Abstract
The power of human language rests upon its intricate links to human cognition. By 3 months of age, listening to language supports infants’ ability to form object categories, a building block of cognition. Moreover, infants display a systematic shift between 3 and 4 months—a shift from familiarity to novelty preferences—in their expression of this link between language and core cognitive processes. Here, we capitalize on this tightly-timed developmental shift in fullterm infants to assess a) whether it also appears in preterm infants and b) whether it reflects infants’ maturational status or the duration of their postnatal experience. Healthy late preterm infants (N = 22) participated in an object categorization task while listening to language. Their performance, coupled with that of fullterm infants, reveals that this developmental shift is evident in preterm infants and unfolds on the same maturational timetable as in their fullterm counterparts.
Keywords: language acquisition, conceptual development, preterm infant, maturation, language and thought
Questions concerning the relative contributions of postnatal experience and maturation are at the core of the developmental sciences. These questions take center stage not only in establishing theories of development, but also in identifying best practices, especially for infants who are born prematurely. Healthy preterm infants, who constitute the largest group of infants born in the United States who will eventually seek therapeutic services, nonetheless remain relatively understudied (Boyle & Boyle, 2013). But the existing research, though sparse, is intriguing: Although healthy preterm infants appear to reach several pediatric milestones on the same maturational timetable as their fullterm peers (Bosworth & Dobkins, 2009; deRegnier, Wewerka, Georgieff, Mattia, & Nelson, 2002; Hitzert et al., 2015; Jando et al., 2012; Mash, Quinn, Dobson, & Narter, 1998; Peña, Pittaluga, & Mehler, 2010; Peña, Werker, & Dehaene-Lambertz, 2012; Ricci et al., 2008; Romeo et al., 2012; Stolarova et al., 2003), many later encounter developmental challenges. Even subtle developmental challenges lead them to seek early intervention services from infancy through school-age (Baron, Litman, Ahronovich, & Baker, 2012; Celik, Demirel, Canpolat, & Dilmen, 2013; Clements, Barfield, Ayadi, & Wilber, 2007; Harijan & Boyle, 2012; Lipkind, Slopen, Pfeiffer, & McVeigh, 2012; Nepomnyaschy, Hegyi, Ostfeld, & Reichman, 2012; Odd, Emond, & Whitelaw, 2012).
Because preterm infants are at risk for a host of developmental challenges—including those engaging language, cognitive, and attentional processing capacities (Agyei, van der Weel, & van der Meer, 2016; Barre, Morgan, Doyle, & Anderson, 2011; Kavšek & Bornstein, 2010; Rose, Feldman, & Jankowski, 2002)—and because early capacities in preverbal infants are especially strong predictors of later capacities (Benasich & Tallal, 2002; Bosch, 2011; Ferguson, Havy, & Waxman, 2015; Jansson-Verkasalo et al., 2010; Kuhl, 2004; Kuhl et al., 2006; Tsao, Liu, & Kuhl, 2004), researchers and clinicians alike have aimed to identify how preterm infants’ earliest language and cognitive capacities unfold. We know that to acquire language, infants must identify which sounds are part of their native language, distinguishing them first from nonlinguistic sounds (Vouloumanos & Werker, 2004) and then from the sounds of other languages (Best, 1991; Kuhl, Williams, Lacerda, Stevens, & Lindblom, 1992; Werker & Tees, 1984).
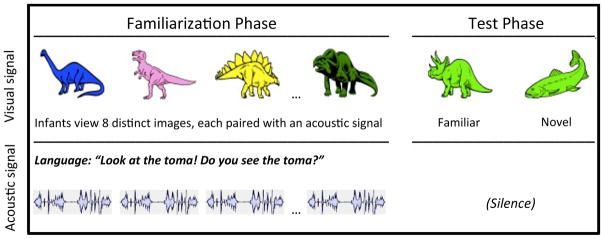
But identifying the sounds of language is not enough: Infants must also discover how the language they hear is linked to the objects and events they encounter. Recent work reveals that by 3 months, fullterm infants have already begun to link language to core conceptual capacities: Listening to language supports object categorization, a building block for cognition (Ferry, Hespos, & Waxman, 2010). This early link was demonstrated using a novelty preference task, one that required infants to integrate visual and acoustic information (Fig. 1; Ferry et al., 2010; Fulkerson & Waxman, 2007). During the Familiarization phase, infants view a series of images of distinct members of a single object category (e.g., dinosaurs), each accompanied by an acoustic signal (e.g., “Look at the modi!”). During the Test phase, infants view two new images, presented in silence: one from the now-familiar category (e.g., dinosaur; “familiar image”) and one from a novel category (e.g., fish; “novel image”). If infants formed an object category during familiarization, then they should distinguish the familiar and novel images presented at test.
Fig. 1.
Experimental design (from Ferry et al., 2010). During the familiarization phase, each infant viewed eight different images, presented sequentially and in conjunction with a sentence. During the test phase, each infant viewed two new images—one from the familiar category and one from a novel category—presented simultaneously in silence.
The results reveal that by 3 months of age, and continuously throughout the first year of life, infants listening to language—but not those listening to other sounds like sine-wave tone sequences—successfully formed object categories (Balaban & Waxman, 1997; Ferry et al., 2010; Fulkerson & Waxman, 2007). These findings highlight that listening to language supports infant cognition (Vouloumanos & Waxman, 2014).
Interestingly, between 3 and 4 months there is a systematic shift in how infants express this link (Ferry et al., 2010). At 3 months, infants listening to language prefer (look longer at) the familiar test image; by 4 months, they prefer the novel test image (Ferry et al., 2010), a preference infants maintain in this task throughout their entire first year (Balaban & Waxman, 1997; Fulkerson & Waxman, 2007).1 Shifts like this, from familiarity to novelty preferences, are ubiquitous in infant research (Colombo & Bundy, 1983; Ferry et al., 2010; Ferry, Hespos, & Waxman, 2013; Hunt, 1970; Hunter & Ames, 1988; Perone & Spencer, 2013; Roder, Bushnell, & Sasseville, 2000; Rose, Feldman, & Jankowski, 2004; Shinskey & Munakata, 2010; Slater, 2004; Uzgiris & Hunt, 1970; Weizmann, Cohen, & Pratt, 1971; Wetherford & Cohen, 1973). Decades of psychophysical evidence suggest that familiarity-to-novelty shifts tend to occur when a task involves complex stimuli (like the paired visual and acoustic stimuli in the infant categorization task) and likely reflect developmental advances in infants’ processing efficiency (Aslin, 2007; Colombo, 2002; Ferry et al., 2010, 2013; Frick, Colombo, & Allen, 2000; Reynolds & Romano, 2016).
