia should be conducted at the beginning of surgical exploration, as in 10–15% of patients the gonad may become palpable and surgical approach can be appropriately tailored |
| The goal of orchidopexy is to locate the gonad in its normal anatomical position, which should be documented on a postoperative followup assessment |
| Surgical procedures should address associated abnormalities, such as a patent processus vaginalis or hernia |
| The role of contralateral prophylactic orchidopexy in unilateral cryptorchidism or monorchidism (to prevent future testicular torsion) is controversial. The rationale for conducting this procedure or not should be disclosed to the family and appropriate warnings given to all families regarding the need for emergent evaluation in case of acute testicular pain |
| The diagnosis of an absent, vanishing, or atrophic testicle is based on surgical exploration. Surgical findings (including the presence of blind ending vas deferens and vessels, absence of testicle or nubbin), and/or pathological evaluation (hemosiderin, testicular tissue, vas deferens, and vessels) should be clearly documented in order to avoid future concerns and need for re-assessment |
| All patients should receive appropriate teaching regarding regular testicular self-exam following orchidopexy and need to alert healthcare providers if palpable abnormalities are noted or if a sudden increase in testicular size is perceived. |
| Patients should be referred for endocrine assessment in cases of delayed puberty and offered evaluation by an infertility specialist if concerned about future fertility potential. This recommendation in particularly important for boys at high risk for hormonal or fertility problems, such as those with bilateral intra-abdominal testicles, cryptorchidism in a solitary gonad, or concern about atrophy after attempted orchidopexy |
Footnotes
Competing interests: The authors report no competing personal or financial interests.
This paper has been peer-reviewed.
References
- 1.Sijstermans K, Hack WWM, Meijer RW, et al. The frequency of undescended testis from birth to adulthood: A review. Int J Androl. 2008;31:1–11. doi: 10.1111/j.1365-2605.2007.00770.x. [DOI] [PubMed] [Google Scholar]
- 2.Berkowitz GS, Lapinski RH, Dolgin SE, et al. Prevalence and natural history of cryptorchidism. Pediatrics. 1993;92:44–9. [PubMed] [Google Scholar]
- 3.Cendron M, Huff DS, Keating MA, et al. Anatomical, morphological and volumetric analysis: A review of 759 cases of testicular maldescent. J Urol. 1993;149:570–3. doi: 10.1016/s0022-5347(17)36151-7. [DOI] [PubMed] [Google Scholar]
- 4.Hack WWM, van der Voort-Doedens LM, Goede J, et al. Natural history and long-term testicular growth of acquired undescended testis after spontaneous descent or pubertal orchidopexy. BJU Int. 2010;106:1052–9. doi: 10.1111/j.1464-410X.2010.09226.x. https://doi.org/10.1111/j.1464-410X.2010.09226.x. [DOI] [PubMed] [Google Scholar]
- 5.Health Canada. Fertility in Canada [Internet] Government of Canada; [Accessed June 1, 2017]. [cited 2014 Nov]. Available from: http://healthycanadians.gc.ca/healthy-living-vie-saine/pregnancy-grossesse/fert-eng.php. [Google Scholar]
