Abstract
Objective
Unlike complete (R0) resection guidelines, current National Comprehensive Cancer Network (NCCN) adjuvant therapy guidelines after incomplete (R1/R2) resection of non-small cell lung cancer (NSCLC) are based on low-level evidence. We attempted to validate them.
Methods
Patients with pathologic stage I-IIIA NSCLC from 2004–2011 in the National Cancer Data Base were stratified by margin status, NCCN-specified stage groupings and adjuvant therapy exposure (none, radiotherapy, chemotherapy or both). Five-year overall survival (OS) and hazard ratios, adjusted for patient and institutional characteristics, were compared. We used a parallel analysis of R0 resections to validate our methodology.
Results
We analyzed 3461 R1/R2, and 78,929 R0 resections. After R0 resection, the NCCN-recommended option was associated with the best survival across all stage groups, supporting our analytic approach. R1/R2 stage IA patients treated with radiation had a 26% OS, compared to 58% with no treatment (p=0.003). In stage IB/IIA (N0) R1/R2 patients, radiation was associated with a 25% OS compared to 47% with no treatment (p=0.025) and 62% with chemotherapy (p<0.007). Chemoradiation was not associated with a survival benefit in either group. Patients with IIA (N1)/IIB and IIIA had better survival with chemotherapy or chemoradiation. No group had a survival benefit with radiation alone.
Conclusions
NCCN adjuvant therapy guidelines after complete resection, based on high-level evidence, are validated, but not guidelines for patients with incompletely resected early stage NSCLC, which are based on low-level evidence. Monomodality postoperative radiotherapy was not validated for any stage. Specific studies are needed to determine optimal management after incomplete resection.
Introduction
Lung cancer accounts for approximately 27% of all annual US cancer deaths.1 Most long-term survivors are among the 29% of patients who have undergone curative-intent surgical resection.2,3 In high-risk patients, adjuvant chemotherapy 4–6 and/or postoperative radiotherapy (PORT) may improve survival.7 The quality of evidence for the benefit of these treatments varies by stage and margin status.7–10
Randomized clinical trials (RCTs) and a pooled analysis have demonstrated the benefit of adjuvant chemotherapy in completely (R0) resected patients with T-category 2b or more advanced primary tumors, and those with nodal metastasis.4–6,11 A large meta-analysis showed the harmfulness of PORT in R0-resected patients without mediastinal nodal metastasis12,13; a retrospective analysis of the US Surveillance, Epidemiology and End Results database and an unplanned retrospective analysis of a clinical trial suggest R0 patients with mediastinal nodal metastasis may benefit from PORT.7,10
Unlike the situation after complete resection, there is no RCT evidence to guide adjuvant management for the 2–17% of non-small-cell lung cancer (NSCLC) resections with microscopic (R1) or macroscopic (R2) positive margins.14–16 However, recipients of incomplete resection are at significantly high risk for early death, irrespective of stage.16–18 Current National Comprehensive Cancer Network (NCCN) guideline recommendations for post-operative management of these patients are based on unverified expert opinion.19 Therefore, the guidelines need validation.
We evaluated the survival impact of four different adjuvant therapy options, after incomplete resection, in the National Cancer Database (NCDB) to determine which options seemed best for patients grouped into stage clusters as in the NCCN guidelines.19
Methods
Cohort selection
We used the NCDB, an oncology database sourced from Commission on Cancer-accredited facilities, which covers approximately 70% of newly diagnosed US cancer cases.20,21 We selected patients with surgically resected pathologic stage I-IIIA NSCLC from 2004–2011 (International Classification of Disease for Oncology, 9th edition [ICD-9-CM] site codes C34.0 – C34.9), excluding patients with missing information on last date of contact, administration (or date of administration) of radiation or chemotherapy, facility or patient location. We also excluded patients with more than one surgical procedure, neoadjuvant radiation or chemotherapy, no (or unknown) nodal examination, adjuvant therapy more than 180 days past date of diagnosis, government insurance and death within 60 days of surgery.
Objectives
The primary objective of this analysis was to compare stage-specific survival between post-operative therapy modalities in patients with incomplete surgical resection (R1/R2) who did not undergo re-resection. We used a parallel analysis of R0 patients to evaluate whether our data and methodology produced results congruent with existing high-level evidence for treatment of R0 patients.
Adjuvant therapy options
We classified post-operative therapy modalities as chemotherapy, radiotherapy, chemoradiation, or no treatment. Therapy administered within six months after surgery, at any dose level, was included as post-operative therapy. The median time from surgery to onset of treatment, by modality, is reported in Supplemental Table I. For combined modality chemoradiation therapy, the second modality had to begin within 2 months of the end of the first. The time from surgery to initiation of adjuvant therapy was evaluated to verify that adjuvant modalities were not typically used for the purpose of salvage therapy in this cohort.
NCCN stage groups and adjuvant therapy guidelines
NCCN recommendations for adjuvant therapy are based on pathologic stage, categorized into the following four groups: 1. Stage IA (T1ab, N0); 2. Stage IB (T2a, N0) and Stage IIA (T2b, N0); 3. Stage IIA (T1ab-T2a, N1) and Stage IIB (T3, N0; T2b N1); 4. Stage IIIA (T1-3, N2; T3, N1). The NCCN-recommended non-surgical adjuvant therapy for group 1 is PORT; for group 2, PORT with or without chemotherapy; for groups 3 and 4, chemoradiation (sequential or concurrent) for R1 and concurrent chemoradiation for R2.19
Variables
Margin status was evaluated as negative (R0) or positive (R1, R2 or positive not otherwise specified), and in subsequent analyses R1 and R2 were evaluated individually. Covariates (detailed in Table 1 and Supplemental Table I) in the analysis included patient demographic (age, sex, race, insurance status, income, rural/urban residence, census region), and clinical characteristics (number of comorbidities [0,1, or ≥2], histology, tumor grade, tumor size, primary site, type of surgery), as well as institutional characteristics (facility type).
Table 1A.
Patient Demographic and Institutional Characteristics Among Margin Positive Patients by Stage Group.