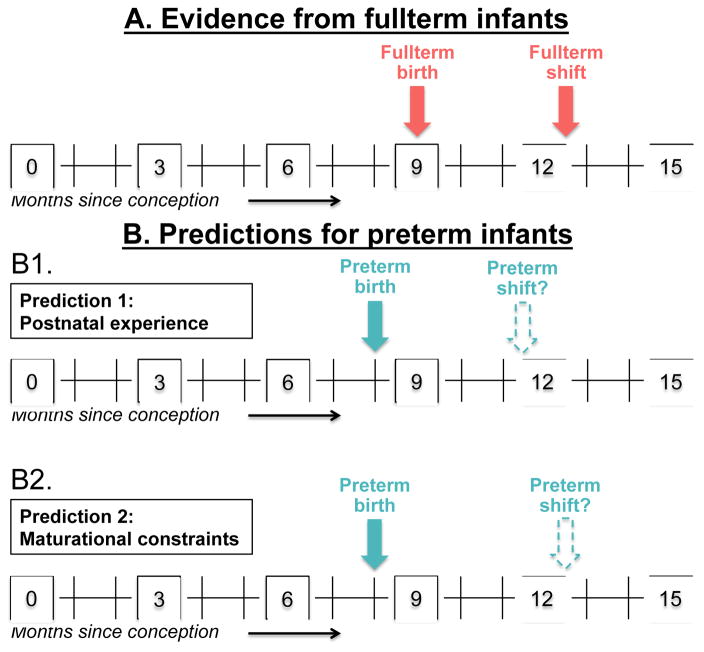
What remains unanswered is whether these early milestones—successful categorization in the context of listening to language at 3 months and a shift from familiarity to novelty preferences at 4 months (Fig. 2A)—reflect infants’ postnatal experience (listening to language, observing objects in their environment) or their maturational status. Evidence from healthy preterm infants, when considered in conjunction with evidence from healthy fullterm infants, provides a unique opportunity to uncouple these effects.
Fig. 2.
The shift from familiarity to novelty preferences. (A) Fullterm infants (from Ferry et al., 2010; Fulkerson & Waxman, 2007) shift from familiarity to novelty preferences between 12 and 13 months post conception (that is, between 3 and 4 months since birth). (B) We make two predictions for preterm infants, considering as an example infants born a month early, at 8 months post conception. (B1) If postnatal experience undergirds the shift from familiarity to novelty preferences, then infants born one month early should show the shift (dotted arrow) between 11 and 12 months post conception (that is, between 3 and 4 months since birth). (B2) If there are maturational constraints on the effect of postnatal experience, then infants born one month early should show the shift between 12 and 13 months post conception (that is, between 4 and 5 months since birth).
Developmental scientists have taken advantage of this opportunity to tease apart the effects of postnatal experience and maturational status in key developmental milestones. The logic of experimental designs comparing preterm and fullterm infants is straightforward. Consider, for example, two infants conceived on the same date. If one is born one month premature, she will accrue a full month of postnatal (or extra-utero) experience before her fullterm counterpart is born. If the developmental trajectory underlying the expression of a particular milestone is guided primarily by the duration of infants’ postnatal experience, then preterm infants should attain the milestone earlier than their fullterm peers, thanks to the additional postnatal experience conferred by their preterm birth. In contrast, if the developmental trajectory is constrained by the maturational status of the infant, then preterm infants should attain the milestone when they reach the same maturational age as their fullterm counterparts.
Peña and her colleagues’ (Peña et al., 2012) seminal work serves as a case-in-point. They focused specifically on a single well-documented developmental milestone: Within their first year, infants become increasingly attuned to the phonetic properties of their native language (Kuhl et al., 1992; Rivera-Gaxiola, Silva-Pereyra, & Kuhl, 2005; Werker & Tees, 1984). By comparing preterm and fullterm infants’ performance in the same speech perception task, they discovered that perceptual tuning to native speech sounds is constrained by infants’ maturational status, rather than postnatal experience.
Designs like this have been instrumental in teasing apart the relative contributions of experience and maturation in several vital perceptual milestones. In some cases, as in Peña et al. (2012), maturation constrains developmental timing (for other evidence of maturation-based effects in audition/speech perception, see deRegnier et al., 2002; Peña et al., 2010; for evidence in vision, see Bosworth & Dobkins, 2009; Hitzert et al., 2015; Jando et al., 2012; Mash et al., 1998; Ricci et al., 2008; Romeo et al., 2012; Stolarova et al., 2003). But in other cases, the evidence reveals just the opposite—that experience, rather than maturation, underlies certain developmental processes (for evidence of experience-based effects in audition/speech perception, see Caskey, Stephens, Tucker, & Vohr, 2011; Gonzalez-Gomez & Nazzi, 2012; for evidence in vision, see Bosworth and Dobkins, 2009; Hitzert et al., 2015; Jando et al., 2012; Matthews, Ellis, and Nelson, 1996; Peña, Arias, and Dehaene-Lambertz, 2014; Ricci et al., 2008; Romeo et al., 2012; Van Hof-Van Duin and Mohn, 1986).
Interestingly, even within a single perceptual modality, there are differences in the relative importance of postnatal experience and maturational status. For example, in auditory/speech perception, some sensitivities (e.g., phonotactic sensitivity) unfold on the basis of postnatal experience (Gonzalez-Gomez & Nazzi, 2012), while others (e.g., phonetic sensitivity) are constrained by maturational status (Peña et al., 2010, 2012). Likewise in vision, some sensitivities (e.g., chromatic contrast) unfold on the basis of infants’ postnatal experience, while others (e.g., luminance contrast) are constrained by maturational status (Bosworth & Dobkins, 2009). Taken together, then, the extant evidence paints a nuanced picture: Both within and across distinct perceptual modalities, some developmental milestones appear to emerge primarily in response to infants’ postnatal experience while others depend more crucially on infants’ maturational status.
In the current investigation, we advance this work in two ways. First, we consider the contributions of postnatal experience and maturational status in a task that requires infants to navigate across modalities (Balaban & Waxman, 1997; Ferry et al., 2010; Fulkerson & Waxman, 2007). This is crucial because in the natural course of events, infants spontaneously integrate information from more than a single modality. For instance, their perception of speech is influenced not only by acoustic information, but also by accompanying visual information (e.g., the mouth shape of the speaker producing the sounds) (Bristow et al., 2009; Burnham & Dodd, 2004; Havy, Foroud, Fais, & Werker, in press; Weikum et al., 2007; Yeung & Werker, 2013) and sensorimotor information (e.g., their own mouth shape while they are listening) (Bruderer, Danielson, Kandhadai, & Werker, 2015). Second, we move beyond infants’ perceptual sensitivities alone to consider the contributions of postnatal experience and maturational status in the unfolding of a link between human language and object categorization, a core cognitive capacity.
We take as our starting point the precisely-timed developmental shift from familiarity (at 3 months) to novelty preferences (at 4 months and thereafter), a shift that until now we have observed only in fullterm infants (Ferry et al., 2010). We compare fullterm and preterm infants’ categorization in the context of listening to language to disentangle the contributions of postnatal experience (listening to language, observing objects) and maturational status as infants’ earliest link between language and cognition unfolds.