- 6.Lee PA. Fertility after cryptorchidism: Epidemiology and other outcome studies. Urology. 2005;66:427–31. doi: 10.1016/j.urology.2005.01.017. https://doi.org/10.1016/j.urology.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Tasian GE, Hittelman AB, Kim GE, et al. Age at orchiopexy and testis palpability predict germ and Leydig cell loss: Clinical predictors of adverse histological features of cryptorchidism. J Urol. 2009;182:704–9. doi: 10.1016/j.juro.2009.04.032. https://doi.org/10.1016/j.juro.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 8.Garner MJ, Turner MC, Ghadirian P, et al. Epidemiology of testicular cancer: An overview. Int J Cancer. 2005;116:331–9. doi: 10.1002/ijc.21032. https://doi.org/10.1002/ijc.21032. [DOI] [PubMed] [Google Scholar]
- 9.Wood HM, Elder JS. Cryptorchidism and testicular cancer: Separating fact from fiction. J Urol. 2009;181:452–61. doi: 10.1016/j.juro.2008.10.074. https://doi.org/10.1016/j.juro.2008.10.074. [DOI] [PubMed] [Google Scholar]
- 10.Cook MB, Akre O, Forman D, et al. A systematic review and meta-analysis of perinatal variables in relation to the risk of testicular cancer—experiences of the son. Int J Epidemiol. 2010;39:1605–18. doi: 10.1093/ije/dyq120. https://doi.org/10.1093/ije/dyq120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trabert B, Zugna D, Richiardi L, et al. Congenital malformations and testicular germ cell tumours. Int J Cancer. 2013;133:1900–4. doi: 10.1002/ijc.28207. https://doi.org/10.1002/ijc.28207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akre O, Pettersson A, Richiardi L. Risk of contralateral testicular cancer among men with unilaterally undescended testis: A meta-analysis. Int J Cancer. 2009;124:687–9. doi: 10.1002/ijc.23936. https://doi.org/10.1002/ijc.23936. [DOI] [PubMed] [Google Scholar]
- 13.Snodgrass W, Bush N, Holzer M, et al. Current referral patterns and means to improve accuracy in diagnosis of undescended testis. Pediatrics. 2011;127:e382–8. doi: 10.1542/peds.2010-1719. https://doi.org/10.1542/peds.2010-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White PC. Neonatal screening for congenital adrenal hyperplasia. Nat Rev Endocrinol. 2009;5:490–8. doi: 10.1038/nrendo.2009.148. https://doi.org/10.1038/nrendo.2009.148. [DOI] [PubMed] [Google Scholar]
- 15.Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680 000 people exposed to computed tomography scans in childhood or adolescence: Data linkage study of 11 million Australians. BMJ. 2013;346:f2360. doi: 10.1136/bmj.f2360. https://doi.org/10.1136/bmj.f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. Laparoscopic management of persistent mullerian duct syndrome. JAMA Pediatr. 2013;167:700–7. doi: 10.1001/jamapediatrics.2013.311. https://doi.org/10.1001/jamapediatrics.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferlin A, Zuccarello D, Zuccarello B, et al. Genetic alterations associated with cryptorchidism. JAMA. 2008;300:2271–6. doi: 10.1001/jama.2008.668. https://doi.org/10.1001/jama.2008.668. [DOI] [PubMed] [Google Scholar]
- 18.Kırlı EA, Karnak İ, Ciftci AO, et al. An unexpected diagnosis in children with male phenotype and bilateral non-palpable gonad: Congenital adrenal hyperplasia with female genotype. Ped Surgery Int. 2013;29:719–24. doi: 10.1007/s00383-013-3319-3. https://doi.org/10.1007/s00383-013-3319-3. [DOI] [PubMed] [Google Scholar]
- 19.Teo AQA, Khan AR, Williams MPL, et al. Is surgical exploration necessary in bilateral anorchia? J Pediatr Urol. 2013;9:e78–81. doi: 10.1016/j.jpurol.2012.09.006. https://doi.org/10.1016/j.jpurol.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Lee MM, Donahoe PK, Silverman BL, et al. Measurements of serum müllerian inhibiting substance in the evaluation of children with non-palpable gonads. N Engl J Med. 1997;336:1480–6. doi: 10.1056/NEJM199705223362102. https://doi.org/10.1056/NEJM199705223362102. [DOI] [PubMed] [Google Scholar]