| Categories | Total | Stage IA(T1ab,N0) | Stage IB (T2a,N0) & IIA (T2b,N0) | Stage IIA(T1ab–T2a,N1) & IIB | Stage IIIA(T1–T3,N2; T3,N1) | p-value |
|---|---|---|---|---|---|---|
| N=3461 | N=369 | N=857 | N=1317 | N=918 | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | ||
| Age Group | ||||||
| 18–49 | 227 (7) | 16 (4) | 40 (5) | 90 (7) | 81 (9) | < .0001 |
| 50–64 | 1190 (34) | 105 (28) | 247 (29) | 476 (36) | 362 (39) | |
| 65–74 | 1230 (36) | 141 (38) | 328 (38) | 458 (35) | 303 (33) | |
| 75–90 | 814 (24) | 107 (29) | 242 (28) | 293 (22) | 172 (19) | |
| Sex | ||||||
| Male | 1851 (53) | 159 (43) | 436 (51) | 766 (58) | 490 (53) | < .0001 |
| Female | 1610 (47) | 210 (57) | 421 (49) | 551 (42) | 428 (47) | |
| Race/Ethnicity | ||||||
| Non-Hispanic, White | 2653 (77) | 287 (78) | 670 (78) | 1000 (76) | 696 (76) | 0.2 |
| Hispanic | 73 (2) | 10 (3) | 14 (2) | 27 (2) | 22 (2) | |
| Black | 342 (10) | 39 (11) | 79 (9) | 123 (9) | 101 (11) | |
| Other | 88 (3) | 6 (2) | 16 (2) | 48 (4) | 18 (2) | |
| Missing | 305 (9) | 27 (7) | 78 (9) | 119 (9) | 81 (9) | |
| Insurance | ||||||
| Uninsured | 87 (3) | 6 (2) | 19 (2) | 40 (3) | 22 (2) | < .0001 |
| Medicaid | 176 (5) | 15 (4) | 36 (4) | 63 (5) | 62 (7) | |
| Younger Medicare | 219 (6) | 22 (6) | 42 (5) | 74 (6) | 81 (9) | |
| Older Medicare | 1758 (51) | 221 (60) | 497 (58) | 635 (48) | 405 (44) | |
| Private | 1184 (34) | 103 (28) | 257 (30) | 485 (37) | 339 (37) | |
| Missing | 37 (1) | 2 (1) | 6 (0) | 20 (2) | 9 (1) | |
| Median Income-Quartile | ||||||
| <$30,000 | 478 (14) | 52 (14) | 117 (14) | 180 (14) | 129 (14) | 0.84 |
| $30,000– $34,999 | 708 (20) | 67 (18) | 175 (20) | 276 (21) | 190 (21) | |
| $35,000– $45,999 | 999 (29) | 100 (27) | 242 (28) | 382 (29) | 275 (30) | |
| $46,000+ | 1095 (32) | 126 (34) | 275 (32) | 407 (31) | 287 (31) | |
| Missing | 181 (5) | 24 (7) | 48 (6) | 72 (5) | 37 (4) | |
| Comorbidity | ||||||
| 0 | 1611 (47) | 144 (39) | 387 (45) | 623 (47) | 457 (50) | 0.014 |
| 1 | 1270 (37) | 149 (40) | 321 (37) | 491 (37) | 309 (34) | |
| 2+ | 580 (17) | 76 (21) | 149 (17) | 203 (15) | 152 (17) | |
| Histology | ||||||
| NOS | 9 (0) | 1 (0) | 1 (0) | 7 (1) | 0 (0) | < .0001 |
| Large Cell | 177 (5) | 14 (4) | 34 (4) | 70 (5) | 59 (6) | |
| Squamous | 1340 (39) | 121 (33) | 335 (39) | 572 (43) | 312 (34) | |
| Other | 248 (7) | 16 (4) | 49 (6) | 110 (8) | 73 (8) | |
| Adenocarcinoma | 1687 (49) | 217 (59) | 438 (51) | 558 (42) | 474 (52) | |
| Tumor Grade | ||||||
| well/moderately differentiated | 1688 (49) | 244 (66) | 477 (56) | 579 (44) | 388 (42) | < .0001 |
| poorly/undifferentiated | 1641 (47) | 105 (28) | 343 (40) | 695 (53) | 498 (54) | |
| Unknown | 132 (4) | 20 (5) | 37 (4) | 43 (3) | 32 (3) | |
| Tumor Size | ||||||
| ≤3cm | 1339 (39) | 356 (96) | 279 (33) | 418 (32) | 286 (31) | < .0001 |
| >3cm-≤5cm | 1156 (33) | 3 (1) | 367 (43) | 457 (35) | 329 (36) | |
| >5cm | 940 (27) | 8 (2) | 207 (24) | 428 (33) | 297 (32) | |
| Unknown | 26 (1) | 2 (1) | 4 (0) | 14 (1) | 6 (1) | |
| Rural/Urban | ||||||
| Rural | 664 (19) | 65 (18) | 165 (19) | 263 (20) | 171 (19) | 0.46 |
| Urban | 2582 (75) | 279 (76) | 631 (74) | 970 (74) | 702 (76) | |
| Unknown | 215 (6) | 25 (7) | 61 (7) | 84 (6) | 45 (5) | |
| Census Region | ||||||
| Northeast | 572 (17) | 72 (20) | 136 (16) | 207 (16) | 157 (17) | 0.58 |
| Midwest | 1100 (32) | 108 (29) | 283 (33) | 409 (31) | 300 (33) | |
| South | 1366 (39) | 150 (41) | 327 (38) | 532 (40) | 357 (39) | |
| West | 423 (12) | 39 (11) | 111 (13) | 169 (13) | 104 (11) | |
| Primary Site | ||||||
| C340- Main bronchus | 54 (2) | 1 (0) | 13 (2) | 15 (1) | 25 (3) | < .0001 |
| C341-upper lobe | 2068 (60) | 224 (61) | 474 (55) | 854 (65) | 516 (56) | |
| C342-Middle lobe | 174 (5) | 22 (6) | 54 (6) | 46 (3) | 52 (6) | |
| C343-Lower lobe | 976 (28) | 113 (31) | 278 (32) | 335 (25) | 250 (27) | |
| C348-Overlapping lesion | 124 (4) | 3 (1) | 28 (3) | 40 (3) | 53 (6) | |
| C349-Lung NOS | 65 (2) | 6 (2) | 10 (1) | 27 (2) | 22 (2) | |
| T category | ||||||
| T1 | 676 (20) | 369 (100) | 0 (0) | 184 (14) | 123 (13) | < .0001 |
| T2 | 1773 (51) | 0 (0) | 857 (100) | 536 (41) | 380 (41) | |
| T3 | 1012 (29) | 0 (0) | 0 (0) | 597 (45) | 415 (45) | |
| N Category | ||||||
| N0 | 1823 (53) | 369 (100) | 857 (100) | 597 (45) | 0 (0) | < .0001 |
| N1 | 964 (28) | 0 (0) | 0 (0) | 720 (55) | 244 (27) | |
| N2 | 674 (19) | 0 (0) | 0 (0) | 0 (0) | 674 (73) | |
| Surgery | ||||||
| Sublobar | 420 (12) | 114 (31) | 95 (11) | 109 (8) | 102 (11) | < .0001 |
| Lobe/bilobectomy | 2643 (76) | 250 (68) | 703 (82) | 1060 (80) | 630 (69) | |
| Pneumonectomy | 398 (12) | 5 (1) | 59 (7) | 148 (11) | 186 (20) | |
| Facility type | ||||||
| Community Cancer Program | 329 (10) | 31 (8) | 75 (9) | 139 (11) | 84 (9) | 0.75 |
| Comprehensive Community Cancer Program | 1772 (51) | 191 (52) | 445 (52) | 679 (52) | 457 (50) | |
| Teaching/Research Cancer Program | 738 (21) | 79 (21) | 188 (22) | 274 (21) | 197 (22) | |
| NCI Program/Network | 299 (9) | 39 (11) | 72 (8) | 104 (8) | 84 (9) | |
| Other | 323 (9) | 29 (8) | 77 (9) | 121 (9) | 96 (10) | |
Statistical Analysis
Overall survival (OS) times were taken from the date of surgery until the date of death or last follow-up. Survival analyses were conducted to compare the four post-operative treatment modalities within each of the four stage groups. OS was estimated using the Kaplan-Meier method and post-operative treatment groups were compared using the log-rank test.
OS comparisons were also evaluated using univariate Cox proportional hazards models and multiple variable Cox proportional hazards models to adjust for covariates. Model-based hazard ratio estimates are reported with 95% confidence intervals. For each model we present unadjusted hazard ratios and hazard ratios adjusted for demographic, clinical, surgical, and institutional characteristics. The proportional hazards assumption was evaluated graphically, using log(−log) survival plots by treatment group. We used ‘no adjuvant treatment’ as the reference adjuvant therapy option, since there is no clinical trial evidence to support adjuvant therapy after incomplete NSCLC resection. P-values less than 0.05 were considered statistically significant with no adjustment for multiple comparison and all analyses were conducted in SAS Version 9.4 (Cary, NC). 22
Sensitivity Analyses
We conducted multiple sensitivity analyses to address specific details of the analysis. First, the specific type of positive resection (R1 or R2) was unknown for some margin-positive patients. We evaluated the sensitivity of our results to margin-positivity of unknown type by conducting multiple analyses in which we grouped them as R1, R2, and eliminated them.
