Abstract
OBJECTIVES
Acute pancreatitis has a highly variable course. Currently there is no widely accepted method to measure disease activity in patients hospitalized for acute pancreatitis. We aimed to develop a clinical activity index that incorporates routine clinical parameters to assist in the measurement, study, and management of acute pancreatitis.
METHODS
We used the UCLA/RAND appropriateness method to identify items for inclusion in the disease activity instrument. We conducted a systematic literature review followed by two sets of iterative modified Delphi meetings including a panel of international experts between November 2014 and November 2015. The final instrument was then applied to patient data obtained from five separate study cohorts across Southern California to assess profiles of disease activity.
RESULTS
From a list of 35 items comprising 6 domains, we identified 5 parameters for inclusion in the final weighted clinical activity scoring system: organ failure, systemic inflammatory response syndrome, abdominal pain, requirement for opiates and ability to tolerate oral intake. We applied the weighted scoring system across the 5 study cohorts comprising 3,123 patients. We identified several distinct patterns of disease activity: (i) overall there was an elevated score at baseline relative to discharge across all study cohorts, (ii) there were distinct patterns of disease activity related to duration of illness as well as (iii) early and persistent elevation of disease activity among patients with severe acute pancreatitis defined as persistent organ failure.
CONCLUSIONS
We present the development and initial validation of a clinical activity score for real-time assessment of disease activity in patients with acute pancreatitis.
INTRODUCTION
Acute pancreatitis is a common cause for hospitalization accounting for over 200,000 hospitalizations in the United States on an annual basis (1,2). Despite the increasing burden of this disease there remains no therapy directed to the molecular pathogenesis that has established efficacy in altering the natural history of this condition. An international panel of experts convened by the National Institutes of Health in Bethesda, MD, concluded in 2012 that despite improvement in our understanding of the mechanistic pathways underlying acute pancreatitis, there has been a lack of progress in development of new clinical therapies (3). In addition to lack of therapies, the field lacks a standardized quantitative system to measure disease activity at any given time during the course of acute pancreatitis.
A key step to testing novel treatments and improving the quality of care for patients with acute pancreatitis is the development of a standardized quantitative method to measure real time clinical disease activity. While established definitions for diagnosis as well as classification of severity exist (4,5), there is currently no widely accepted method to objectively assess a patient’s level of disease activity during different phases of acute pancreatitis. The ability to accurately and reliably assess a patient’s level of disease activity has important clinical as well as research implications. From a clinical perspective it is important to define a patient’s phase of illness in order to determine appropriate timing for interventions such as fluid resuscitation, re-introduction of oral nutrition or safe discharge from an inpatient care unit. From a research standpoint, a validated dynamic clinical activity score would help identify the impact of an intervention on the natural history of disease.
In this article, we describe the process of development and initial validation of an acute Pancreatitis Activity Scoring System (PASS) that incorporates both clinical parameters and patient reported symptoms for the assessment of disease activity in patients with acute pancreatitis.
METHODS
Study overview
Adapting previous definitions applied in other disease areas (6), we defined disease activity as “reversible clinical manifestations of acute pancreatitis”. We used the RAND/UCLA appropriateness model (7) to identify potentially appropriate parameters for inclusion in the newly developed activity scoring system. The RAND/UCLA appropriateness model is a validated method to synthesize medical literature and gather expert-opinion for application to issues pertaining to health care. In applying the RAND/UCLA appropriateness method we conducted a modified Delphi process incorporating two rounds of in-person meetings to identify and refine the list of potential parameters to be included. This was followed by a subsequent validation phase during which individual centers were asked to apply the system within their patient populations. A debriefing session was held 1-year later to allow feedback based on initial experience in applying the new scoring system.
Item generation and refinement
We defined a priori that components included in the clinical activity score should meet the following criteria:
Individual components should have content validity for the assessment of patients with acute pancreatitis.
Individual components should be widely available and provide highly reliable as well as reproducible measurement.
Collection of the individual components of the score should not require additional testing or incur further cost beyond the routine care of patients with acute pancreatitis.
The composite score should reflect the dynamic nature of acute pancreatitis and therefore provide opportunity for serial measurement.
The composite score should have a wide dynamic range to capture the full spectrum of illness.
The composite score should include both established clinical parameters and patient reported symptoms.
Finally, the working group proposed that the final score should be recalculated at 12-h increments to capture rapid changes in a patient’s clinical course while still providing feasible real-time prospective as well as retrospective data ascertainment.
Literature review
To identify items for potential inclusion in the activity score we performed a systematic literature review of clinical outcome and assessment measures used in previous randomized controlled trials in acute pancreatitis (8). Specifically, we conducted a search of PubMed, Embase and the Cochrane database of studies published from 1996 to 2014 that met the following criteria: randomized control trials, English language, use of Human subjects, and studies that evaluated the effect of therapy in acute pancreatitis. We excluded prevention studies either primary, for example, post-ERCP pancreatitis or secondary prevention of recurrent acute pancreatitis due to gallstones or alcohol. Primary or main outcomes of each study were analyzed. In addition, we reviewed currently validated prognostic clinical scoring systems in acute pancreatitis (9) to identify additional clinical parameters to potentially be included in the activity score. The information was collated and discussed at meetings of the Southern California Pancreas Study Group in advance of the joint meeting of the American Pancreatic Association (APA)/Japanese Pancreas Society (JPS) meeting in November, 2014.