Method
We recruited healthy preterm infants to ask whether the developmental trajectory observed in fullterm infants (successful categorization in the context of listening to language at 3 months and their shift from familiarity to novelty preferences at 4 months and thereafter) reflects postnatal experience or maturational status. The procedure, stimuli, and coding are identical to those reported in Ferry et al. (2010) and in Fulkerson and Waxman (2007).
We reason as follows: If the familiarity-to-novelty shift (Fig. 2B) can be attributed to postnatal experience alone, then preterm infants should reveal this shift once they have attained the same duration of postnatal experience (at the same experiential age) as their fullterm counterparts and should maintain it thereafter. In contrast, if the effects of postnatal experience are constrained by maturation (Fig. 2C), then preterm infants should exhibit the shift at the same maturational age as their fullterm counterparts. Note that it is also possible that, unlike fullterm infants, preterm infants will fail to form object categories while listening to language at this early a point in development.
Participants
Forty-three healthy late preterm infants from predominantly college-educated, white families living in the Greater Chicago Area participated. Late preterm infants are ideal because they permit us to examine the relative contributions of postnatal experience and maturational status without the confounding factors of medical or neurologic illness. We therefore adopted rigorous inclusion criteria, including only infants born between 32-37 weeks post conception (for comparison, the obstetric definition of fullterm infants are those born 38-42 weeks post conception) who spent little to no time in the NICU. These criteria insured that our participants met the obstetric criteria of healthy late preterm infants (Boyle & Boyle, 2013).
All infants participated when they were between 3-8 months post birth. As in Ferry et al. (2010) and Fulkerson and Waxman (2007), all came from families where English was the predominant language spoken in the home (>50% exposure to English). All procedures were approved by the Northwestern University Institutional Review Board and informed consent was obtained from a parent of each infant. Twenty-one infants were excluded for inattentiveness (looking less than 25% during familiarization; N = 9)2, looking exclusively to only one test image (N = 7), or failing to complete the task (N = 5). The final sample included 22 preterm infants (12 female), all born between 32 and 36 weeks post conception (34.77+/-1.23 weeks). At the time of testing, the preterm infants had a mean experiential age (time since birth) of 5.24 +/- 1.27 months and a mean maturational age (time since conception) of 13.06 +/- 1.33 months.
Stimuli
Visual
Identical to Ferry, et al., 2010; Fulkerson and Waxman, 2007. Line-drawn images of dinosaurs and fish formed two 8-item familiarization sets and two test pairs. Within each familiarization set, images varied in color; within each test pair, images were matched in color. Images (15 cm2) were projected onto a white screen 100 cm from the infant’s eyes.
Auditory
Identical to Ferry, et al., 2010; Fulkerson and Waxman, 2007. A labelling phrase spoken in infant-directed speech, ~2.2 s (from Ferry et al., 2010), was played from a hidden speaker located 35 cm below the screen.
Procedure
Identical to Ferry, et al., 2010; Fulkerson and Waxman, 2007. Infants were seated on a caregiver’s lap facing a screen (located 86 cm above the floor; 64 cm high × 127 cm wide). The visual images were projected onto the screen. The left and right positions of the projected images were separated by 35 cm. Caregivers, who wore opaque glasses, were instructed not to talk to their infants or influence their attention in any way. Infants’ responses were recorded by a video camera (hidden 35 cm below the screen).
Familiarization phase
Visual stimuli (either 8 distinct dinosaurs or fish) were presented on alternating sides of the screen (20 s each). The left/right position of the first familiarization image was counterbalanced across infants. Acoustic stimuli were presented as each image appeared and were repeated 8 s later.
Test phase
Two images appeared side-by-side, in silence, and remained visible for 20 s. The left/right position of the test images was counterbalanced across infants.
Coding
Identical to Ferry, et al., 2010; Fulkerson and Waxman, 2007. Infants’ looking time at test served as our dependent measure. Infants’ left-right eye gaze directions were coded frame-by-frame by trained coders blind to the hypotheses. Reliability between two trained coders was 97%.
Analyses
As in prior analyses using this task (Ferry et al., 2010, 2013; Fulkerson & Waxman, 2007), we calculated the proportion of time infants spent looking at the novel image at test (total time looking to novel image / total time looking to novel and familiar image), focusing our analyses on each infant’s first 10 s of accumulated looking. Proportions less than 0.5 indicate a preference for the familiar image (familiarity preference); proportions greater than 0.5 indicate a preference for the novel image (novelty preference).
Results
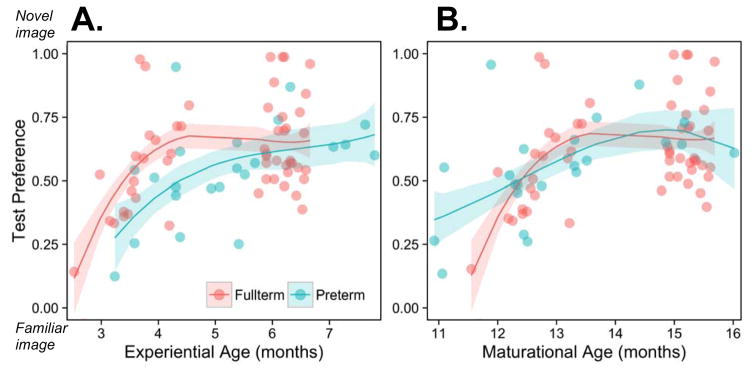
The results, depicted in Figures 3 and 4, reveal that like fullterm infants, preterm infants exhibit a developmental shift from familiarity to novelty preferences. To assess whether the timing of this shift is better predicted by infants’ postnatal experience or maturational status, we used growth curve models.
Fig. 3.
Individual data and lines of best fit for models for preterm (green) and fullterm (red) infants’ experiential ages (A) and maturational ages (B). Shading represents +/- 1 SE for each model’s estimates. Notice that when infants are matched on the basis of postnatal experience (3A), preterm infants’ growth curve looks ‘delayed’ relative to those of their fullterm counterparts, but that when infants are matched on the basis of maturational status (3B), preterm and fullterm infants’ growth curves are indistinguishable.
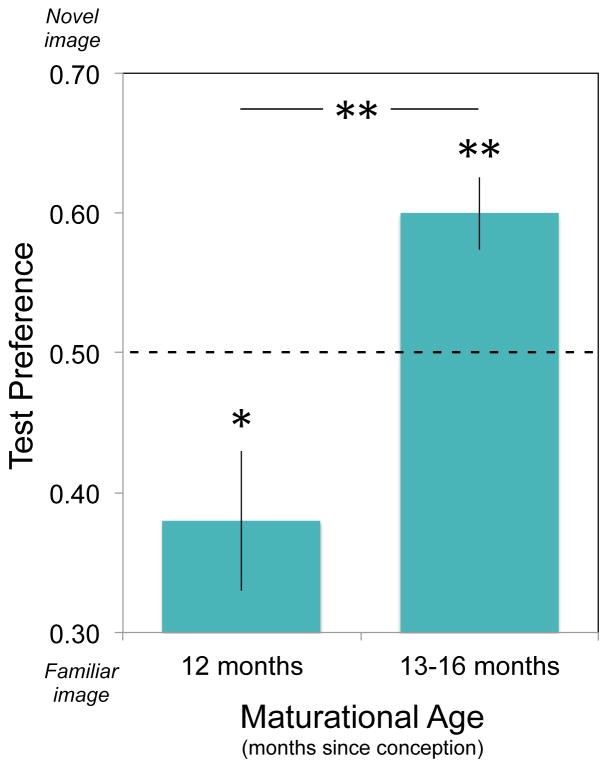
Fig. 4.