Additional sensitivity analyses were conducted to evaluate if departures from proportional hazards or the large number of covariates adjusted for in each model could impact the observed results from primary analysis. In these analyses, propensity-score adjusted models were used to control for demographic, clinical, surgical, and institutional characteristics with a propensity score, which was entered into the model as a covariate.23
Finally, we evaluated the potential impact of departures from the proportional hazards assumption by re-evaluating the multiple variable Cox models after eliminating any exposure groups where the assumption was questionable.
Results
A total of 82,440 patients were eligible: 3461 (4%) with incomplete resection, the primary analysis group of interest (Figure 1), and 78,979 (96%) with R0 resection (Supplemental Figure I), used to validate our analytic approach. The demographic and clinical characteristics of these patients, stratified by NCCN stage group (Table 1A [non-R0] and Supplemental Table IIA [R0]) and adjuvant therapy exposure (Table 1B [non-R0] and Supplemental Table IIB [R0]), are presented.
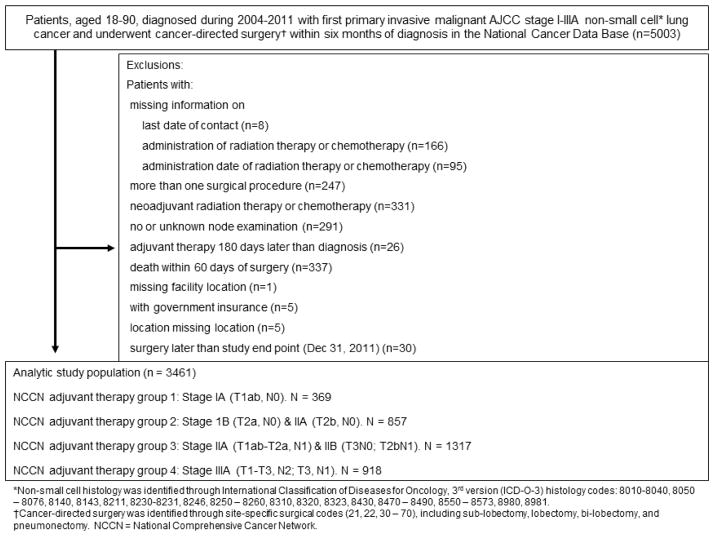
Figure 1.
Study consort diagram for margin positive patients
Patient selection schema among margin-positive NSCLC patients
*Non-small cell histology was identified through International Classification of Diseases for Oncology, 3rd version (ICD-O-3) histology codes: 8010-8040, 8050–8076,8140, 8143,8211, 8230-8231, 8246, 8250–8260, 8310, 8320, 8323, 8430, 8470–8490, 8550–8573, 8980, 8981.
†Cancer-directed surgery was identified through site-specific surgical codes (21, 22, 30–70), including sub-lobectomy, lobectomy, bi-lobectomy, and pneumonectomy. NCCN = National Comprehensive Cancer Network.
Table 1B.
Patient Demographic and Institutional Characteristics Among Margin Positive Patients by Adjuvant Therapy.
| Categories | Total | No Treatment | Chemotherapy | Radiation therapy | Chemoradiation | p-value |
|---|---|---|---|---|---|---|
| N=3461 | N=1406 | N=645 | N=447 | N=963 | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | ||
| Stage Group | ||||||
| Stage IA | 369 (11) | 265 (19) | 19 (3) | 60 (13) | 25 (3) | < .0001 |
| Stage IB & IIA | 857 (25) | 477 (34) | 142 (22) | 119 (27) | 119 (12) | |
| Stage IIA & IIB | 1317 (38) | 419 (30) | 284 (44) | 199 (45) | 415 (43) | |
| Stage IIIA | 918 (27) | 245 (17) | 200 (31) | 69 (15) | 404 (42) | |
| Age Group | ||||||
| 18–49 | 227 (7) | 66 (5) | 44 (7) | 14 (3) | 103 (11) | < .0001 |
| 50–64 | 1190 (34) | 390 (28) | 271 (42) | 106 (24) | 423 (44) | |
| 65–74 | 1230 (36) | 508 (36) | 227 (35) | 170 (38) | 325 (34) | |
| 75–90 | 814 (24) | 442 (31) | 103 (16) | 157 (35) | 112 (12) | |
| Sex | ||||||
| Male | 1851 (53) | 735 (52) | 337 (52) | 240 (54) | 539 (56) | 0.31 |
| Female | 1610 (47) | 671 (48) | 308 (48) | 207 (46) | 424 (44) | |
| Race/Ethnicity | ||||||
| Non-Hispanic, White | 2653 (77) | 1087 (77) | 475 (74) | 350 (78) | 741 (77) | 0.18 |
| Hispanic | 73 (2) | 35 (2) | 15 (2) | 8 (2) | 15 (2) | |
| Black | 342 (10) | 147 (10) | 67 (10) | 34 (8) | 94 (10) | |
| Other | 88 (3) | 29 (2) | 18 (3) | 17 (4) | 24 (2) | |
| Missing | 305 (9) | 108 (8) | 70 (11) | 38 (9) | 89 (9) | |
| Insurance | ||||||
| Uninsured | 87 (3) | 37 (3) | 14 (2) | 9 (2) | 27 (3) | < .0001 |
| Medicaid | 176 (5) | 69 (5) | 23 (4) | 23 (5) | 61 (6) | |
| Younger Medicare | 219 (6) | 77 (5) | 44 (7) | 16 (4) | 82 (9) | |
| Older Medicare | 1758 (51) | 823 (59) | 275 (43) | 296 (66) | 364 (38) | |
| Private | 1184 (34) | 388 (28) | 281 (44) | 96 (21) | 419 (44) | |
| Missing | 37 (1) | 12 (1) | 8 (1) | 7 (2) | 10 (1) | |
| Median Income-Quartile | ||||||
| <$30,000 | 478 (14) | 196 (14) | 79 (12) | 72 (16) | 131 (14) | 0.25 |
| $30,000– $34,999 | 708 (20) | 281 (20) | 139 (22) | 81 (18) | 207 (22) | |
| $35,000– $45,999 | 999 (29) | 405 (29) | 171 (27) | 132 (30) | 291 (30) | |
| $46,000+ | 1095 (32) | 448 (32) | 211 (33) | 139 (31) | 297 (31) | |
| Missing | 181 (5) | 76 (5) | 45 (7) | 23 (5) | 37 (4) | |
