Alcoholic liver disease (ALD) is a major type of chronic liver disease worldwide ranging from simple fatty liver to more severe forms of liver injury, including alcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma (HCC). So far, there are no FDA-approved drugs for the treatment of ALD. Increased inflammation due to over-activation of Kupffer cells is an important mechanism contributing to the pathogenesis of ALD, and has been actively investigated as a therapeutic target for the treatment of ALD. Many potential targets against inflammation have been identified in preclinical studies of ALD,(1) but none of them have been approved to be effective in clinic for the treatment of patients with ALD yet. In this issue of HEPATOLOGY, Saikia et al.(2) add two more new targets including a small specific-sized hyaluronic acid (HA) 35 (HA35) and microRNA (miRNA)-181b-3p that may have therapeutic potential for the treatment of ALD. By screening a series of small specific-sized HA fragments including HA7, HA12, HA59, HA35 and HA74 for their immunoregulatory effects on Kupffer cells from ethanol-fed rats, the authors identified that only small specific-sized HA of ~35kD (HA35) completely normalized the over-activated Kupffer cells from ethanol-fed rats in vitro and in vivo. Furthermore, oral supplementation with HA35 ameliorated ethanol-induced liver injury and inflammation in mice. These findings suggest that HA35 is a potent negative regulator to control Kupffer cell over-activation in ethanol-fed animals.
HA, also called hyaluronan, is an anionic, nonsulfated glycosaminoglycan that is distributed in all bodily tissues and fluids and is a key component of the extracellular matrix. HA is known to play an important role in the control of cell proliferation and migration, but its role in modulating inflammation is complicated.(3) Dependent on the size and matrix structure of the HA molecules, and interaction with various types of HA binding proteins and receptors, HA can act either as an anti-inflammatory mediator or function as a pro-inflammatory factor in response to tissue damage.(3) The study by Saikia et al.(2) clearly demonstrated that HA35, not several other small-sized HAs they tested, acts as a potent anti-inflammatory mediator to ameliorate ethanol-induced over-activation of Kupffer cells in vivo and in vitro. Further mechanistic studies demonstrated that the inhibitory effect of HA35 on hepatic inflammation is mediated by restoring miRNA-181b-3p expression in Kupffer cells that is downregulated by ethanol.
MicroRNAs (miRNAs), which are endogenous small noncoding RNAs (~22 nucleotides) that work at the posttranscriptional level to regulate gene expression through complementary base pairing with the 3′ untranslated region (3′ UTR) of target mRNA. Over the last decade, a large number of miRNAs have been demonstrated to play a critical role in the pathogenesis of various types of liver diseases including ALD, and many of them exert their functions in cell-specific manners (Figure 1).(4) For example, miR-122 is mainly expressed in hepatocytes, playing a crucial role in lipid metabolism and liver carcinogenesis. MiR-155 is highly expressed in macrophages/Kupffer cells and is further increased in isolated Kupffer cells after alcohol feeding, playing a pro-inflammatory role in promoting alcoholic liver injury.(5) In contrast, miR-223, which is highly expressed in neutrophils, is a critical inhibitor to limit over-activation of neutrophils in ethanol or drug-induced hepatotoxicity.(6, 7) Moreover, Delia et al.(8) have demonstrated that miR-182 is the most highly upregulated miRNA in alcoholic hepatitis and mainly expressed in ductular reaction cells, playing an important role in promoting bile acid accumulation and inflammation. The study by Saikia et al.(2) identified miR-181b-3p as a critical negative regulator for TLR4 signaling in Kupffer cells. By using Next Generation Sequencing analysis, the authors demonstrated that 30 miRNAs were markedly downregulated by more than two-fold in Kupffer cells from ethanol-fed rats compared to those from pair-fed animals, and three miRNAs from these 30 downregulated miRNAs were restored by HA35 treatment. Among these three miRNAs, the miR-181b-3p was the most highly downregulated by ethanol. Further studies from this paper demonstrated that importin α5, a protein involved in the activation of NF-κB signaling, is a downstream target of miR-181b-3p, and that miR-181b-3p inhibited importin α5 expression, thereby attenuating NF-κB signaling activation. Ethanol feeding downregulated miR-181b-3p expression and subsequently upregulated importin α5 and NF-κB signaling activation. Interestingly, all of these effects were normalized by HA35 treatment.
Figure 1. MiRNAs play important roles in the control of ALD development and progression in cell-specific manners.