Preterm infants’ Test Preferences mirror those of their fullterm counterparts (Ferry et al., 2010; Fulkerson & Waxman, 2007) when the two groups are matched for maturational status. A maturational age of 12 months in preterm infants corresponds to an experiential age of 3 months in fullterm infants. Maturational ages of 13-16 months in preterm infants correspond to experiential ages of 4-7 months in fullterm infants.
To calculate growth curve models, we first transformed3 each infant’s preference score (a proportion: looking to novel test image / looking to novel and familiar test images) to create a dependent measure that is better suited to analyzing raw proportions with linear models (Jaeger, 2008). We then entered the transformed data for each infant into R to create growth curve models (Mirman, Dixon, & Magnuson, 2008). Recall that prior research (Ferry et al., 2010; Fulkerson & Waxman, 2007) documented that fullterm infants’ test preferences changed in a non-linear fashion, following a sigmoid curve (from familiarity preferences at 3 months to stable novelty preferences at 4 months and thereafter). Therefore, to create growth curve models that permitted us to capture the two ‘bends’ in the fullterm infants’ sigmoid-shaped developmental trajectory, we generated first-, second-, and third-order orthogonal polynomials corresponding to infant age (either experiential or maturational age, depending on the model, see below). Including these orthogonal polynomials simultaneously in our models allowed us to estimate developmental change corresponding to linear, quadratic, and cubic functions, respectively. In other words, including these polynomials insured that our growth curve models were sufficiently precise to capture the fullterm infants’ sigmoid-shaped developmental trajectory.
We used the data from the preterm infants to create two different growth curve models, one (Model A) using infants’ experiential age (time since birth) and the other (Model B) using their maturational age (time since conception).4 We then compared the fit of each model (Models A and B) for preterm infants to the model for fullterm infants (Fig. 3A and 3B, respectively). To do so, we computed Bayesian Information Criterion (BIC) scores (Raftery, 1995). We selected BIC scores because these are ideally suited to the requirements of the current analysis. First, these models do not make strong assumptions about the shape of growth/change over developmental time. As a result, by comparing different models, this analysis can identify which factors (maturation, experience) best explain the growth curves the infants produce, regardless of the shape they take. Second, BIC models are designed for comparisons in which a) the two models for preterm infants’ data (Models A (maturation) and B (experience)) are non-nested, and b) each of these independent models will be compared to growth curves generated by a known data set (Ferry et al.’s (2010) and Fulkerson and Waxman’s (2007) evidence from fullterm infants) (Vrieze, 2012). More standard tests (e.g., ANOVAs, log-likelihood ratio tests) require the models under comparison to be nested; BIC score comparisons can accommodate non-nested models. In BIC score comparisons, the model comparison yielding the lowest score reflects the model of best fit (Raftery, 1995).5
Our model comparisons indicated that infants’ maturational age best predicts the timing of their shift from familiarity to novelty preferences. We obtained BIC scores of 310.58 for Model A (the experiential model) and 308.08 for Model B (the maturational model). This BIC difference of 2.50 in BIC scores is comparable to a p-value of .028 and Bayes Factor of 3.49 (Raftery, 1995). Thus, our model comparison constitutes positive evidence for Model B.
After determining that Model B (maturational age) better fit the data, we assessed the predictive value of adding an additional Group (preterm, fullterm) factor to both models.6 A -2 log-likelihood ratio test demonstrated that Group did not significantly improve either Model A (χ2(4) = 12.22, p = .35) or Model B (χ2(4) = 4.66, p = .79). Since adding infants’ status as fullterm or preterm to the models provided no additional explanatory value, this suggests that maturational age alone is sufficient to predict the developmental timing of the shift from familiarity to novelty preferences.
A subsequent series of analyses provided converging evidence that maturation, rather than postnatal exposure, best predicts the developmental shift from familiarity to novelty preferences in preterm infants (Fig. 4). For these analyses, following prior work with this method (Ferry et al., 2010, 2013; Fulkerson & Waxman, 2007), we used each infant’s raw mean test preference, aggregating over the first 10 s of accumulated looking.7,8
At a maturational age corresponding to that of 3-month-old fullterm infants, preterm infants exhibited significant familiarity preferences, M = .358, SD = .167; t(10) = -2.83, p = .018, d = .85. Moreover, 82% (9/11) of the preterm infants at this maturational age exhibited familiarity preferences (p = .033, binomial comparison). At a maturational age corresponding to that of 4- to 7-month-old fullterm infants, preterm infants shifted to reveal significant novelty preferences, M = .601, SD = .081; t(8) = 3.77, p = .005, d = 1.23; 89% (8/9) of the preterm infants at this maturational age exhibited novelty preferences (p = .020, binomial comparison). Finally, the magnitude of the novelty preference was as strong in preterm infants at a maturational age of 4 months (M = .581, SD = .097) as in those at a maturational age of 5 to 7 months (M = .627, SD = 056), t(6.53) = .902, p = .40. This lends additional assurances that the shift from familiarity to novelty preferences in preterm infants occurs when they are at the same maturational age as their fullterm peers.
Discussion
These results illuminate the crucial contribution of maturational status as very young infants integrate the language that they hear with the objects they observe. Capitalizing on a precisely-timed developmental shift—documented thus far only in fullterm infants (Ferry et al., 2010)—we examined healthy preterm infants’ performance to ascertain whether this shift reflects infants’ postnatal experience (seeing objects, listening to language) or maturational status. Our results provide the first evidence that this foundational link between language and categorization unfolds along the same maturational timetable in healthy preterm infants as in their fullterm peers. When preterm infants’ ages were adjusted for maturational status (time since conception, or gestational age), their categorization responses mirrored precisely those of fullterm infants: They successfully formed object categories while listening to language, exhibiting reliable familiarity preferences at a maturational age of 12 months (post conception) and shifting to reliable novelty preferences at a maturational age of 13 months (post conception). Thus, although our preterm infants had been exposed to a longer period of postnatal experience than their maturationally equivalent fullterm counterparts, this additional exposure did not confer an advantage in the developmental trajectory underlying the link between language and object categorization. Instead, infants’ maturational status constrains the effect of their postnatal experience.