- 21.Kaefer M, Diamond D, Hendren WH, et al. The incidence of intersexuality in children with cryptorchidism and hypospadias: Stratification based on gonadal palpability and meatal position. J Urol. 1999;162:1003–6. doi: 10.1016/S0022-5347(01)68048-0. discussion 1006–7. [DOI] [PubMed] [Google Scholar]
- 22.Cox MJ, Coplen DE, Austin PF. The incidence of disorders of sexual differentiation and chromosomal abnormalities of cryptorchidism and hypospadias stratified by meatal location. J Urol. 2008;180:2649–52. doi: 10.1016/j.juro.2008.08.058. discussion 2652. [DOI] [PubMed] [Google Scholar]
- 23.Köhler B, Biebermann H, Friedsam V, et al. Analysis of the Wilms’ tumour suppressor gene (WT1) in patients 46, XY disorders of sex development. J Clin Endocrinol Metab. 2011;96:E1131–6. doi: 10.1210/jc.2010-2804. https://doi.org/10.1210/jc.2010-2804. [DOI] [PubMed] [Google Scholar]
- 24.Parelkar SV, Gupta RK, Oak S, et al. Laparoscopic management of persistent Mullerian duct syndrome. J Pediatr Surg. 2009;44:e1–3. doi: 10.1016/j.jpedsurg.2009.05.033. https://doi.org/10.1016/j.jpedsurg.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 25.Farikullah J, Ehtisham S, Nappo S, et al. Persistent Mullerian duct syndrome: Lessons learned from managing a series of eight patients over a 10-year period and review of literature regarding malignant risk from the Mullerian remnants. BJU Int. 2012;110:E1084–9. doi: 10.1111/j.1464-410X.2012.11184.x. https://doi.org/10.1111/j.1464-410X.2012.11184.x. [DOI] [PubMed] [Google Scholar]
- 26.Penson D, Krishnaswami S, Jules A, et al. Effectiveness of hormonal and surgical therapies for cryptorchidism: A systematic review. Pediatrics. 2013;131:e1897–907. doi: 10.1542/peds.2013-0072. https://doi.org/10.1542/peds.2013-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christiansen P, Müller J, Buhl S, et al. Treatment of cryptorchidism with human chorionic gonadotropin or gonadotropin-releasing hormone. A double-blind controlled study of 243 boys. Horm Res. 1988;30:187–92. doi: 10.1159/000181058. https://doi.org/10.1159/000181058. [DOI] [PubMed] [Google Scholar]
- 28.Biers SM, Malone PS. A critical appraisal of the evidence for improved fertility indices in undescended testes after gonadotrophin-releasing hormone therapy and orchidopexy. J Pediatr Urol. 2010;6:239–46. doi: 10.1016/j.jpurol.2010.02.203. https://doi.org/10.1016/j.jpurol.2010.02.203. [DOI] [PubMed] [Google Scholar]
- 29.Jallouli M, Rebai T, Abid N, et al. Neoadjuvant gonadotropin-releasing hormone therapy before surgery and effect on fertility index in unilateral undescended testes: A prospective, randomized trial. Urology. 2009;73:1251–4. doi: 10.1016/j.urology.2008.10.078. https://doi.org/10.1016/j.urology.2008.10.078. [DOI] [PubMed] [Google Scholar]
- 30.Schwentner C, Oswald J, Kreczy A, et al. Neoadjuvant gonadotropin-releasing hormone therapy before surgery may improve the fertility index in undescended testes: A prospective, randomized trial. J Urol. 2005;173:974–7. doi: 10.1097/01.ju.0000153562.07287.77. https://doi.org/10.1097/01.ju.0000153562.07287.77. [DOI] [PubMed] [Google Scholar]
- 31.Ritzén EM. Undescended testes: A consensus on management. Eur J Endocrinol. 2008;159( Suppl 1):S87–90. doi: 10.1530/EJE-08-0181. https://doi.org/10.1530/EJE-08-0181. [DOI] [PubMed] [Google Scholar]