The list of potential components was then reviewed via an electronic survey completed by attendees at the joint APA/JPS meeting in Honolulu, HI in November, 2014. All survey respondents also had the opportunity to propose additional candidate parameters for inclusion.
Modified delphi process
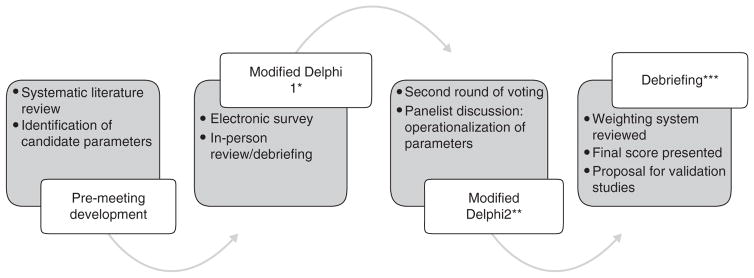
We then invited a panel of international experts from the United States, Europe, Japan and New Zealand to participate in a modified Delphi process incorporating two rounds of face-to-face meetings held in Washington, DC May 2015 and San Diego, CA October 2015. In accordance with the UCLA/RAND appropriateness model we applied an iterative process to refine the elements of the activity score. Each of the panelists was asked to rate items on a 1–9 scale with 1 indicating highly irrelevant and 9 meaning most relevant in the clinical assessment of patients with acute pancreatitis. A score of 4–6 indicated ambivalence or uncertainty regarding the relevance of any given parameter. Panelists were first asked to rate each item independently during the initial Delphi meeting. The results of initial voting were then collated and distributed to the panelists with allowance for further discussion related to relevance of each of the measures to disease activity and the general availability of candidate measures in clinical practice. This discussion was followed by another round of scoring. During the second Delphi meeting in San Diego, CA panelists further discussed relevance and availability of candidate markers in clinical practice. Only parameters receiving a score of 8 or 9 among a majority of respondents were considered for final inclusion in the activity score. Following each Delphi meeting, a subcommittee reviewed the results of voting and incorporated recommendations from the panel discussions. Following the second Delphi meeting, a sub-committee was tasked to assign a relative value to each of the final parameters on a 100-point scale. The weighting scale was then refined through an iterative process with application to several case scenarios. The finalized scoring system with proposed weighting scale was presented to the full panel at the annual meeting of the American Pancreatic Association in October 2015. A 1-year validation phase was then proposed to assess feasibility of the scoring system across health systems as well as allow opportunity for open commentary regarding individual parameters and/or weighting scale. The findings of the validation phase were reviewed at a debriefing session held during the October 2016 meeting of the American Pancreatic Association, Boston, MA. The steps involved in the process of development the scoring system and the final score are outlined in Figure 1.
Figure 1.
Development of the acute Pancreatitis Activity Scoring System (PASS). The figure presents the steps in development of the PASS. *, Joint meeting of the American Pancreatic Association/Japanese Pancreas Society, November 2014 Honolulu, HI. **, Digestive Disease Week, May 2015 Washington, DC. ***, Annual meeting of the American Pancreatic Association, November 2015, San Diego, CA.
Score validation
To demonstrate feasibility as well as reproducibility of the new activity scoring system we applied the index across several health systems in Southern California. Specifically, we applied the activity scoring system on data obtained from five major health systems serving Southern California: Kaiser Permanente Southern California, Cedars-Sinai Medical Center (CSMC), Los Angeles County hospital/University of Southern California, University of California Los Angeles Harbor Medical Center and University of California San Francisco Fresno Medical Center.
All care provided within each of the medical centers was provided according to institutional standards with management decisions at the discretion of each patient’s primary treatment team. Each of the participating sites received approval to conduct the study from their respective institutional review boards. A brief description of the health systems, the medical centers they comprise and individual study cohorts is included below:
Kaiser Permanente Southern California
Kaiser Permanente Southern California (KPSC) is a regional integrated healthcare system comprising 13 acute care hospitals. We performed a retrospective cohort study of patients hospitalized for acute pancreatitis from January 2006 to December 2013 using data from the KPSC system. For the KPSC acute pancreatitis study, cases were identified based on ICD-9 code 577.0 with additional confirmation by elevation in amylase or lipase ≥3 times upper limit of normal. This strategy for case identification has been previously demonstrated to yield >95% positive predictive value for true cases of acute pancreatitis based on clinical criteria from manual chart abstraction (10). Patients with history of opiate prescription within 90 days prior to admission, treatment with analgesic patch during hospitalization or diagnosis of chronic pancreatitis or pancreatic cancer prior to their hospitalization for acute pancreatitis were excluded. Patients discharged within 24 h of hospitalization as well as those without a pain score recorded during the baseline period were further excluded. In the KPSC cohort, activity scores were electronically captured based on data contained within the electronic health record.