This outcome falls in line with previous evidence that some neurological and perceptual milestones are guided more by maturation than by postnatal exposure (Bosworth & Dobkins, 2009; deRegnier et al., 2002; Hitzert et al., 2015; Jando et al., 2012; Mash et al., 1998; Peña et al., 2010, 2012; Ricci et al., 2008; Romeo et al., 2012; Stolarova et al., 2003). But the current work takes us further: It illuminates the importance of maturation in a task that requires infants to navigate across the auditory and visual modalities, and to do so in the service of linking language and cognition.
This raises a compelling question. If preterm infants are maturationally on par with their fullterm counterparts, successfully forming object categories in the context of listening to language, then why do they later encounter developmental obstacles, as indexed by their increased enrollment in early language and cognitive interventions (Baron et al., 2012; Celik et al., 2013; Clements et al., 2007; Harijan & Boyle, 2012; Lipkind et al., 2012; Nepomnyaschy et al., 2012; Odd et al., 2012)? Certainly the evidence reported here cannot resolve this important and pernicious problem. But a careful review of the literature, considered within a developmental framework, offers a new theoretical framework that permits us to address it. Central to this framework is the observation that infants’ early advances in language and cognition are quintessentially developmental processes. They involve a cascade of sensitive periods (Hensch, 2004; Johnson & Newport, 1989; Newport, 1990; Newport, Bavelier, & Neville, 2001; Werker & Hensch, 2014; Werker & Tees, 1984) that unfold continuously over developmental time, and require coordination among a suite of interdependent capacities (including perceptual, social, and memory capacities) in which early-emerging capacities serve as the foundation for those that follow (Kuhl, 2007; Waxman & Lidz, 2006; Werker & Tees, 2005). Notice, then, that if some of infants’ early-emerging capacities are coupled tightly to maturational status and others are tied more closely to postnatal experience, then the timing in which the foundational elements unfold in preterm infants may not correspond precisely to the timing in fullterm infants.
These nuanced developmental cascades are especially relevant to questions concerning how the link between language and cognition unfolds. In future research, it will be important to identify whether maturation guides the effect of experience on infants’ language processing (auditory modality), their object categorization (visual modality), both, or the link between the two. It will also be important to examine preterm infants throughout the first year to clarify how postnatal experience and maturation interact as infants continue to refine the link between the language they hear and the objects and events they witness. That is, in future work it will be important to specify when and how distinct elements of language, visual, and cognitive processes unfold, how differences in timing might alter the developmental cascade in preterm infants, and how this might bear on later delays or deficits observed in preterm infants. This will further our understanding of how maturation and experience interact to sculpt the developing brain and its capacity for language.
In conclusion, we document that healthy late preterm infants are maturationally on par with their fullterm counterparts in a crucial developmental foundation: forming object categories in the context of listening to language. This advances our understanding of early developmental success in a vulnerable population. It also advances a strong theoretical framework for identifying infants’ earliest links between language and cognition and how they unfold.
Research Highlights.
Healthy preterm infants were compared with 3- and 4-month-old fullterm infants to identify whether the developmental timing of an early link between language and object categorization, documented thus far only in fullterm infants, unfolds on the basis of postnatal experience or maturation.
Preterm infants listening to language in an object categorization task revealed the same developmental trajectory as fullterm infants when they were matched for maturational age.
Infants’ expression of this link was dependent on their maturational age and not the duration of their postnatal experience.
Infants’ earliest link between language and categorization unfolds on the same maturational timetable in healthy late preterm infants and their fullterm counterparts.
Acknowledgments
The research reported here was supported by the National Institute of Child Health and Human Development of the National Institutes of Health under award number R01HD08310. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
At 3 and 4 months of age, language is not the only acoustic signal that promotes object categorization: Nonhuman primate vocalizations (Madagascar blue-eyed lemur: Eulemur macaco flavifrons) also support object categorization (Ferry et al., 2013). Moreover, infants listening to lemur vocalizations reveal the same shift from familiarity preferences at 3 months to novelty preferences at 4 months. Yet by 6 months, infants have established a more precise link between human language and categorization: Vocalizations of nonhuman primates no longer effectively boost infant categorization (Ferry et al., 2013). Here, we focus exclusively on preterm infants’ categorization in the context of listening to human language, an effect that in fullterm infants is evident throughout the first year of life (Balaban & Waxman, 1997; Ferry et al., 2010; Fulkerson & Waxman, 2007).
Ferry and colleagues (Ferry et al., 2010, 2013) imposed a more stringent inclusion criteria (50% looking during familiarization) with fullterm infants. Here, we imposed a 25% criterion in an effort to increase the likelihood that preterm infants would be retained for analysis. We note here that preterm infants’ patterns of results are identical whether we use 25% or 50% as our criterion for inclusion.
We used the following equation (in R) to transform raw proportions into a DV more appropriate for linear models (logits): TestPreference_logit = log((TestPreference + .001)/(1 – TestPreference + .001)). By adding .001 to the raw proportions in the numerator and denominator, we avoid the analytic problems associated with log-transforming zeros.
We used the following equations (in R): Model A: TestPreference_logit ~ ExperientialAge_ot1 + ExperientialAge _ot2 + ExperientialAge _ot3. Model B: TestPreference_logit ~ MaturationalAge_ot1 + MaturationalAge _ot2 + MaturationalAge _ot3.
BIC score analyses have been instrumental in developmental work (e.g., Connell & Frye, 2006; Hirsh-Pasek & Burchinal, 2006), including analyses of preterm infants (Schwichtenberg, Anders, Vollbrecht, & Poehlmann, 2012).
We used the following equations (in R): Model A: TestPreference_logit ~ (ExperientialAge_ot1 + ExperientialAge _ot2 + ExperientialAge _ot3)*group. Model B: TestPreference_logit ~ (MaturationalAge_ot1 + MaturationalAge _ot2 + MaturationalAge _ot3)*group.
The same patterns of results emerge in an analysis of looking-time throughout the full 20 s test period.
Following standard procedure in this work (c.f., Ferguson & Waxman, 2016; Perszyk & Waxman, 2016), infants with test preferences greater than 2 SD from the mean (N = 2) were excluded from these analyses.