- 32.American Academy of Pediatrics. Timing of elective surgery on the genitalia of male children with particular reference to the risks, benefits, and psychological effects of surgery and anesthesia. Pediatrics. 1996;97:590–4. [PubMed] [Google Scholar]
- 33.Kolon TF, Herndon CA, Baker LA, et al. Evaluation and treatment of cryptorchidism: AUA guideline. J Urol. 2014;192:337–45. doi: 10.1016/j.juro.2014.05.005. https://doi.org/10.1016/j.juro.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Hutson JM, Li R, Southwell BR, Petersen BL, et al. Germ cell development in the postnatal testis: The key to prevent malignancy in cryptorchidism? Front Endocrinol (Lausanne) 2012;3:176. doi: 10.3389/fendo.2012.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kollin C, Karpe B, Hesser U, et al. Surgical treatment of unilaterally undescended testes: Testicular growth after randomization to orchiopexy at age nine months or three years. J Urol. 2007;178:1589–93. doi: 10.1016/j.juro.2007.03.173. discussion 1593. [DOI] [PubMed] [Google Scholar]
- 36.Bianchi A, Squire BR. Transscrotal orchidopexy: Orchidopexy revised. Ped Surgery Int. 1989;4:189–92. [Google Scholar]
- 37.Gordon M, Cervellione RM, Morabito A, et al. 20 years of transcrotal orchidopexy for undescended testis: Results and outcomes. J Pediatr Urol. 2010;6:506–12. doi: 10.1016/j.jpurol.2009.10.016. https://doi.org/10.1016/j.jpurol.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Callewaert PRH, Rahnama’i MS, Biallosterski BT, et al. Scrotal approach to both palpable and impalpable undescended testes: should it become our first choice? Urology. 2010;76:73–6. doi: 10.1016/j.urology.2009.09.096. https://doi.org/10.1016/j.urology.2009.09.096. [DOI] [PubMed] [Google Scholar]
- 39.Yucel S, Celik O, Kol A, et al. Initial prescrotal approach for palpable cryptorchid testis: Results during a three-year period. J Urol. 2011;185:669–72. doi: 10.1016/j.juro.2010.09.117. https://doi.org/10.1016/j.juro.2010.09.117. [DOI] [PubMed] [Google Scholar]
- 40.Bassel YS, Scherz HC, Kirsch AJ. Scrotal incision orchiopexy for undescended testes with or without a patent processus vaginalis. J Urol. 2007;177:1516–8. doi: 10.1016/j.juro.2006.11.075. https://doi.org/10.1016/j.juro.2006.11.075. [DOI] [PubMed] [Google Scholar]
- 41.Cloutier J, Moore K, Nadeau G, et al. Modified scrotal (Bianchi) mid-raphe single incision orchiopexy for low palpable undescended testis: Early outcomes. J Urol. 2011;185:1088–92. doi: 10.1016/j.juro.2010.10.039. https://doi.org/10.1016/j.juro.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 42.Novaes HFF, Carneiro Neto JA, Macedo A, et al. Single scrotal incision orchiopexy—a systematic review. Int Braz J Urol. 2013;39:305–11. doi: 10.1590/S1677-5538.IBJU.2013.03.02. https://doi.org/10.1590/S1677-5538.IBJU.2013.03.02. [DOI] [PubMed] [Google Scholar]
- 43.Al-Mandil M, Khoury AE, et al. Potential complications with the prescrotal approach for the palpable undescended testis? A comparison of single prescrotal incision to the traditional inguinal approach. J Urol. 2008;180:686–9. doi: 10.1016/j.juro.2008.04.040. https://doi.org/10.1016/j.juro.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 44.Na SW, Kim S-O, Hwang EC, et al. Single scrotal incision orchiopexy for children with palpable low-lying undescended testis: Early outcome of a prospective, randomized, controlled study. Korean J Urol. 2011;52:637–41. doi: 10.4111/kju.2011.52.9.637. https://doi.org/10.4111/kju.2011.52.9.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nazem M, Hosseinpour M, Shahbandari M. Evaluation of orchidopexy with or without opening the external oblique fascia in children with superficial inguinal undescended testis. Eur J Pediatr Surg. 2011;21:255–7. doi: 10.1055/s-0031-1275724. https://doi.org/10.1055/s-0031-1275724. [DOI] [PubMed] [Google Scholar]