| Comorbidity | ||||||
| 0 | 1611 (47) | 634 (45) | 331 (51) | 183 (41) | 463 (48) | 0.005 |
| 1 | 1270 (37) | 511 (36) | 225 (35) | 179 (40) | 355 (37) | |
| 2+ | 580 (17) | 261 (19) | 89 (14) | 85 (19) | 145 (15) | |
| Histology | ||||||
| NOS | 9 (0) | 3 (0) | 2 (0) | 1 (0) | 3 (0) | |
| Large Cell | 177 (5) | 57 (4) | 38 (6) | 23 (5) | 59 (6) | < .0001 |
| Squamous | 1340 (39) | 524 (37) | 214 (33) | 216 (48) | 386 (40) | |
| Other | 248 (7) | 90 (6) | 46 (7) | 32 (7) | 80 (8) | |
| Adenocarcinoma | 1687 (49) | 732 (52) | 345 (53) | 175 (39) | 435 (45) | |
| Tumor Grade | ||||||
| well/moderately differentiated | 1688 (49) | 753 (54) | 307 (48) | 208 (47) | 420 (44) | |
| poorly/undifferentiated | 1641 (47) | 590 (42) | 312 (48) | 221 (49) | 518 (54) | < .0001 |
| Unknown | 132 (4) | 63 (4) | 26 (4) | 18 (4) | 25 (3) | |
| Tumor Size | ||||||
| ≤3cm | 1339 (39) | 654 (47) | 212 (33) | 172 (38) | 301 (31) | |
| >3cm-≤5cm | 1156 (33) | 417 (30) | 223 (35) | 161 (36) | 355 (37) | < .0001 |
| >5cm | 940 (27) | 323 (23) | 207 (32) | 112 (25) | 298 (31) | |
| Unknown | 26 (1) | 12 (1) | 3 (0) | 2 (0) | 9 (1) | |
| Rural/Urban | ||||||
| Rural | 664 (19) | 285 (20) | 110 (17) | 90 (20) | 179 (19) | 0.014 |
| Urban | 2582 (75) | 1023 (73) | 482 (75) | 337 (75) | 740 (77) | |
| Unknown | 215 (6) | 98 (7) | 53 (8) | 20 (4) | 44 (5) | |
| Census Region | ||||||
| Northeast | 572 (17) | 234 (17) | 106 (16) | 79 (18) | 153 (16) | < .0001 |
| Midwest | 1100 (32) | 386 (27) | 222 (34) | 144 (32) | 348 (36) | |
| South | 1366 (39) | 586 (42) | 246 (38) | 158 (35) | 376 (39) | |
| West | 423 (12) | 200 (14) | 71 (11) | 66 (15) | 86 (9) | |
| Primary Site | ||||||
| C340- Main bronchus | 54 (2) | 16 (1) | 8 (1) | 12 (3) | 18 (2) | 0.22 |
| C341-upper lobe | 2068 (60) | 825 (59) | 384 (60) | 280 (63) | 579 (60) | |
| C342-Middle lobe | 174 (5) | 76 (5) | 34 (5) | 14 (3) | 50 (5) | |
| C343-Lower lobe | 976 (28) | 412 (29) | 185 (29) | 122 (27) | 257 (27) | |
| C348-Overlapping lesion | 124 (4) | 45 (3) | 22 (3) | 16 (4) | 41 (4) | |
| C349-Lung NOS | 65 (2) | 32 (2) | 12 (2) | 3 (1) | 18 (2) | |
| T category | ||||||
| T1 | 676 (20) | 368 (26) | 82 (13) | 89 (20) | 137 (14) | < .0001 |
| T2 | 1773 (51) | 757 (54) | 388 (60) | 191 (43) | 437 (45) | |
| T3 | 1012 (29) | 281 (20) | 175 (27) | 167 (37) | 389 (40) | |
| N Category | ||||||
| N0 | 1823 (53) | 912 (65) | 247 (38) | 309 (69) | 355 (37) | < .0001 |
| N1 | 964 (28) | 320 (23) | 248 (38) | 94 (21) | 302 (31) | |
| N2 | 674 (19) | 174 (12) | 150 (23) | 44 (10) | 306 (32) | |
| Surgery | ||||||
| Sublobar | 420 (12) | 180 (13) | 56 (9) | 78 (18) | 106 (11) | < .0001 |
| Lobe/bilobectomy | 2643 (76) | 1077 (77) | 482 (75) | 335 (75) | 749 (78) | |
| Pneumonectomy | 398 (12) | 149 (11) | 107 (17) | 34 (8) | 108 (11) | |
| Facility type | ||||||
| Community Cancer Program | 329 (10) | 123 (9) | 55 (9) | 33 (7) | 118 (12) | < .0001 |
| Comprehensive Community Cancer Program | 1772 (51) | 687 (49) | 328 (51) | 249 (56) | 508 (53) | |
| Teaching/Research Cancer Program | 738 (21) | 343 (24) | 131 (20) | 97 (22) | 167 (17) | |
| NCI Program/Network | 299 (9) | 133 (9) | 66 (10) | 32 (7) | 68 (7) | |
| Other | 323 (9) | 120 (9) | 65 (10) | 36 (8) | 102 (11) | |
Early-stage patients with incomplete resection: NCCN groups 1 and 2
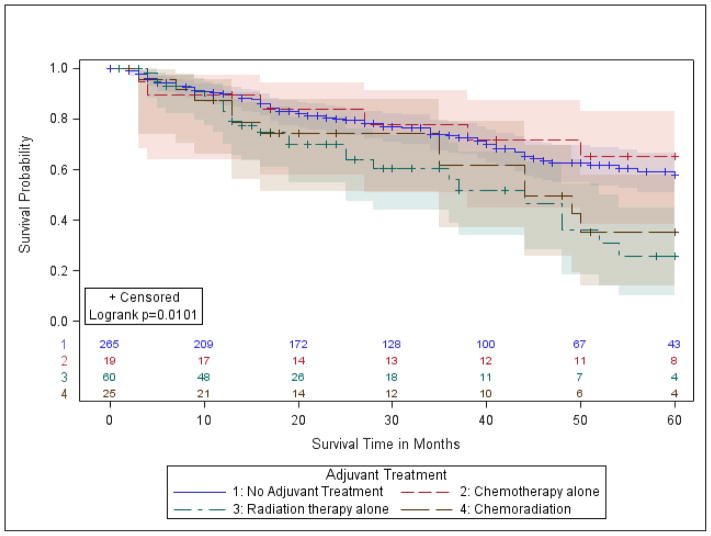
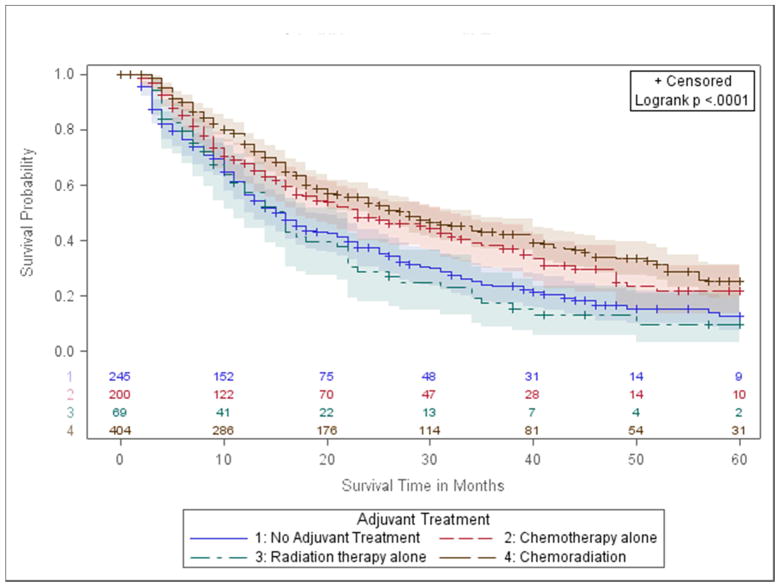
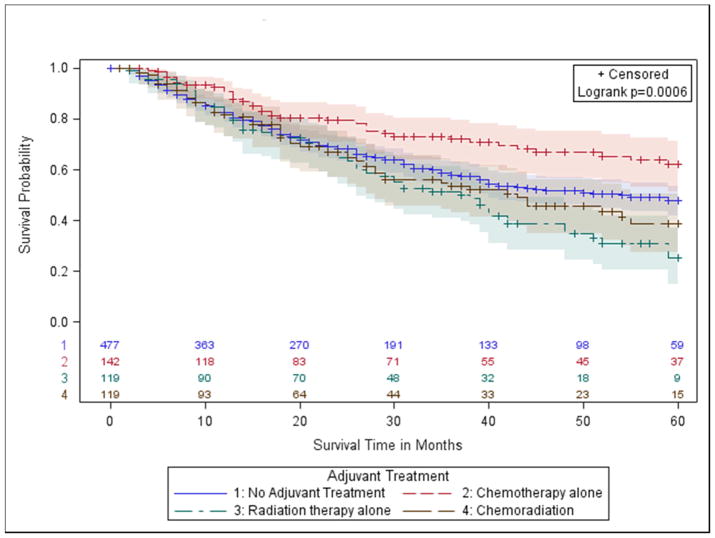
OS estimates were compared by treatment modality in margin-positive patients with stage IA (T1ab, N0) and stages IB/IIA (T2a, N0 and T2b, N0). Margin-positive stage IA patients who received PORT alone had significantly lower OS compared to those with no treatment (5-Year OS: 26% vs. 58%, p-value=0.0030, Table 2, Figure 2A). This result trended towards statistical significance in the fully adjusted model (aHR: 1.7, p-value=0.0551, Table II). Similarly, for stage IB/IIA patients, the 5-year OS was 47% with no treatment, and 25% with PORT (p-value=0.0251; aHR 1.28, p-value=0.12) (Table 2, Figure 2B).