References
- Agyei SB, van der Weel FR (Ruud), van der Meer ALH. Longitudinal study of preterm and full-term infants: High-density EEG analyses of cortical activity in response to visual motion. Neuropsychologia. 2016;84:89–104. doi: 10.1016/j.neuropsychologia.2016.02.001. http://doi.org/10.1016/j.neuropsychologia.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Aslin RN. What’s in a look? Developmental Science. 2007;10(1):48–53. doi: 10.1111/J.1467-7687.2007.00563.X. http://doi.org/10.1111/j.1467-7687.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban MT, Waxman SR. Do words facilitate object categorization in 9-month-old infants? Journal of Experimental Child Psychology. 1997;64:3–26. doi: 10.1006/jecp.1996.2332. http://doi.org/10.1006/jecp.1996.2332. [DOI] [PubMed] [Google Scholar]
- Baron IS, Litman FR, Ahronovich MD, Baker R. Late preterm birth: A review of medical and neuropsychological childhood outcomes. Neuropsychology Review. 2012;22(4):438–450. doi: 10.1007/s11065-012-9210-5. http://doi.org/10.1007/s11065-012-9210-5. [DOI] [PubMed] [Google Scholar]
- Barre N, Morgan A, Doyle LW, Anderson PJ. Language abilities in children who were very preterm and/or very low birth weight: A meta-analysis. Journal of Pediatrics. 2011;158(5) doi: 10.1016/j.jpeds.2010.10.032. http://doi.org/10.1016/j.jpeds.2010.10.032. [DOI] [PubMed] [Google Scholar]
- Benasich AA, Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behavioural Brain Research. 2002;136(1):31–49. doi: 10.1016/s0166-4328(02)00098-0. http://doi.org/10.1016/S0166-4328(02)00098-0. [DOI] [PubMed] [Google Scholar]
- Best CT. The emergence of native-language phonological influences in infants: A perceptual assimilation model. The Development of Speech Perception: The Transition from Speech Sounds to Spoken Words. 1991;167:167–224. [Google Scholar]
- Bosch L. Precursors to language in preterm infants: Speech perception abilities in the first year of life. In: Braddick O, Atkinson J, Innocenti G, editors. Progress in brain research. 1. Vol. 189. Elsevier B.V; 2011. pp. 239–57. http://doi.org/10.1016/B978-0-444-53884-0.00028-2. [DOI] [PubMed] [Google Scholar]
- Bosworth RG, Dobkins KR. Chromatic and luminance contrast sensitivity in fullterm and preterm infants. Journal of Vision. 2009;9(13):1–16. doi: 10.1167/9.13.15. http://doi.org/10.1167/9.13.15.Introduction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle JD, Boyle EM. Born just a few weeks early: Does it matter? Archives of Disease in Childhood - Fetal and Neonatal Edition. 2013;98(1):85–88. doi: 10.1136/archdischild-2011-300535. http://doi.org/10.1136/archdischild-2011-300535. [DOI] [PubMed] [Google Scholar]
- Bristow D, Dehaene-Lambertz G, Mattout J, Soares C, Gliga T, Baillet S, Mangin JF. Hearing faces: how the infant brain matches the face it sees with the speech it hears. Journal of Cognitive Neuroscience. 2009;21(5):905–21. doi: 10.1162/jocn.2009.21076. http://doi.org/10.1162/jocn.2009.21076. [DOI] [PubMed] [Google Scholar]
- Bruderer AG, Danielson DK, Kandhadai P, Werker JF. Sensorimotor influences on speech perception in infancy. Proceedings of the National Academy of Sciences. 2015;112(44):1–6. doi: 10.1073/pnas.1508631112. http://doi.org/10.1073/pnas.1508631112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham D, Dodd B. Auditory-visual speech integration by prelinguistic infants: Perception of an emergent consonant in the McGurk effect. Developmental Psychobiology. 2004;45(4):204–220. doi: 10.1002/dev.20032. http://doi.org/10.1002/dev.20032. [DOI] [PubMed] [Google Scholar]
- Caskey M, Stephens B, Tucker R, Vohr B. Importance of parent talk on the development of preterm infant vocalizations. Pediatrics. 2011;128(5):1–7. doi: 10.1542/peds.2011-0609. http://doi.org/10.1542/peds.2011-0609. [DOI] [PubMed] [Google Scholar]
- Celik IH, Demirel G, Canpolat FE, Dilmen U. A common problem for neonatal intensive care units: Late preterm infants, a prospective study with term controls in a large perinatal center. Journal of Maternal-Fetal and Neonatal Medicine. 2013;26(5):459–462. doi: 10.3109/14767058.2012.735994. http://doi.org/10.3109/14767058.2012.735994. [DOI] [PubMed] [Google Scholar]
- Clements KM, Barfield WD, Ayadi MF, Wilber N. Preterm birth–associated cost of early intervention services: An analysis by gestational age. Pediatrics. 2007 doi: 10.1542/peds.2006-1729. http://doi.org/10.1542/peds.2006-1729. [DOI] [PubMed]
- Colombo J. Infant attention grows up: The emergence of a developmental cognitive neuroscience perspective. Current Directions in Psychological Science. 2002;11(6):196–200. http://doi.org/10.1111/1467-8721.00199. [Google Scholar]
- Colombo J, Bundy RS. Infant response to auditory familiarity and novelty. Infant Behavior and Development. 1983;6(2–3):305–311. http://doi.org/10.1016/S0163-6383(83)80039-3. [Google Scholar]
- Connell AM, Frye AA. Growth mixture modelling in developmental psychology: Overview and demonstration of heterogeneity in developmental trajectories of adolescent antisocial behaviour. Infant and Child Development. 2006;15(6):609–621. http://doi.org/10.1002/icd.481. [Google Scholar]
- deRegnier RA, Wewerka S, Georgieff MK, Mattia F, Nelson CA. Influences of postconceptional age and postnatal experience on the development of auditory recognition memory in the newborn infant. Developmental Psychobiology. 2002;41(3):216–225. doi: 10.1002/dev.10070. http://doi.org/10.1002/dev.10070. [DOI] [PubMed] [Google Scholar]
- Ferguson B, Havy M, Waxman SR. The precision of 12-month-old infants’ link between language and categorization predicts vocabulary size at 12 and 18 months. Frontiers in Psychology. 2015;6(August):1319. doi: 10.3389/fpsyg.2015.01319. http://doi.org/10.3389/fpsyg.2015.01319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry AL, Hespos SJ, Waxman SR. Categorization in 3- and 4-month-old infants: An advantage of words over tones. Child Development. 2010;81(2):472–9. doi: 10.1111/j.1467-8624.2009.01408.x. http://doi.org/10.1111/j.1467-8624.2009.01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry AL, Hespos SJ, Waxman SR. Nonhuman primate vocalizations support categorization in very young human infants. Proceedings of the National Academy of Sciences of the United States of America. 2013:1–5. doi: 10.1073/pnas.1221166110. http://doi.org/10.1073/pnas.1221166110. [DOI] [PMC free article] [PubMed]
- Frick JE, Colombo J, Allen JR. Temporal sequence of global-local processing in 3-month-old infants. Infancy. 2000;1(3):375–386. doi: 10.1207/S15327078IN0103_6. http://doi.org/10.1207/S15327078IN0103_6. [DOI] [PubMed] [Google Scholar]
- Fulkerson AL, Waxman SR. Words (but not tones) facilitate object categorization: Evidence from 6- and 12-month-olds. Cognition. 2007;105(1):218–28. doi: 10.1016/j.cognition.2006.09.005. http://doi.org/10.1016/j.cognition.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Gomez N, Nazzi T. Phonotactic acquisition in healthy preterm infants. Developmental Science. 2012;15(6):885–94. doi: 10.1111/j.1467-7687.2012.01186.x. http://doi.org/10.1111/j.1467-7687.2012.01186.x. [DOI] [PubMed] [Google Scholar]