- 46.Cisek LJ, Peters CA, Atala A, et al. Current findings in diagnostic laparoscopic evaluation of the non-palpable testis. J Urol. 1998;160:1145–9l. discussion 1150. [PubMed] [Google Scholar]
- 47.Snodgrass WT, Yucel S, Ziada A. Scrotal exploration for unilateral non-palpable testis. J Urol. 2007;178:1718–21. doi: 10.1016/j.juro.2007.05.089. https://doi.org/10.1016/j.juro.2007.05.089. [DOI] [PubMed] [Google Scholar]
- 48.Storm D, Redden T, Aguiar M, et al. Histological evaluation of the testicular remnant associated with the vanishing testes syndrome: Is surgical management necessary? Urology. 2007;70:1204–6. doi: 10.1016/j.urology.2007.08.020. https://doi.org/10.1016/j.urology.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 49.Kirsch AJ, Escala J, Duckett JW, et al. Surgical management of the non-palpable testis: The Children’s Hospital of Philadelphia experience. J Urol. 1998;159:1340–3. https://doi.org/10.1016/S0022-5347(01)63613-9. [PubMed] [Google Scholar]
- 50.Fowler R, Stephens FD. The role of testicular vascular anatomy in the salvage of high undescended testes. Aust N Z J Surg. 1959;29:92–106. doi: 10.1111/j.1445-2197.1959.tb03826.x. https://doi.org/10.1111/j.1445-2197.1959.tb03826.x. [DOI] [PubMed] [Google Scholar]
- 51.Baker LA, Docimo SG, Surer I, et al. A multi-institutional analysis of laparoscopic orchidopexy. BJU Int. 2001;87:484–9. doi: 10.1046/j.1464-410x.2001.00127.x. https://doi.org/10.1046/j.1464-410X.2001.00127.x. [DOI] [PubMed] [Google Scholar]
- 52.Esposito C, Caldamone AA, Settimi A, et al. Management of boys with non-palpable undescended testis. Nat Clin Pract Urol. 2008;5:252–60. doi: 10.1038/ncpuro1102. https://doi.org/10.1038/ncpuro1102. [DOI] [PubMed] [Google Scholar]
- 53.Caldamone AA, Amaral JF. Laparoscopic stage 2 Fowler-Stephens orchiopexy. J Urol. 1994;152:1253–6. doi: 10.1016/s0022-5347(17)32562-4. [DOI] [PubMed] [Google Scholar]
- 54.Dénes FT, Saito FJ, Silva FA, et al. Laparoscopic diagnosis and treatment of non-palpable testis. Int Braz J Urol. 2008;34:329–34. doi: 10.1590/s1677-55382008000300010. discussion 335. [DOI] [PubMed] [Google Scholar]
- 55.Dave S, Manaboriboon N, Braga LHP, et al. Open vs. laparoscopic staged Fowler-Stephens orchiopexy: Impact of long loop vas. J Urol. 2009;182:2435–9. doi: 10.1016/j.juro.2009.07.050. https://doi.org/10.1016/j.juro.2009.07.050. [DOI] [PubMed] [Google Scholar]
- 56.Robertson SA, Munro FD, Mackinlay GA. Two-stage Fowler-Stephens orchidopexy preserving the gubernacular vessels and a purely laparoscopic second stage. J Laparoendosc Adv Surg Tech A. 2007;17:101–7. doi: 10.1089/lap.2006.0565. https://doi.org/10.1089/lap.2006.0565. [DOI] [PubMed] [Google Scholar]
- 57.Braga LH, DeMaria J. Laparoscopic orchidopexy preserving the cremasteric vessels and using the inguinal canal as a pathway for testicular descent. Can Urol Assoc J. 2009;3:560. [Google Scholar]
- 58.DeMaria J. Surgical maneuvers to improve testicular survival after a single stage Fowler-Stephens orchidopexy. Poster Presentation Annual Congress of the European Society of Pediatric Urology; 2003. [Google Scholar]
- 59.Hay SA. Collateral circulation after spermatic vessel ligation for abdominal testis and its impact on staged laparoscopically assisted orchiopexy. J Laparoendosc Adv Surg Tech A. 2007;17:124–7. doi: 10.1089/lap.2006.0508. https://doi.org/10.1089/lap.2006.0508. [DOI] [PubMed] [Google Scholar]