Cedars-Sinai medical center
The Cedars-Sinai Health System includes an 886 bed regional tertiary academic hospital facility as well as the Cedars-Sinai Health network that comprises the Cedars-Sinai Medical Group as well as Cedars-Sinai Health Associates. Patients hospitalized for acute pancreatitis at CSMC from January 2014 to November 2014 were identified in a retrospective fashion by International Classification and Diagnosis (ICD-9) code 577.0. All cases were manually reviewed to confirm the presence of at least 2 of the 3 following criteria: typical upper abdominal pain symptoms; elevation in serum amylase or lipase ≥3 times normal; and/or imaging features consistent with acute pancreatitis on radiographic imaging. Activity scores were calculated from data manually abstracted from the medical record in the CSMC study cohort.
Los Angeles County+University of Southern California medical center
As a 600-bed tertiary acute care academic hospital, Los Angeles County+University of Southern California (USC) Medical Center is one of the largest public hospitals in the United States. Patients hospitalized for acute pancreatitis at USC between March 2015–March 2016 were prospectively identified based on the aforementioned criteria consistent with acute pancreatitis. Acute pancreatitis activity scores were retrospectively calculated based on prospectively collected data obtained in the LAC/USC pancreatitis study cohort.
University of California Los Angeles, Harbor Medical Center
As a 570 bed academic hospital, Harbor University of California Los Angeles (UCLA) is one of the main level 1 trauma centers serving the greater South Bay area of Los Angeles County. Patients hospitalized for acute pancreatitis at Harbor UCLA between 1 January 2015 and 31 December 2015 were identified based on the presence of at least two of the previously indicated criteria for Acute Pancreatitis. Acute pancreatitis activity scores were retrospectively calculated based on prospectively collected data obtained in the Harbor-UCLA pancreatitis study cohort.
University of California San Francisco, Fresno Medical Center
As a 685 bed academic hospital, University of California San Francisco, Fresno Medical Center (UCSF Fresno) is the only Level 1 Trauma Center serving a multi-county area of Central California and is the fifth largest, third busiest hospital in the state. Patients hospitalized for acute pancreatitis at UCSF Fresno between 1 January 2015 and 30 June 2015 were identified based on aforementioned clinical criteria for acute pancreatitis. Acute pancreatitis activity scores were retrospectively calculated based on prospectively collected data obtained in the UCSF Fresno pancreatitis study cohort.
For each of the centers, individual hospitalizations were divided into discrete 12-h increments with variables obtained from the emergency department incorporated into the initial (baseline) 12-h assessment. For parameter assessment, the most extreme vital sign or laboratory value obtained during an individual 12-h window was used. If no laboratory value was available during the assigned window, the most recent prior laboratory value was carried forward.
The final components of the clinical activity score were then ascertained for each of the 12-h windows for each patient’s hospitalization.
Profiles of disease activity in acute pancreatitis
We performed three sets of analyses to assess the ability of the acute PASS system to capture distinct profiles of disease activity in patients with acute pancreatitis.
First, we evaluated the distribution of activity scores at baseline as well as discharge across the five study cohorts.
We then tested the ability of the scoring system to reflect differences in disease activity profiles in the KPSC study cohort based on hospital length of stay (self-limited disease/early resolution: <3 days, intermediate: 3–7 days, extended disease: length-of-stay beyond 7 days).
Finally, we plotted profiles of disease activity for patients based on severity of disease. Severe acute pancreatitis was defined as multi-organ or persistent organ failure in accordance to the revised Atlanta Classification system for acute pancreatitis (4). Organ failure was defined as a score of 2 or higher in any organ system according to the Modified Marshall organ failure system (4). Persistent organ failure was defined as the presence of organ failure for ≥48 h. In addition, mechanical ventilation or in-hospital dialysis was also considered as representing persistent organ failure. Moderate pancreatitis was defined as the presence of transient organ failure. Mild pancreatitis was defined as the absence of either organ failure or local complications of pancreatitis. We then plotted activity scores stratified by severity of acute pancreatitis.
All statistical analysis was performed in SAS version 9.3 (Cary, NC). All reported P -values are two-sided with alpha 0.05 significance.