- Harijan P, Boyle EM. Health outcomes in infancy and childhood of moderate and late preterm infants. Seminars in Fetal and Neonatal Medicine. 2012;17(3):159–162. doi: 10.1016/j.siny.2012.02.002. http://doi.org/10.1016/j.siny.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Havy M, Foroud A, Fais L, Werker J. The role of auditory and visual speech in word-learning at 18 months and in adulthood. Child Development. doi: 10.1111/cdev.12715. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical Period Regulation. Annual Review of Neuroscience. 2004;27(1):549–579. doi: 10.1146/annurev.neuro.27.070203.144327. http://doi.org/10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hirsh-Pasek K, Burchinal M. Mother and Caregiver Sensitivity Over Time: Predicting Language and Academic Outcomes With Variable- and Person-Centered Approaches. Merrill-Palmer Quarterly. 2006;52(3):449–485. http://doi.org/10.1353/mpq.2006.0027. [Google Scholar]
- Hitzert MM, van Geert PLC, Hunnius S, Van Braeckel KNJa, Bos AF, Geuze RH. Associations between developmental trajectories of movement variety and visual attention in fullterm and preterm infants during the first six months postterm. Early Human Development. 2015;91(1):89–96. doi: 10.1016/j.earlhumdev.2014.12.006. http://doi.org/10.1016/j.earlhumdev.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Hunt JM. Attentional preference and experience: I. Introduction. Journal of Genetic Psychology. 1970;117(1):99–107. http://doi.org/10.1080/00221325.1970.10533940. [Google Scholar]
- Hunter MA, Ames EW. A multifactor model of infant preferences for novel and familiar stimuli. Advances in Infancy Research. 1988;5:69–95. [Google Scholar]
- Jaeger TF. Categorical data analysis: Away from ANOVAs (transformation or not) and towards Logit Mixed Models. Journal of Memory and Language. 2008;59(4):434–446. doi: 10.1016/j.jml.2007.11.007. http://doi.org/10.1016/j.jml.2007.11.007.Categorical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jando G, Miko-Barath E, Marko K, Hollody K, Torok B, Kovacs I. Early-onset binocularity in preterm infants reveals experience-dependent visual development in humans. Proceedings of the National Academy of Sciences. 2012;109(27):11049–11052. doi: 10.1073/pnas.1203096109. http://doi.org/10.1073/pnas.1203096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson-Verkasalo E, Ruusuvirta T, Huotilainen M, Alku P, Kushnerenko E, Suominen K, … Hallman M. Atypical perceptual narrowing in prematurely born infants is associated with compromised language acquisition at 2 years of age. BMC Neuroscience. 2010;11:88. doi: 10.1186/1471-2202-11-88. http://doi.org/10.1186/1471-2202-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JS, Newport EL. Critical period effects in second language learning: The influence of maturational state on the acquisition of English as a second language. Cognitive Psychology. 1989 doi: 10.1016/0010-0285(89)90003-0. http://doi.org/10.1016/0010-0285(89)90003-0. [DOI] [PubMed]
- Kavšek M, Bornstein MH. Visual habituation and dishabituation in preterm infants: A review and meta-analysis. Research in Developmental Disabilities. 2010 doi: 10.1016/j.ridd.2010.04.016. http://doi.org/10.1016/j.ridd.2010.04.016. [DOI] [PMC free article] [PubMed]
- Kuhl PK. Early language acquisition: cracking the speech code. Nature Reviews. Neuroscience. 2004;5(11):831–43. doi: 10.1038/nrn1533. http://doi.org/10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Is speech learning “gated” by the social brain? Developmental Science. 2007;10(1):110–120. doi: 10.1111/j.1467-7687.2007.00572.x. http://doi.org/10.1111/j.1467-7687.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Stevens E, Hayashi A, Deguchi T, Kiritani S, Iverson P. Infants show a facilitation effect for native language phonetic perception between 6 and 12 months. Developmental Science. 2006;9(2):F13–F21. doi: 10.1111/j.1467-7687.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Williams KA, Lacerda F, Stevens KN, Lindblom B. Linguistic experience alters phonetic perception in infants by 6 months of age. Science. 1992;255:606–608. doi: 10.1126/science.1736364. [DOI] [PubMed] [Google Scholar]
- Lipkind HS, Slopen ME, Pfeiffer MR, McVeigh KH. School-age outcomes of late preterm infants in New York City. American Journal of Obstetrics and Gynecology. 2012;206(3):222.e1–222.e6. doi: 10.1016/j.ajog.2012.01.007. http://doi.org/10.1016/j.ajog.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Mash C, Quinn PC, Dobson V, Narter DB. Global influences on the development of spatial and object perceptual categorization abilities: Evidence from preterm infants. Developmental Science. 1998;1(1):84–102. http://doi.org/10.1111/1467-7687.00017. [Google Scholar]
- Matthews A, Ellis AE, Nelson CA. Development of preterm and full-term infant ability on AB, recall memory, transparent barrier detour, and means-end tasks. Child Development. 1996;67(6):2658–2676. http://doi.org/10.1111/j.1467-8624.1996.tb01881.x. [PubMed] [Google Scholar]
- Mirman D, Dixon J, Magnuson J. Statistical and computational models of the visual world paradigm: Growth curves and individual differences. Journal of Memory and Language. 2008;59(4):475–494. doi: 10.1016/j.jml.2007.11.006. http://doi.org/10.1016/j.jml.2007.11.006.Statistical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepomnyaschy L, Hegyi T, Ostfeld BM, Reichman NE. Developmental outcomes of late-preterm infants at 2 and 4 years. Maternal and Child Health Journal. 2012;16(8):1612–1624. doi: 10.1007/s10995-011-0853-2. http://doi.org/10.1007/s10995-011-0853-2. [DOI] [PubMed] [Google Scholar]
- Newport EL. Maturational constraints on language learning. Cognitive Science. 1990;14:11–28. [Google Scholar]
- Newport EL, Bavelier D, Neville H. Critical thinking about critical periods: Perspectives on a critical period for language acquisition. In: Dupoux E, editor. Language, brain, and cognitive development. Cambridge, Massachusettes: MIT Press; 2001. [Google Scholar]
- Odd DE, Emond A, Whitelaw A. Long-term cognitive outcomes of infants born moderately and late preterm. Developmental Medicine and Child Neurology. 2012;54(8):704–9. doi: 10.1111/j.1469-8749.2012.04315.x. http://doi.org/10.1111/j.1469-8749.2012.04315.x. [DOI] [PubMed] [Google Scholar]
- Peña M, Arias D, Dehaene-Lambertz G. Gaze Following Is Accelerated in Healthy Preterm Infants. Psychological Science. 2014;25(10):1884–1892. doi: 10.1177/0956797614544307. http://doi.org/10.1177/0956797614544307. [DOI] [PubMed] [Google Scholar]
- Peña M, Bonatti LL, Nespor M, Mehler J. Signal-driven computations in speech processing. Science (New York, NY) 2002 Oct;298:604–607. doi: 10.1126/science.1072901. http://doi.org/10.1126/science.1072901. [DOI] [PubMed] [Google Scholar]
- Peña M, Pittaluga E, Mehler J. Language acquisition in premature and full-term infants. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3823–3828. doi: 10.1073/pnas.0914326107. http://doi.org/10.1073/pnas.0914326107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña M, Werker JF, Dehaene-Lambertz G. Earlier speech exposure does not accelerate speech acquisition. The Journal of Neuroscience. 2012;32(33):11159–63. doi: 10.1523/JNEUROSCI.6516-11.2012. http://doi.org/10.1523/JNEUROSCI.6516-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perone S, Spencer JP. Autonomous visual exploration creates developmental change in familiarity and novelty seeking behaviors. Frontiers in Psychology. 2013 Sep;4 doi: 10.3389/fpsyg.2013.00648. http://doi.org/10.3389/fpsyg.2013.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery AE. Bayesian Model Selection in Social Research. Sociological Methodology. 1995 http://doi.org/10.2307/271063.