- 60.Docimo SG. The results of surgical therapy for cryptorchidism: A literature review and analysis. J Urol. 1995;154:1148–52. https://doi.org/10.1016/S0022-5347(01)67015-0. [PubMed] [Google Scholar]
- 61.Ferro F, Spagnoli A, Zaccara A, et al. Is preoperative laparoscopy useful for impalpable testis? J Urol. 1999;162:995–6. doi: 10.1016/S0022-5347(01)68044-3. discussion 997. [DOI] [PubMed] [Google Scholar]
- 62.Chandrasekharam VVSS. Laparoscopy vs. inguinal exploration for non-palpable undescended testis. Indian J Pediatr. 2005;72:1021–3. doi: 10.1007/BF02724403. https://doi.org/10.1007/BF02724403. [DOI] [PubMed] [Google Scholar]
- 63.Abolyosr A. Laparoscopic vs. open orchiopexy in the management of abdominal testis: A descriptive study. Int J Urol. 2006;13:1421–4. doi: 10.1111/j.1442-2042.2006.01582.x. https://doi.org/10.1111/j.1442-2042.2006.01582.x. [DOI] [PubMed] [Google Scholar]
- 64.Moursy EE, Gamal W, Hussein MM. Laparoscopic orchiopexy for non-palpable testes: Outcome of two techniques. J Pediatr Urol. 2011;7:178–81. doi: 10.1016/j.jpurol.2010.04.010. https://doi.org/10.1016/j.jpurol.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 65.Rogers E, Teahan S, Gallagher H, et al. The role of orchiectomy in the management of postpubertal cryptorchidism. J Urol. 1998;159:851–4. https://doi.org/10.1016/S0022-5347(01)63752-2. [PubMed] [Google Scholar]
- 66.Ankarberg-Lindgren C, Westphal O, Dahlgren J. Testicular size development and reproductive hormones in boys and adult males with Noonan syndrome: A longitudinal study. Eur J Endocrinol. 2011;165:137–44. doi: 10.1530/EJE-11-0092. https://doi.org/10.1530/EJE-11-0092. [DOI] [PubMed] [Google Scholar]
- 67.Dada R, Kumar R, Kucheria K. A two-year-old baby with Downs syndrome, cryptorchidism, and testicular tumour. Eur J Med Genet. 2006;49:265–8. doi: 10.1016/j.ejmg.2005.08.002. https://doi.org/10.1016/j.ejmg.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 68.Noseworthy J. Recurrent undescended testes. Semin Pediatr Surg. 2003;12:90–3. doi: 10.1016/s1055-8586(02)00017-3. https://doi.org/10.1016/S1055-8586(02)00017-3. [DOI] [PubMed] [Google Scholar]
- 69.McIntosh LA, Scrimgeour D, Youngson GG, et al. The risk of failure after primary orchidopexy: An 18-year review. J Pediatr Urol. 2013;9:759–62. doi: 10.1016/j.jpurol.2012.09.002. https://doi.org/10.1016/j.jpurol.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Walsh TJ, Dall’Era MA, Croughan MS, et al. Prepubertal orchiopexy for cryptorchidism may be associated with lower risk of testicular cancer. J Urol. 2007;178:1440–6. doi: 10.1016/j.juro.2007.05.166. discussion1446. https://doi.org/10.1016/j.juro.2007.05.166. [DOI] [PubMed] [Google Scholar]
- 71.Oh J, Landman J, Evers A, et al. Management of the postpubertal patient with cryptorchidism: An updated analysis. J Urol. 2002;167:1329–33. https://doi.org/10.1016/S0022-5347(05)65293-7. [PubMed] [Google Scholar]
- 72.Barthold JS, Gonzalez R. The epidemiology of congenital cryptorchidism, testicular ascent, and orchiopexy. J Urol. 2003;170:2396–401. doi: 10.1097/01.ju.0000095793.04232.d8. https://doi.org/10.1097/01.ju.0000095793.04232.d8. [DOI] [PubMed] [Google Scholar]
- 73.Stec AA, Thomas JC, DeMarco RT, et al. Incidence of testicular ascent in boys with retractile testes. J Urol. 2007;178:1722–4. doi: 10.1016/j.juro.2007.05.091. https://doi.org/10.1016/j.juro.2007.05.091. [DOI] [PubMed] [Google Scholar]