RESULTS
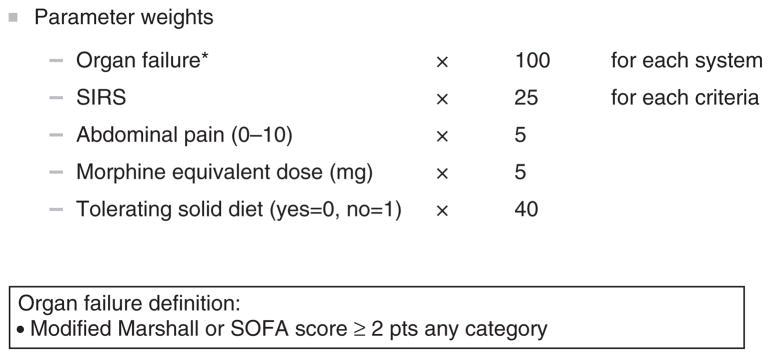
Results of the systematic review and a list of candidate parameters for inclusion in the score have been previously reported (8). From an initial review of 345 abstracts, we identified 69 studies that met full inclusion and exclusion criteria. Following further review of clinical outcome measures and prognostic markers, we compiled a list of 35 items comprising 6 domains: patient symptoms, physical signs, nutrition/oral intake, inflammatory markers, complications and laboratory tests (Supplementary Figure S1 online). Following the 2 Delphi meetings, the list of candidate markers was narrowed to five parameters rated as highly relevant in the assessment of acute pancreatitis: organ failure, systemic inflammatory response syndrome, abdominal pain, analgesic requirement and tolerance of oral intake. Measurement of the individual components of the activity scoring system was then operationalized as follows:
Organ failure: each system (respiratory, circulatory or renal) to be counted based on a modified Marshall organ failure score of 2 or higher (Supplementary Figure S2). In the absence of arterial blood gas measurement, a requirement for mechanical ventilation, vasopressor support or acute inpatient dialysis are also considered evidence of organ failure. The presence of any of these criteria for any portion of time during a 12-h measurement period was counted.
Systemic Inflammatory Response Syndrome (SIRS): each component of the SIRS system is measured independently and weighted independently in the PASS system. Criteria for SIRS are presented in Supplementary Figure S2.
Abdominal pain: a patient reported response to a numeric rating scale from 0 (no pain) to 10 (worst pain) is used to assess patient perceived level of pain. The highest recorded pain score within a 12-h block is used for calculation of the activity score. If a patient was sleeping at the time of assessment the pain level is assumed to be zero.
Morphine equivalent dose: to identify as well standardize a patient’s degree of requirement for opiate analgesic, a total morphine equivalent dose (MED) was calculated for each 12-hour block. To calculate the MED, the dose of opiate analgesics was first converted to oral morphine equivalents using a conversion factor based on the standard of 30 mg oral morphine=10 MED (Supplementary Figure S2). The total oral morphine equivalent dose was then summed for each 12-h block and divided by a factor of 3 to convert to the intravenous morphine equivalent dose.
Tolerance of solid diet: Successful resumption of oral intake was defined as consumption of any type of solid meal without subsequent increase in abdominal pain or vomiting. Any oral intake satisfying the above conditions within a 12-hour time frame is considered tolerance of a solid diet for the given assessment period.
Parameter weights: the components of the scoring system along with weighting scales are presented in Figure 2.
Figure 2.
Acute Pancreatitis Activity Scoring System with parameter weights.
Score performance and validation
Demographic and baseline clinical features for all three study cohorts are presented in Table 1.
Table 1.
Demographic and Clinical Characteristics of Southern California Pancreas Study Group Acute Pancreatitis Cohorts
| January 2006–December 2013 | January 2014–November 2014 | March 2015–March 2016 | January 2015–December 2015 | January 2015–June 2015 | |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| KPSC (N =2,282) | CSMC (N =222) | USC (N =291) | Harbor UCLA (N =152) | UCSF Fresno (N =176) | |
| Age at diagnosis | |||||
|
| |||||
| Median (IQR) | 52 (38, 65) | 52 (37, 68) | 44 (33, 53) | 44 (37, 55) | 50 (38, 63) |
|
| |||||
| Female sex | 1,181 (51.8%) | 115 (55.3%) | 134 (46.2%) | 81 (53%) | 74 (42%) |
|
| |||||
| Race/ethnicity a | |||||
|
| |||||
| NHW | 825 (36.2%) | — | 19 (6.6%) | 25 (16%) | 94 (53%) |
|
| |||||
| NHB | 210 (9.2%) | — | 18 (6.2%) | 18 (12%) | 0 (0%) |
|
| |||||
| Asian | 157 (6.9%) | — | 14 (4.8%) | 4 (3%) | 0 (0%) |
|
| |||||
| Other | 35 (1.5%) | — | 6 (2.0%) | 0 (0%) | 1 (1%) |
|
| |||||
| Hispanic | 1,055 (46.2%) | 48 (23.2%) | 234 (80.7%) | 105 (69%) | 81 (46%) |
|
| |||||
| Body mass index, median (IQR) | 28.6 (24.7, 33.0) | 25.2 (21.8, 30.0) | 28.0 (24.4,33.0) | 29.2 (24.6–34.1) | 26.6 (23.3,31.5) |
|
| |||||
| Etiology a | |||||
|
| |||||
| Gallstone | 630 (27.6%) | 62 (30.0%) | 126 (44.2%) | 72 (47%) | 31 (18%) |
|
| |||||
| Alcohol related | 550 (24.1%) | 33 (16.0%) | 75 (26.3%) | 36 (24%) | 78 (44%) |
|
| |||||
| Other | 1,102 (48.3%) | 127 (54.0%) | 90 (29.5%) | 44 (29%) | 67 (38%) |
|
| |||||
| Lengh of stay | |||||
|
| |||||
| Median (IQR) | 3.5 (2.3, 5.3) | 3.5 (1.5, 7.0) | 4 (2.0, 7.0) | 5 (3, 7) | 5 (3, 7) |
|
| |||||
| Range | 1.0–137 | 0.5–33 | 1.0–60 | (0–133) | 0–37 |
|
| |||||
| Charlson score a | |||||
|
| |||||
| 0 | 973 (42.6%) | — | 142 (49.1%) | — | — |
|
| |||||
| 1–2 | 961 (42.1%) | — | 90 (31.1%) | — | — |
|
| |||||
| 3 or more | 348 (15.2%) | — | 57 (19.7%) | — | — |
CSMC, Cedars Sinai Medical Center; IQR, interquartile range; KPSC, Kaiser Permanente Southern California; NHB, non-Hispanic Black; NHW, non-Hispanic White; UCLA, University of California Los Angeles; UCSF, University of California San Francisco; USC, Los Angeles County+University of Southern California.