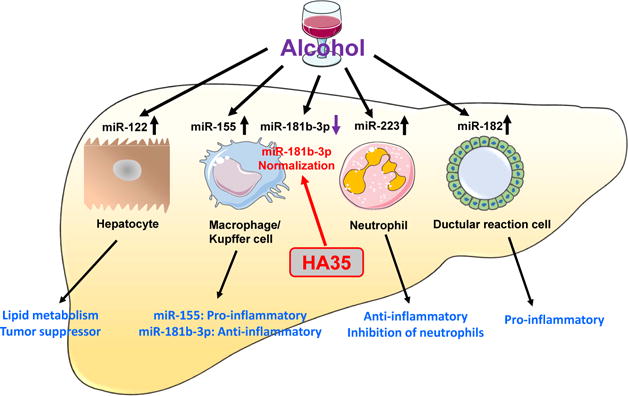
For example, miR-181b-3p acts as anti-inflammatory mediator to inhibit Kupffer cell activation; whereas miR-155 promotes Kupffer cell activation. Ethanol downregulates miR-181b-3p in Kupffer cells, thereby inducing Kupffer cell activation. Treatment of HA35 normalizes ethanol-induced miR-181b-3p downregulation in Kupffer cells and subsequently ameliorates ethanol-induced liver injury and inflammation.
As discussed above, many miRNAs including miR-181b-3p play important roles in the pathogenesis of ALD. The obvious question is whether miRNAs can be used as therapeutic targets for the treatment of ALD. To our knowledge, there have been no reports on miRNA-based therapy in ALD. However, miRNAs-based therapeutic approach has been tested in other types of liver diseases. For example, the miR-122 inhibitor miravirsen was tested in a Phase 2a study for the treatment of hepatitis C (HCV), and showed that five weekly treatments with the miR-122 inhibitor miravirsen resulted in a dose-dependent and prolonged reduction in HCV RNA levels with no evidence of viral resistance.(9) In addition, Femke et al.(10) indicated that a single dose of RG-101, which is a GalNAc conjugated anti-miR-122 oligonucleotide, caused a significant viral load reduction in HCV patients, which was associated with normalization of NK cell proportions and reduction of NK cell activation receptor expression without eliciting systemic immune response. All the above clinical results strongly highlighted that miRNAs can be considered as novel and prospective therapeutic targets for the treatment of liver diseases. Given an important role of miR-181b-3p in the control of Kupffer cell activation and HA35 restoration of miR-181b-3p as demonstrated by Saikia et al.,(2) HA35, miR-181b-3p, or combination may have therapeutic potential for the treatment of ALD. However, before testing these targets in clinical studies, more experiments should be performed to examine the effects of miR-181b-3p and HA35 on other types of immune cells such as neutrophils that also play important roles in the pathogenesis of ALD.
References
- 1.Gao B, Tsukamoto H. Inflammation in Alcoholic and Nonalcoholic Fatty Liver Disease: Friend or Foe? Gastroenterology. 2016;150:1704–1709. doi: 10.1053/j.gastro.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saikia P, Bellos D, McMullen MR, Pollard KA, de la Motte C, Nagy LE. miR181b-3p and its target importin alpha5 regulate TLR4 signaling in Kupffer cells and liver injury in mice in response to ethanol. Hepatology. 2017 doi: 10.1002/hep.29144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Front Immunol. 2014;5:101. doi: 10.3389/fimmu.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDaniel K, Herrera L, Zhou T, Francis H, Han Y, Levine P, Lin E, et al. The functional role of microRNAs in alcoholic liver injury. J Cell Mol Med. 2014;18:197–207. doi: 10.1111/jcmm.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bala S, Csak T, Saha B, Zatsiorsky J, Kodys K, Catalano D, Satishchandran A, et al. The pro-inflammatory effects of miR-155 promote liver fibrosis and alcohol-induced steatohepatitis. J Hepatol. 2016;64:1378–1387. doi: 10.1016/j.jhep.2016.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, He Y, Zhou Z, Ramirez T, Gao Y, Gao Y, Ross RA, et al. MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6-p47phox-oxidative stress pathway in neutrophils. Gut. 2017;66:705–715. doi: 10.1136/gutjnl-2016-311861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He Y, Feng D, Li M, Gao Y, Ramirez T, Cao H, Kim SJ, et al. Hepatic mtDNA-TLR9-microRNA-223 forms a negative feedback loop to limit neutrophil over-activation and acetaminophen hepatotoxicity. Hepatology. 2017 doi: 10.1002/hep.29153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaya D, Coll M, Rodrigo-Torres D, Vila-Casadesus M, Altamirano J, Llopis M, Graupera I, et al. Integrative microRNA profiling in alcoholic hepatitis reveals a role for microRNA-182 in liver injury and inflammation. Gut. 2016;65:1535–1545. doi: 10.1136/gutjnl-2015-311314. [DOI] [PubMed] [Google Scholar]
- 9.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 10.Stelma F, van der Ree MH, Sinnige MJ, Brown A, Swadling L, de Vree JML, Willemse SB, et al. Immune phenotype and function of NK and T cells in chronic hepatitis C patients who received a single dose of anti-miR-122, RG-101. Hepatology. 2017:n/a–n/a. doi: 10.1002/hep.29148. [DOI] [PMC free article] [PubMed] [Google Scholar]