Table 2.
Kaplan Meier Survival Analysis and Proportional Hazards Models by Stage Group for Margin Positive Patients.
| Post-Op Treatment | Margin Positive
|
|||||
|---|---|---|---|---|---|---|
| N | 5 Year Overall Survival (%)(Logrank P-Value*) | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio** (95% CI) | P-Value* | ||
| Group 1: Stage IA (T1ab, N0) | No Treatment | 265 | 58 (Referent) | 1.00 (Referent) | 1.00 (Referent) | |
| Chemo Only | 19 | 65 (0.6687) | 0.81 (0.35–1.86) | 1.27 (0.48–3.38) | 0.6369 | |
| Radiation Only | 60 | 26 (0.0030) | 1.97 (1.25–3.11) | 1.68 (0.99–2.84) | 0.0551 | |
| Chemo + Rad | 25 | 35 (0.0895) | 1.69 (0.92–3.12) | 0.96 (0.47–1.98) | 0.9176 | |
|
| ||||||
| Group 2: Stage IB (T2a, N0) & Stage IIA (T2b, N0) | No Treatment | 477 | 47 (Referent) | 1.00 (Referent) | 1.00 (Referent) | |
| Chemo Only | 142 | 62 (0.0065) | 0.61 (0.43–0.87) | 0.58 (0.40–0.84) | 0.004 | |
| Radiation Only | 119 | 25 (0.0251) | 1.39 (1.04–1.86) | 1.28 (0.94–1.74) | 0.1185 | |
| Chemo + Rad | 119 | 39 (0.3571) | 1.15 (0.85–1.57) | 0.97 (0.70–1.35) | 0.8678 | |
|
| ||||||
| Group 3: Stage IIA (T1ab-T2a, N1) & Stage IIB (T3, N0;T2b N1) | No Treatment | 419 | 27 (Referent) | 1.00 (Referent) | 1.00 (Referent) | |
| Chemo Only | 284 | 36 (0.0001) | 0.65 (0.53–0.81) | 0.72 (0.58–0.90) | 0.0041 | |
| Radiation Only | 199 | 26 (0.5907) | 1.06 (0.85–1.32) | 0.94 (0.74–1.18) | 0.5878 | |
| Chemo + Rad | 415 | 37 (<.0001) | 0.68 (0.56–0.83) | 0.76 (0.62–0.93) | 0.0083 | |
|
| ||||||
| Group 4: Stage IIIA (T1-3, N2; T3, N1) | No Treatment | 245 | 12 (Referent) | 1.00 (Referent) | 1.00 (Referent) | |
| Chemo Only | 200 | 21 (0.0048) | 0.70 (0.55–0.90) | 0.77 (0.60–1.00) | 0.0466 | |
| Radiation Only | 69 | 10 (0.5215) | 1.11 (0.81–1.51) | 1.03 (0.74–1.43) | 0.8729 | |
| Chemo + Rad | 404 | 25 (<.0001) | 0.59 (0.48–0.72) | 0.63 (0.51–0.79) | <.0001 | |
P-values compare each treatment to referent (no treatment)
Adjusted for Age, Sex, Race/Ethnicity, Insurance, Median Income, Comorbidity, Histology, Tumor Grade, Tumor Size, Rural/Urban, Census Region, Primary Site, T Category, N Category, Surgery Facility type, Facility Surgical % Lung Cancer, Facility % Medicaid or Uninsured
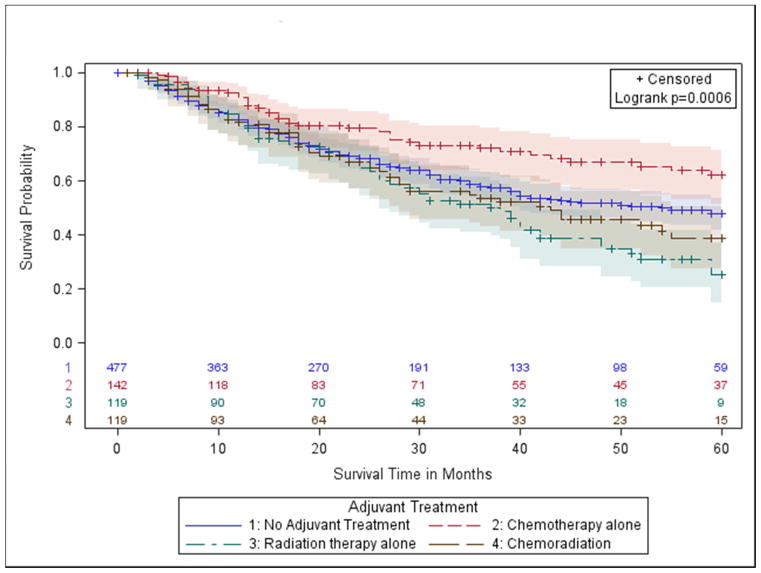
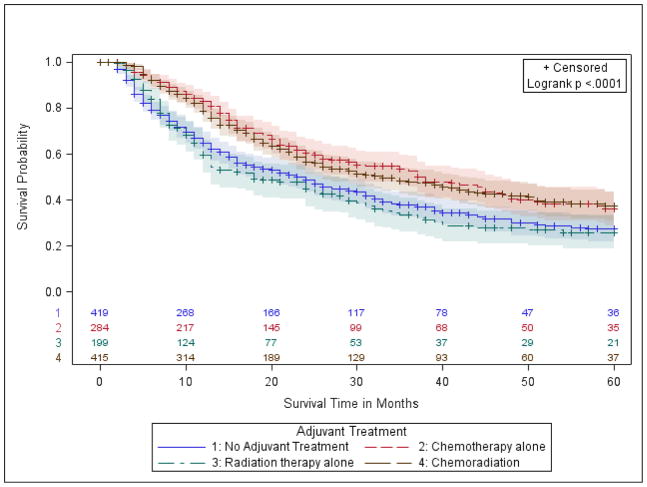
Figure 2.
Kaplan Meier survival curves for margin positive patients categorized by National Comprehensive Cancer Network adjuvant therapy stage groups. The log-rank p-value tests the null hypothesis that all 4 groups have similar survival.
a.) Group 1- stage IA (T1ab, N0);
b.) Group 2- stage IB (T2a, N0) and Stage IIA (T2b, N0);
c.) Group 3- stage IIA (T1ab-T2a, N1) and stage IIB (T3, N0; T2b N1);
d.) Group 4- stage IIIA (T1-3, N2; T3, N1).
We found no significant association between chemotherapy and survival in stage IA patients with positive margins. However, survival was significantly higher in persons with stages IB-IIA who received post-operative chemotherapy compared to no treatment (5-Year OS: 62% vs. 47%, p-value=0.0065, Table 2, Fig 2B). These results remained statistically significant in fully-adjusted models (aHR 0.58, p-value=0.0040, Table 2). Sensitivity analysis using propensity score-adjusted models (Supplemental Table III) and those that did not consider treatment groups where the proportional hazards assumption may be violated (Supplemental Table IV) provided consistent results. Survival with chemoradiation was not significantly different from no adjuvant treatment in group 1 or 2 patients (Table 2).