Data reported where available.
KPSC cohort study
We identified a total of 4,421 patients hospitalized for acute pancreatitis within KPSC during the study period. Following application of the study exclusion criteria there were 2,282 patients in the final study cohort. A total of 193 (6.1%) of patients developed organ failure. Among these patients, renal failure (61.1%) was the most common form of organ failure followed by respiratory failure (28.5%). Median length of stay was 3.5 days (interquartile range interquartile range 2.3, 5.3).
Cedars-Sinai Medical Center
Among 425 potentially eligible patients based on diagnosis codes, 222 met further criteria for a clinical diagnosis of acute pancreatitis. A total of 79 (36%) of patients developed organ failure during their hospitalization. Median length of stay was 3.5 days (interquartile range 1.5, 7.0).
Los Angeles County+University of Southern California
A total of 291 patients were included in the USC acute pancreatitis study cohort. A total of 9 (3.1%) of patients developed persistent organ failure with 50 (17.0%) of patients experiencing moderately severe pancreatitis characterized by either transient organ failure or local complications. Median length of stay in the USC study cohort was 4 days (2.0, 7.0).
University of California Los Angeles, Harbor Medical Center
A total of 152 patients were included in the Harbor UCLA acute pancreatitis study cohort. Among patients in the study cohort, 11 (7%) of patients developed persistent organ failure and 56 (37%) of patients experienced moderately severe pancreatitis characterized by either transient organ failure or local complications. Median length of stay in the Harbor UCLA study cohort was 5 days.
University of California San Francisco, Fresno medical center
A total of 176 patients were included in the UCSF Fresno acute pancreatitis study cohort. A total of 35 (20%) of patients developed persistent organ failure with 66 (38%) of patients experiencing moderately severe pancreatitis characterized by either transient organ failure or local complications. Median length of stay in the UCSF Fresno study cohort was 5 days.
Profiles of disease in acute pancreatitis
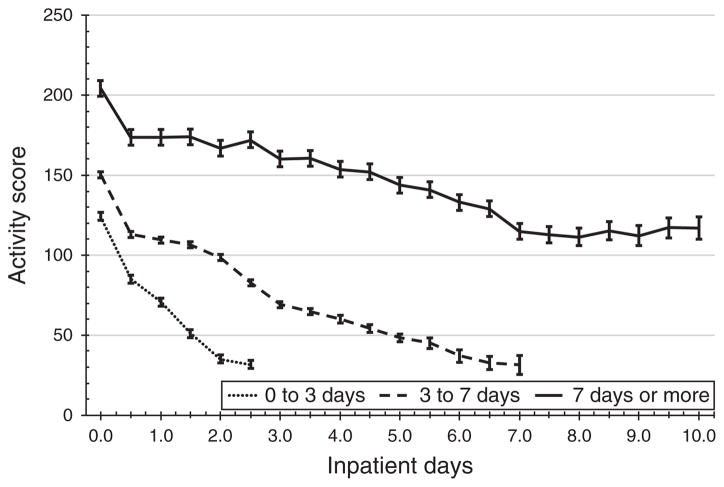
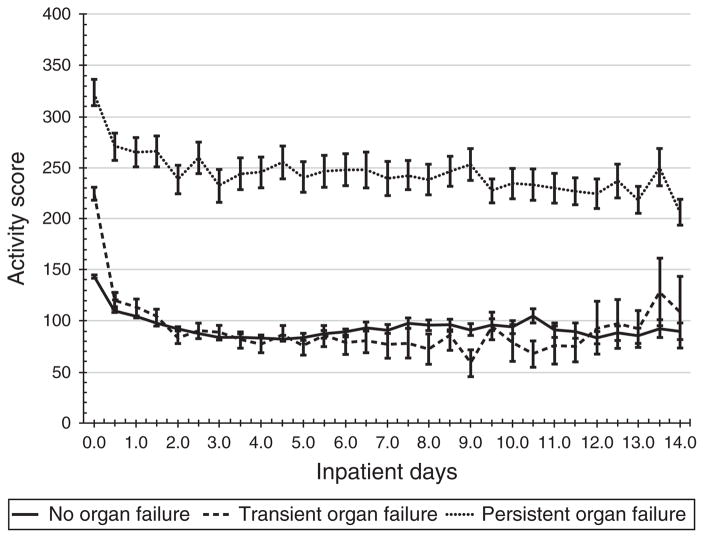
A summary of the individual components of the activity score at baseline and discharge across the study cohorts is presented in Table 2. There were several differences among the study cohorts. Specifically, a higher prevalence of SIRS and organ failure was noted in the Cedars-Sinai, Harbor UCLA and UCSF Fresno study cohorts at baseline. Table 3 depicts the distribution of activity scores at baseline and discharge. As expected, there was a significant reduction in overall score at discharge across all the study cohorts. A plot of the trajectory of acute PASS scores stratified by length of stay (0–3 days, 4–7 days or >7 days) as well as disease severity (mild, moderate (transient organ failure) or severe acute pancreatitis) is presented in Figures 3 and 4, respectively. From these plots there were distinct profiles of disease activity: patients