Late-stage patients with incomplete resection: NCCN groups 3 and 4
In margin-positive NCCN group 3, patients with Stage IIA (T1ab-T2a, N1) or Stage IIB (T3, N0;T2b N1), those who received radiation had a similar survival experience to those who received no treatment (5-Year OS: 26% vs. 27%, p-value= 0.59, Figure 2C, Table 2). Recipients of chemotherapy or chemoradiation had superior survival (p-values<0.0010, Table 2, Figure 2C). Results were similar in fully adjusted models, where the chemotherapy group had 0.72 times the hazard of death compared to no treatment (p-value=0.0041), and the chemoradiation group had 0.74 times the hazard of death (p-value=0.0083).
Subsequent analysis found no substantial differences in survival in the chemoradiation group based on the order in which therapies were administered (Supplemental Table V). When evaluated separately, patients receiving chemotherapy first and then radiation had 37% 5-Year OS compared to 36% for patients receiving radiation first and then chemotherapy and 38% for those receiving both concurrently (Supplemental Table V).
Consistent with NCCN guidelines, margin-positive patients with stage IIA or stage IIB were further delineated based on the specific type of incomplete resection, R1, R2, or unknown (margin-positive, but type not specified). Although potentially limited by smaller sample sizes, results were largely consistent with those observed for all margin-positive patients combined (Supplemental Table VI).
In margin-positive NCCN group 4, patients with Stage IIIA (T1-3, N2; T3, N1), 5-year OS was similar between patients who received PORT (10%) and no treatment (12%, p-value=0.52, Fig 2d). However, compared with no treatment, patients with chemotherapy alone had higher 5-year OS (21% vs. 12%, p-value=0.0045), as did those with chemoradiation (25%, p-value <0.0001). Fully adjusted models confirmed these findings (Table 2). Specifically, the patients had a lower hazard of death in both the chemotherapy group (aHR=0.77, p-value= 0.0466) and the chemoradiation group (aHR 0.63, p-value <0.0001), compared to no treatment.
Analysis of margin-positive patients with stage IIIA, after further stratification into R1 or R2 subsets, yielded similar results to the combined cohort (Supplemental Table VI). Similar to group 3 patients, we found no meaningful difference in survival in Stage IIIA patients based on the order that chemoradiation was received (Supplemental Table V).
Validation analysis with margin-negative resections
We applied the same analysis to the R0 resection cohort in a parallel analysis. Five-year OS, unadjusted proportional hazards models, and adjusted proportional hazards models in this cohort are presented in Table 3 and Supplemental Figure II. We further delineated the stage IIIA margin-negative survival analysis by pN-category (N0/N1 vs. N2) to match the NCCN guidelines subsets and evaluated their comparative OS based on adjuvant therapy exposure (Supplemental Table VII). The pattern of adjuvant therapy benefit in our analysis matched up with the evidence-based NCCN guidelines for R0 resection (Table 4).
Table 3.
Kaplan Meier Survival Analysis and Proportional Hazards Models by Stage Group for Margin Negative Patients.
| Post-Op Treatment | Margin Negative
|
|||||
|---|---|---|---|---|---|---|
| N | 5 Year Overall Survival (%)(log-rank P-Value*) | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio** (95% CI) | P-Value* | ||
| Group 1: Stage IA (T1ab, N0) | No Treatment | 33780 | 71 (Referent) | 1.00 (Referent) | 1.00 (Referent) | |
| Chemo Only | 789 | 74 (0.2946) | 0.92 (0.78–1.08) | 1.02 (0.87–1.20) | 0.816 | |
| Radiation Only | 136 | 44 (<.0001) | 2.89 (2.22–3.73) | 2.18 (1.67–2.85) | <.0001 | |
| Chemo + Rad | 76 | 40 (<.0001) | 3.19 (2.21–4.59) | 2.99 (2.07–4.31) | <.0001 | |
|
| ||||||
| Group 2: Stage IB (T2a, N0) & Stage IIA (T2b, N0) | No Treatment | 19281 | 57 (Referent) | 1.00 (Referent) | 1.00 (Referent) | |
| Chemo Only | 4568 | 68 (<.0001) | 0.68 (0.63–0.72) | 0.74 (0.69–0.80) | <.0001 | |
| Radiation Only | 250 | 38 (<.0001) | 1.92 (1.60–2.31) | 1.8 (1.49–2.16) | <.0001 | |
| Chemo + Rad | 215 | 47 (0.0003) | 1.47 (1.19–1.81) | 1.41 (1.14–1.74) | 0.0016 | |
|
| ||||||
| Group 3: Stage IIA (T1ab-T2a, N1) & Stage IIB (T3, N0;T2b N1) | No Treatment | 6101 | 37 (Referent) | 1.00 (Referent) | 1.00 (Referent) | |
| Chemo Only | 5788 | 53 (<.0001) | 0.59 (0.56–0.63) | 0.66 (0.62–0.70) | <.0001 | |
| Radiation Only | 354 | 28 (<.0001) | 1.35 (1.17–1.57) | 1.36 (1.18–1.58) | <.0001 | |
| Chemo + Rad | 895 | 40 (0.1772) | 0.93 (0.84–1.03) | 1.04 (0.93–1.16) | 0.4811 | |
|
| ||||||
| Group 4: Stage IIIA (T1-3, N2; T3, N1) | No Treatment | 2119 | 24 (Referent) | 1.00 (Referent) | 1.00 (Referent) | |
| Chemo Only | 2520 | 39 (<.0001) | 0.59 (0.55–0.65) | 0.63 (0.58–0.69) | <.0001 | |
| Radiation Only | 248 | 18 (0.0747) | 1.18 (0.99–1.39) | 1.15 (0.97–1.37) | 0.1025 | |
| Chemo + Rad | 1859 | 38 (<.0001) | 0.62 (0.57–0.68) | 0.69 (0.63–0.76) | <.0001 | |
P-values compare each treatment to referent (no treatment)
Adjusted for Age, Sex, Race/Ethnicity, Insurance, Median Income, Comorbidity, Histology, Tumor Grade, Tumor Size, Rural/Urban, Census Region, Primary Site, T Category, N Category, Surgery Facility type, Facility Surgical % Lung Cancer, Facility % Medicaid or Uninsured
Table 4.
Comparative survival impact of post-operative adjuvant therapy in patients with completely and incompletely resected stage I – IIIA NSCLC in the NCDB, current NCCN adjuvant therapy recommendations (‘recs’), and objective results from our analysis.
| Stage | Margin Status | Chemo | Radiation | chemoXRT | NCCN | NCDB Data |
|---|---|---|---|---|---|---|
| Group 1: Stage IA | Negative | Neutral | Worse | Worse | Observe | Supports observation |
| Positive | Neutral | Worse | Neutral | Radiation | Supports Observation | |
| Group 2: Stage IB & Stage IIA | Negative | Better | Worse | Worse | Observe or Chemo | Supports Chemo Only |
| Positive | Better | Neutral | Neutral | RT+/−Chemo | Supports Chemo Only | |
| Group 3: Stage IIA & Stage IIB | Negative | Better | Worse | Neutral | Chemo | Supports Chemo Only |
| Positive | Better | Neutral | Better | |||
| R1 | Better | Neutral | Better | Chemo+RT | Supports Chemo+/−RT | |
| R2 | Insufficient Data (Supplemental Table I) | Chemo+RT | ||||
| Group 4: Stage IIIA | Negative | Better | Neutral | Better | ||
| Non-N2 | Better | Neutral | Neutral | Chemo | Supports chemo only | |
| N2 | Better | Worse | Better | Chemo+RT | Supports Chemo+/−RT | |
| Group 4: Stage IIIA | Positive | Better | Neutral | Better | ||
| R1 | Neutral | Neutral | Better | Chemo+RT | Supports chemo+RT | |
| R2 | Insufficient Data (Supplemental Table I) | |||||
Chemo= chemotherapy; RT= radiation therapy; Chemo+RT= combined-modality chemotherapy and radiation therapy.