with self-limited disease (hospitalization <3 days) presented with lower activity scores and experienced a more rapid decline in score. By contrast, patients with prolonged illness presented with higher scores at baseline and exhibited persistent elevation in scores during the initial stage of hospitalization (Figure 4). Likewise, patients that went on to experience multiple or persistent organ failure had on average an increased baseline PASS score that remained elevated during the initial phase of hospitalization whereas those with milder forms of illness had lower scores at baseline with a more rapid decline. For patients that remained hospitalized beyond 7 days disease activity profiles were similar for patients with transient or no organ failure (Figure 4).
Table 2.
Individual Parameters of the acute Pancreatitis Activity Scoring System (PASS) at Baseline and Discharge across study sites
| Parameter | KPSC (N =2,282) | CSMC | USC | Harbor UCLA | UCSF Fresno | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Baseline | Discharge | Baseline | Discharge | Baseline | Discharge | Baseline | Discharge | Baseline | Discharge | |
| Organ Failure | 161 (7.1%) | 47 (2.1%) | 34 (15.3%) | 19 (8.6%) | 18 (6.2%) | 4 (1.4%) | 51 (34%) | 16 (11%) | 63 (36%) | 39 (22%) |
|
| ||||||||||
| SIRS | 750 (32.9%) | 6 (0.3%) | 104 (46.9%) | 32 (14.4%) | 94 (32.3%) | 21 (7.2%) | 107 (70%) | 79 (52%) | 118 (67%) | 44 (25%) |
|
| ||||||||||
| Pain Median (IQR) | 8 (5, 10) | 0 (0, 5) | 9 (7, 10) | 3 (0, 7) | 8 (6, 10) | 0 (0, 7.5) | 9 (7, 10) | 0 (0, 6) | 6 (3, 9) | 0 (0, 0) |
|
| ||||||||||
| iv MED Median (IQR) | 8 (4, 16) | 4 (2, 8) | 10 (4, 25) | 0 (0, 6.5) | 4 (0, 10) | 0 (0, 3.3) | 1.5 (0, 3) | 1 (0, 1) | 0 (0, 0.6) | 0 (0, 0) |
|
| ||||||||||
| NPO/liquid only diet | 2,143 (96.7%) | 764 (33.5%) | 212 (95.5%) | 109 (49%) | 197 (88.7%) | 40 (4.9%) | 145 (96%) | 27 (18%) | 165 (94%) | 18 (10%) |
CSMC, Cedars Sinai Medical Center; IQR, interquartile range; Iv MED, intravenous morphine equivalent dose; KPSC, Kaiser Permanente Southern California; UCLA, University of California Los Angeles; UCSF, University of California San Francisco; USC, Los Angeles County+University of Southern California.
Data presented as n (%), unless otherwise indicated.
Table 3.
Activity Scores at Baseline and Discharge
| KPSC (N =2,282) | CSMC (N =222) | USC (N =291) | Harbor UCLA (N =152) | UCSF Fresno (N =176) | |
|---|---|---|---|---|---|
| Baseline Median (IQR) | 148 (100, 182) | 188 (133, 269) | 130 (92, 169) | 145 (100, 110) | 121 (90, 180) |
| Discharge Median (IQR) | 28 (0, 42) | 75 (40, 118) | 32 (0, 65) | 40 (18, 76) | 2 (0, 50) |
CSMC, Cedars Sinai Medical Center; IQR, interquartile range; KPSC, Kaiser Permanente Southern California; UCLA, University of California Los Angeles; UCSF, University of California San Francisco; USC, Los Angeles County+University of Southern California.
Figure 3.
Trajectory of clinical activity scores stratified by length-of-stay: short stay (<3 days), intermediate (3–7 days) and longer duration (>7 days hospitalization) from the Kaiser Permanente Acute Pancreatitis study cohort.
Figure 4.
Trajectory of clinical activity scores stratified by organ failure status (none, transient, multiple or persistent by revised Atlanta Classification) from the Kaiser Permanente Southern California acute pancreatitis study cohort.
DISCUSSION
We report the development and initial validation of a dynamic disease-specific clinical assessment instrument for use in acute pancreatitis. This tool was developed as a collaborative initiative representing the collective effort of an international working group as well as multiple clinical centers across Southern California. The instrument was predicated on the need for an objective means to monitor a patient’s condition in acute pancreatitis and therefore incorporates exclusively parameters used in the context of routine patient care. The five components identified through the consensus-based Delphi process (organ failure, SIRS, abdominal pain, opiate requirement, and tolerance of oral intake) are routine parameters used in the clinical assessment of patients with acute pancreatitis. In the validation phase of the study we demonstrate how the newly developed scoring system can be used to track patients across the full spectrum of illness ranging from mild, self-limited disease to protracted and/or severe illness. We have also demonstrated the feasibility of calculating the activity score using a variety of methods including retrospective manual chart abstraction (CSMC), prospective data collection (USC, Harbor UCLA, and UCSF Fresno) as well as automated electronic data capture (KPSC).
While numerous prognostic scoring systems (9) have been developed since the original Ranson score, this report presents the first attempt to develop a tool for objective measurement of real time disease activity in acute pancreatitis that can be used during the entire episode of inpatient care. It is important to distinguish the separate objectives of a prognostic scoring system compared to assessment of disease activity. While the primary objective of a prognostic scoring system is to risk-stratify patients for a particular outcome, e.g., severity or mortality, the purpose of measuring disease activity is to assess the status of a patient’s condition at any given moment in time as well as evaluate response to current treatment. This type of dynamic assessment is intended to enable providers to objectively monitor a patient’s disease course to help guide decisions on the need for further investigation or treatment as well as suitability for potential discharge.
The present activity scoring system should also be distinguished from the classification systems that exist for defining disease severity in acute pancreatitis (4,5). This distinction is reflected in our definition of disease activity as “reversible manifestations of disease”. Severity as defined in acute pancreatitis is a fixed state or outcome such that once a patient satisfies the criteria for severe acute pancreatitis they are considered to have had “severe acute pancreatitis”. In contrast, disease activity fluctuates during the course of disease. This was demonstrated in our mapping of disease activity profiles. Patients with self-limited disease (<3 days hospital stay) tended to experience a rapid decline in activity scores. Meanwhile patients with prolonged illness (hospital length of stay >7 days) demonstrated on average a high level of disease activity throughout early course of illness.
Studying the various parameters of the activity scoring system across the study cohorts revealed several important insights regarding practice patterns in acute pancreatitis. First, differences in the study populations were readily apparent based on the increased prevalence of SIRS as well as organ failure at baseline in the CSMC, Harbor UCLA and UCSF Fresno study cohorts. This likely reflects the population cared for by these tertiary referral centers. In addition, although pain scores were similar at baseline, it is interesting to note variation in morphine requirements at baseline as well as discharge across the study cohorts. Variation in these parameters highlights differences in practice patterns regarding pain management (standard clinical practice at Harbor UCLA and UCSF Fresno is to avoid use of narcotic analgesics whenever possible) as well as differences in discharge criteria from an inpatient setting.
The development of a widely accepted disease activity scoring system has several potential clinical and research applications in acute pancreatitis. We present a few examples here for consideration. One clinical example would be to associate the score at the time of discharge with 30-day re-admission rates for pancreatitis. We hypothesize that a study to address this issue would identify a threshold score above which the re-admission rate would be unacceptably high and inconsistent with good clinical practice. On the other hand, rates of re-admission for patients with high scores could be decreased by professional home care or rehabilitation level care. As another example, acute PASS scores could be used for defining level of care in the early stages of a pancreatitis episode including short term observation followed by home care at the lower activity levels and increased levels of care including monitoring in an intensive care unit for high activity levels.
The PASS system could also be used in research studies to assess the effect of an intervention on the natural history of pancreatitis. Specifically, an intervention might accelerate a decrease in the activity score or reduce the time-to-resolution of disease. As such, further investigation is needed to determine a threshold PASS score at which point an episode of acute pancreatitis is considered resolved. In addition, further studying the relative contribution of the various components of the scoring system at different phases of illness can help reveal insights into the natural history of disease as well as highlight further differences in clinical care.
A limitation of the PASS system is that measures are all clinically derived from patient and clinician observations. It does not include liquid biopsy measures of inflammatory or necrotic pancreatic tissue, which are known patho-biologic processes of the disease. Future studies can be performed to determine if such liquid biopsy measures or additional biomarkers provide advantages compared to the clinical PASS. It is also possible that certain liquid biopsy measures combined with the PASS system would provide enhanced information than either alone. The inclusion of opiate analgesic requirement was felt to be important to reflect a patient’s ongoing need for pain control. Inclusion of this parameter may limit the generalizability of the scale for patients with chronic use of opiates. In addition, there may be an element of bias introduced based on institutional treatment protocols. Ultimately, we believe that variation in opiate use observed in the present study illustrates the importance of inclusion of this parameter in the scoring system to provide an accurate overall assessment of a patient’s pain level at any given point during hospitalization.