Comparison with NCCN Recommendations
Results from margin-positive and margin-negative analyses by stage groups are summarized qualitatively in Table 4, and are compared with the current NCCN recommendations.
Discussion
We compared OS between post-operative adjuvant therapy modalities in patients with completely and incompletely resected NSCLC, to determine if current NCCN recommendations are supported by a robust nationally-representative dataset. Our primary interest was in the patients with incomplete resection, but we used the R0 cohort to validate our methodology, and the suitability of the NCDB for this purpose. This analysis consistently corroborated NCCN guidelines backed by high-level clinical trial evidence, but did not support current recommendations in several scenarios after incomplete resection, where the available evidence is sparse.
In patients with completely resected stage IA NSCLC, RCT have shown no benefit from adjuvant therapy.4,6 In stage IB-IIB, RCTs and a pooled analysis including the five largest studies, have shown an increase in overall and relapse-free survival with postoperative Cisplatin-based chemotherapy compared to observation.4–6,11 Our analysis of the R0 cohort is consistent with this evidence. Specifically, patients with completely resected stage IB-IIA NSCLC who received chemotherapy had results superior to all other treatment groups. In patients with completely resected stage IIIA NSCLC, current evidence supports chemotherapy for those with N0 or N1, and chemotherapy or chemoradiation for those with N2, which is the current NCCN recommendation.19 The R0 cohort analysis supports the use of chemotherapy in N0 and N1 patients, and chemotherapy with or without radiation in patients with N2.
Incomplete resections occur relatively infrequently, and adjuvant therapy trials specifically exclude these patients.4–7,10,11,24 Therefore, there is no definitive evidence on the best choice of post-operative therapy in this situation.16–18 NCCN guidelines currently recommend PORT for group 1 (stage IA), PORT with or without chemotherapy for group 2 (stage IB and IIA), and chemoradiation for groups 3 (stage IIA with N1 and IIB) and 4 (T3N1 and T1-3, N2).19 Our analysis supports observation for group 1, chemotherapy only for group 2, chemotherapy with or without radiation for group 3, and chemoradiation therapy for group 4. This analysis supports the NCCN recommendations for groups 3 and 4, but suggests that the current recommendations may be harmful to patients in groups 1 and 2. It also does not support the use of PORT alone in any subset.
Recent publications using the NCDB have provided conflicting results on the value of PORT after incomplete resection. Hancock found that chemotherapy or chemotherapy plus PORT provided superior results for stages I-III.18 However patients who received PORT alone after incomplete resection had unimproved (stage II-III) or worse (stage I) survival. Wang reported slightly longer survival in patients completing a full regime of PORT at 50–74 Gy post-operatively.25 Key differences in our study may explain the conflicting results.
Our analysis of the NCDB used the NCCN adjuvant therapy stage groupings in an attempt to validate the treatment guidelines. Therefore, we further delineated stage I patients by pT-category and stage IIA patients by pN-category. This delineation, coupled with the broader timeline (2004–2011 vs. 2003–2006), may explain the subtle difference between our findings and those of Hancock.18 Both studies found that early-stage patients receiving PORT alone had shorter survival. However, we found the best survival for early-stage (NCCN group 2) patients was with chemotherapy alone compared with Hancock’s findings that chemotherapy with or without PORT both showed similar survival that was superior to no adjuvant treatment or PORT alone for the undilineated group of stage II and III patients.18
The report by Wang, supporting the use of PORT in Stage II-III patients with incomplete resection, differed from our work by evaluating only patients with an optimal PORT experience.25 Specifically, Wang excluded all patients who died within 120 days of surgery, and only included patients who completed optimal-dose radiation. A less optimal classification of PORT use is more pragmatic and provides better information for treatment of patients, whose ability to receive a full treatment regime of PORT cannot be known at the time of treatment decision. Patients who died as a result of acute radiation complications would have been excluded from their analysis. Another difference is that they treated chemotherapy as a confounding variable rather than a separate treatment option as we, and Hancock, have done.
Our PORT analysis group included all persons who survived 60 days post-surgery and received treatment with PORT within 6 months of surgery. Patients who discontinued PORT or received PORT at a less-than-optimal dose were included to adhere to the intention-to-treat principle and avoid potential selection bias. Because treatment with PORT alone may be carried out differently than PORT with chemotherapy, we considered these two treatment options separately to better represent clinical practice and to avoid the potential for residual confounding by controlling for chemotherapy use exclusively through statistical modeling.
This retrospective study has several limitations. We have expressly excluded the primary recommendation of re-resection for non-R0 resections because of the relatively small number of such patients in the database. Ideally, PORT is preferably commenced within 60 days. We used a 6-month eligibility window, as others have done in these types of analyses, to reflect the practical reality that some patients start adjuvant therapy late.18,25 The median time to onset of PORT alone was 52 days, and 75% of patients initiated therapy within 74 days. This suggests that PORT was used adjuvantly, and not for salvage therapy after disease progression. However, it is impossible to verify the clinical circumstances around any of the treatments.
The NCDB covers 70% of all lung cancer cases in the US, drawing from a diverse group of hospitals. However, results may not apply directly to substantially different institutions. Although the NCDB is thorough, incomplete and inaccurate data are still potential problems. Although we addressed this limitation for critical variables by validating our results with sensitivity analyses, unequal assignment of post-operative treatment modalities may have impacted our results and the sample size of some analysis subsets may be too small for meaningful statistical inference. Outside a well-executed RCT, this remains a potential explanation for differences observed in all studies of this question. We have addressed this limitation, as well as possible, with extensive adjustment by statistical analysis.
The lack of observed benefit from PORT or chemoradiation in early-stage patients after incomplete resection parallels the current evidence in completely resected patients; the impact of radiation therapy in reducing the increased cancer-related mortality risk after incomplete resection does not seem to overcome the excessive treatment-related mortality risk of PORT.26 Chemotherapy appears to be valuable to some degree across stage groups; patients with mediastinal nodal metastasis seem to benefit from chemotherapy or combined-modality chemoradiation.
Well-conducted retrospective evaluations can lead to conflicting conclusions based on selection criteria for assigning treatment groups after the fact. An inherent imbalance between treatment groups prior to treatment initiation is likely when treatment is selected based on physician decision after individual patient assessment. Statistical adjustment is unlikely to completely eliminate such confounding-by-indication.
This study provides the most comprehensive evaluation of NCCN guidelines for postoperative therapy to date. Results are largely consistent with high-level evidence available after complete surgical resection. In patients with incomplete resection, where the available evidence is far less, these data did not support the use of PORT in early-stage patients. All available evidence in incompletely-resected patients is lower-level, and results are discrepant. Only RCTs can definitively determine the best adjuvant therapy for incompletely resected NSCLC.
Such a trial will be challenging to execute because of the relatively low incidence of incomplete resections, and the practical reality that incomplete resections are least frequent in the types of institutions that typically conduct clinical trials.16 However, infrastructure such as the National Cancer Institute’s Community Oncology Research Program can be harnessed to support such a trial. The possibility of patient harm in the existing evidence void should stimulate the political will to resolve this question.
Supplementary Material
Central Figure.
Kaplan Meier Survival Curves for Margin Positive Patients in Stage IB (T2a, N0) and Stage IIA (T2b, N0).
Perspective Statement.
NCCN guidelines for post-operative chemotherapy and radiation after complete surgical resection for NSCLC, based on high-level evidence, are validated in this analysis. Current guidelines for post-operative therapy after incomplete resection of stage I-II NSCLC, which are based on lower-level evidence, are not supported by this analysis.
Central Message.
NCCN guidelines for post-operative therapy after incomplete surgical resection in stage I-II patients should be prospectively evaluated.
Acknowledgments
This study used the National Cancer Data Base. The authors acknowledge the efforts of the American College of Surgeons, the Commission on Cancer, and the American Cancer Society in the creation of the National Cancer Data Base. All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the American College of Surgeons, the Commission on Cancer, the American Cancer Society, and the Patent-Centered Outcomes Research Institute, its board of governors, or its methodology committee. Dr. Chun Chieh Lin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding:
Dr. Osarogiagbon was partially supported by RO1CA172253 and PCORI IH-1304-6147. Drs. Jemal and Lin were supported by the American Cancer Society intramural research funding. Dr. Kong was supported by R01CA142840, P01CA59827 and R01CA66948.
Abbreviations
- NCCN
National Comprehensive Cancer Network
- PORT
Postoperative radiotherapy
- RCTs
Randomized clinical trials
- NSCLC
Non-small-cell lung cancer
- NCDB
National Cancer Database
- IQR
Interquartile range
- OS
Overall survival
- HR
Hazard Ratio
Footnotes
Disclosure:
Dr. Osarogiagbon has served as a consultant for Roche/Genentech and Eli Lilly, and speaker for Pfizer and Roche/Genentech. Dr. Kong has received research funding and travel support and speaker’s honorarium from Varian Medical System. The remaining authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Pfannschmidt J, Muley T, Bulzebruck H, Hoffmann H, Dienemann H. Prognostic assessment after surgical resection for non-small cell lung cancer: experiences in 2083 patients. Lung cancer. 2007;55(3):371–377. doi: 10.1016/j.lungcan.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Little AG, Rusch VW, Bonner JA, Gaspar LE, Green MR, Webb WR, Stewart AK. Patterns of surgical care of lung cancer patients. The Annals of thoracic surgery. 2005;80(6):2051–2056. doi: 10.1016/j.athoracsur.2005.06.071. discussion 2056. [DOI] [PubMed] [Google Scholar]
- 4.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 5.Winton T, Livingston R, Johnson D, Rigas J, Johnston M, Butts C, Cormier Y, Goss G, Inculet R, Vallieres E, Fry W, Bethune D, Ayoub J, Ding K, Seymour L, Graham B, Tsao MS, Gandara D, Kesler K, Demmy T, Shepherd F. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352(25):2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 6.Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell R, Seymour L, Spiro SG, Rolland E, Fossati R, Aubert D, Ding K, Waller D, Le Chevalier T. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(21):3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 7.Lally BE, Zelterman D, Colasanto JM, Haffty BG, Detterbeck FC, Wilson LD. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(19):2998–3006. doi: 10.1200/JCO.2005.04.6110. [DOI] [PubMed] [Google Scholar]
- 8.Keller SM, Adak S, Wagner H, Herskovic A, Komaki R, Brooks BJ, Perry MC, Livingston RB, Johnson DH. A randomized trial of postoperative adjuvant therapy in patients with completely resected stage II or IIIA non-small-cell lung cancer. Eastern Cooperative Oncology Group. N Engl J Med. 2000;343(17):1217–1222. doi: 10.1056/NEJM200010263431703. [DOI] [PubMed] [Google Scholar]
- 9.Weisenburger TH. Effects of postoperative mediastinal radiation on completely resected stage II and stage III epidermoid cancer of the lung. LCSG 773. Chest. 1994;106(6 Suppl):297S–301S. [PubMed] [Google Scholar]
- 10.Douillard JY, Rosell R, De Lena M, Riggi M, Hurteloup P, Mahe MA. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. International journal of radiation oncology, biology, physics. 2008;72(3):695–701. doi: 10.1016/j.ijrobp.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 11.Butts CA, Ding K, Seymour L, Twumasi-Ankrah P, Graham B, Gandara D, Johnson DH, Kesler KA, Green M, Vincent M, Cormier Y, Goss G, Findlay B, Johnston M, Tsao MS, Shepherd FA. Randomized phase III trial of vinorelbine plus cisplatin compared with observation in completely resected stage IB and II non-small-cell lung cancer: updated survival analysis of JBR-10. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28(1):29–34. doi: 10.1200/JCO.2009.24.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. PORT Meta-analysis Trialists Group. Lancet. 1998;352(9124):257–263. [PubMed] [Google Scholar]
- 13.Burdett S, Stewart L Group PM-a. Postoperative radiotherapy in non-small-cell lung cancer: update of an individual patient data meta-analysis. Lung cancer. 2005;47(1):81–83. doi: 10.1016/j.lungcan.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Lequaglie C, Conti B, Brega Massone PP, et al. Unsuspected residual disease at the resection margin after surgery for lung cancer: fate of patients after long-term follow up. Eur J Cardiothorac Surg. 2003;23(2):229–32. doi: 10.1016/s1010-7940(02)00750-9. [DOI] [PubMed] [Google Scholar]
- 15.Kayser K, Anyanwu E, Bauer HG, et al. Tumor presence at resection boundaries and lymph-node metastasis in bronchial carcinoma patients. Thorac Cardiovasc Surgeon. 1993;41(5):308–11. doi: 10.1055/s-2007-1013878. [DOI] [PubMed] [Google Scholar]
- 16.Osarogiagbon RU, Lin CC, Smeltzer MP, Jemal A. Prevalence, Prognostic Implications, and Survival Modulators of Incompletely Resected Non-Small Cell Lung Cancer in the U.S. National Cancer Data Base. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2016;11(1):e5–e16. doi: 10.1016/j.jtho.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wind J, Smit EJ, Senan S, Eerenberg JP. Residual disease at the bronchial stump after curative resection for lung cancer. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2007;32(1):29–34. doi: 10.1016/j.ejcts.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Hancock JG, Rosen JE, Antonicelli A, Moreno A, Kim AW, Detterbeck FC, Boffa DJ. Impact of adjuvant treatment for microscopic residual disease after non-small cell lung cancer surgery. Ann Thorac Surg. 2015;99(2):406–413. doi: 10.1016/j.athoracsur.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 19.National Compreshensive Cancer Network. [Accessed December 16, 2014];Non-Small Cell Lung Cancer NCCN guidelines Version 2. 2015 www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 20.American College of Surgeons. [Accessed December 16, 2014];Programs: National Cancer Data Base. 2011 http://www.facs.org/cancer/ncdb/index.html.
- 21.Lerro CC, Robbins AS, Phillips JL, Stewart AK. Comparison of cases captured in the national cancer data base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20(6):1759–1765. doi: 10.1245/s10434-013-2901-1. [DOI] [PubMed] [Google Scholar]
- 22.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–6. [PubMed] [Google Scholar]
- 23.Austin P. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Effects of postoperative mediastinal radiation on completely resected stage II and stage III epidermoid cancer of the lung. The Lung Cancer Study Group. The New England journal of medicine. 1986;315(22):1377–1381. doi: 10.1056/NEJM198611273152202. [DOI] [PubMed] [Google Scholar]
- 25.Wang EH, Corso CD, Rutter CE, Park HS, Chen AB, Kim AW, Wilson LD, Decker RH, Yu JB. Postoperative Radiation Therapy Is Associated With Improved Overall Survival in Incompletely Resected Stage II and III Non-Small-Cell Lung Cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(25):2727–2734. doi: 10.1200/JCO.2015.61.1517. [DOI] [PubMed] [Google Scholar]
- 26.Lally BE, Detterbeck FC, Geiger AM, Thomas CR, Jr, Machtay M, Miller AA, Wilson LD, Oaks TE, Petty WJ, Robbins ME, Blackstock AW. The risk of death from heart disease in patients with nonsmall cell lung cancer who receive postoperative radiotherapy: analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2007;110(4):911–917. doi: 10.1002/cncr.22845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.