In conclusion, the present study provides a clinical activity score for real-time measurement of disease activity in patients with acute pancreatitis. The Pancreatitis Activity Scoring System (PASS) has been developed by international expert opinion leaders in the field and validated across five separate health care organizations in Southern California.
Supplementary Material
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Acute pancreatitis has a highly variable clinical course.
Assessment of disease status is currently based on individual clinician judgement
An objective approach to measure disease activity is needed to help develop new therapy and standardize treatment in acute pancreatitis
WHAT IS NEW HERE
The pancreatitis activity scoring system (PASS) is an objective method to monitor disease activity developed by an international panel of experts
Distinct profiles of disease activity can be identified based on the PASS system
Individual components of the PASS system can be used to characterize differences in baseline clinical characteristics as well as variation in approaches to care for patients with acute pancreatitis across health systems.
Acknowledgments
Financial support: This study was funded in part through a research grant from Shire pharmaceuticals (Dublin, Ireland). The sponsor provided a research grant to support bio-statistical effort for analyses conducted at the Kaiser Permanente Southern California site. The sponsor did not participate in the study design, collection, analysis, or interpretation of the data or in the writing of the report.
We thank the following panel of international experts for their participation in the development of the Pancreatitis Activity Scoring System: Darwin Conwell, MD, MSc (USA). Timothy Gardner, MD (USA). Ishiguro Hiroshi, MD (Japan). Karl Kwok, MD (USA). Wen Li, MD (United Kingdom). Simon Lo, MD (USA). Julia Mayerle, MD (Germany). Nicholas Nissen, MD (USA). Georgious Papachristou, MD (USA). Walter Park, MD (USA). Tooru Shimosegawa MD (Japan). Vikesh Singh, MD (USA). Robert Sutton, MD (United Kingdom). Mayumi Toshihiko, MD (Japan). Saanthi Vege, MD (USA). Wahid Wassaf, MD, MPH (USA). David Whitcomb, MD, PhD (USA). Jeremy Wilson, MD, PhD (Australia). John Windsor MD (New Zealand). Dhiraj Yadav, MD, MPH (USA).
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
CONFLICT OF INTEREST
Guarantor of the article: Bechien Wu, MD, MPH.
Specific author contributions: Wu: study concept, study design, data interpretation, drafting of manuscript, critical revision of manuscript. Batech: study design, data analysis, data interpretation, critical revision of manuscript. Quezada: data acquisition, study design, data analysis, critical revision of manuscript. Lew: data acquisition, study design, data analysis, critical revision of manuscript. Fujikawa: data acquisition, data analysis, critical revision of the manuscript. Kung: data acquisition, data analysis, critical revision of the manuscript. Jamil, Afghani, and Reicher: study concept, study design, data interpretation, critical revision of manuscript. Chen: study design, data analysis, data interpretation, critical revision of manuscript. Afghani: study concept, study design, data interpretation, critical revision of manuscript. Reicher: study concept, study design, data interpretation, critical revision of manuscript. Buxbaum: study concept, study design, data acquisition, data interpretation, critical revision of manuscript. Pandol: study concept, study design, data interpretation, critical revision of manuscript. All authors reviewed and approved the final draft of the manuscript submitted.
Potential competing interests: None.
References
- 1.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179– 87. e1–3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252– 61. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasca di Magliano M, Forsmark C, Freedman S, et al. Advances in acute and chronic pancreatitis: from development to inflammation and repair. Gastroenterology. 2013;144:e1– e4. doi: 10.1053/j.gastro.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2012;62:102– 11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger EP, Forsmark CE, Layer P, et al. Determinant-Based Classification of Acute Pancreatitis Severity. Ann Surg. 2012;256:1. doi: 10.1097/SLA.0b013e318256f778. [DOI] [PubMed] [Google Scholar]
- 6.Anderson J, Caplan L, Yazdany J, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken) 2012;64:640– 7. doi: 10.1002/acr.21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitch, Kathryn, Bernstein SJ, et al. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: RA; 2001. [accessed May 1, 2014]. Available at: http://www.rand.org/pubs/monograph_reports/MR1269.html. [Google Scholar]
- 8.Afghani E, Pandol SJ, Shimosegawa T, et al. Acute pancreatitis-progress and challenges: a report on an international symposium. Pancreas. 2015;44:1195– 210. doi: 10.1097/MPA.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mounzer R, Langmead CJ, Wu BU, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142:1476– 82. doi: 10.1053/j.gastro.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Wu BU, Pandol SJ, Amy Liu I-L. Simvastatin is associated with reduced risk of acute pancreatitis: findings from a regional integrated healthcare system. Gut. 2014;64:1– 6. doi: 10.1136/gutjnl-2013-306564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.