- Reynolds GD, Romano AC. The Development of Attention Systems and Working Memory in Infancy. Frontiers in Systems Neuroscience. 2016;10(March):1–12. doi: 10.3389/fnsys.2016.00015. http://doi.org/10.3389/fnsys.2016.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci D, Cesarini L, Romeo DMM, Gallini F, Serrao F, Groppo M, … Mercuri E. Visual function at 35 and 40 weeks’ postmenstrual age in low-risk preterm infants. Pediatrics. 2008;122(6):e1193–8. doi: 10.1542/peds.2008-1888. http://doi.org/10.1542/peds.2008-1888. [DOI] [PubMed] [Google Scholar]
- Rivera-Gaxiola M, Silva-Pereyra J, Kuhl PK. Brain potentials to native and non-native speech contrasts in 7- and 11-month-old American infants. Developmental Science. 2005;8(2):162–72. doi: 10.1111/j.1467-7687.2005.00403.x. http://doi.org/10.1111/j.1467-7687.2005.00403.x. [DOI] [PubMed] [Google Scholar]
- Roder BJ, Bushnell EW, Sasseville AM. Infants ’ Preferences for Familiarity and Novelty During the Course of Visual Processing. Infancy. 2000;1(4):491–507. doi: 10.1207/S15327078IN0104_9. http://doi.org/10.1207/S15327078IN0104_9. [DOI] [PubMed] [Google Scholar]
- Romeo DM, Ricci D, Serrao F, Gallini F, Olivieri G, Cota F, … Mercuri E. Visual function assessment in late-preterm newborns. Early Human Development. 2012;88(5):301–5. doi: 10.1016/j.earlhumdev.2011.08.024. http://doi.org/10.1016/j.earlhumdev.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Processing speed in the 1st year of life: a longitudinal study of preterm and full-term infants. Developmental Psychology. 2002;38(6):895–902. doi: 10.1037//0012-1649.38.6.895. http://doi.org/10.1037/0012-1649.38.6.895. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Infant visual recognition memory. Developmental Review. 2004;24(1):74–100. doi: 10.1037/0012-1649.39.3.563. http://doi.org/10.1016/j.dr.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Schwichtenberg AJ, Anders TF, Vollbrecht M, Poehlmann J. Daytime sleep and parenting interactions in infants born preterm. Journal of Developmental Behavioral Pediatrics. 2012;32(1):8–17. doi: 10.1097/DBP.0b013e3181fa57e4. http://doi.org/10.1097/DBP.0b013e3181fa57e4.Daytime. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinskey JL, Munakata Y. Something old, something new: A developmental transition from familiarity to novelty preferences with hidden objects. Developmental Science. 2010;13(2):378–384. doi: 10.1111/j.1467-7687.2009.00899.x. http://doi.org/10.1111/j.1467-7687.2009.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater A. Novelty, familiarity, and infant reasoning. Infant and Child Development. 2004 http://doi.org/10.1002/icd.356.
- Stolarova M, Whitney H, Webb SJ, DeRegnier RA, Georgieff MK, Nelson Ca. Electrophysiological brain responses of six-month-old low risk premature infants. Infancy. 2003;4(3):437–450. http://doi.org/10.1207/S15327078IN0403. [Google Scholar]
- Tsao FM, Liu HM, Kuhl PK. Speech perception in infancy predicts language development in the second year of life: A longitudinal study. Child Development. 2004;75(4):1067–1084. doi: 10.1111/j.1467-8624.2004.00726.x. http://doi.org/10.1111/j.1467-8624.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Uzgiris IC, Hunt JM. Attentional preference and experience: II. An exploratory longitudinal study of the effect of visual familiarity and responsiveness. Journal of Genetic Psychology. 1970;117(1):109–121. http://doi.org/10.1080/00221325.1970.10533941. [Google Scholar]
- Van Hof-Van Duin J, Mohn G. The development of visual acuity in normal fullterm and preterm infants. Vision Research. 1986;26(6):909–916. doi: 10.1016/0042-6989(86)90149-5. http://doi.org/10.1016/0042-6989(86)90149-5. [DOI] [PubMed] [Google Scholar]
- Vouloumanos A, Waxman SR. Listen up! Speech is for thinking during infancy. Trends in Cognitive Sciences. 2014;18(12):642–646. doi: 10.1016/j.tics.2014.10.001. http://doi.org/10.1016/j.tics.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouloumanos A, Werker JF. Tuned to the signal: the privileged status of speech for young infants. Developmental Science. 2004;7(3):270–6. doi: 10.1111/j.1467-7687.2004.00345.x. [DOI] [PubMed] [Google Scholar]
- Vrieze SI. Model selection and psychological theory: a discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) Psychological Methods. 2012;17(2):228–43. doi: 10.1037/a0027127. http://doi.org/10.1037/a0027127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SR, Lidz JL. Early word learning. In: Kuhn D, Siegler R, editors. Handbook of Child Psychology. 6. Vol. 2. Hoboken NJ: Wiley; 2006. pp. 299–335. [Google Scholar]
- Weikum WM, Vouloumanos A, Navarra J, Soto-faraco S, Sebastián-gallés N, Werker JF. Visual Language Discrimination in Infancy. Science. 2007 May; doi: 10.1126/science.1137686. [DOI] [PubMed] [Google Scholar]
- Weizmann F, Cohen LB, Pratt RJ. Novelty, familiarity, and the development of infant attention. Developmental Psychology. 1971;4(2):149–154. http://doi.org/10.1037/h0030432. [Google Scholar]
- Werker JF, Hensch TK. Critical Periods in Speech Perception: New Directions. Annual Review of Psychology. 2014 Sep;:1–24. doi: 10.1146/annurev-psych-010814-015104. http://doi.org/10.1146/annurev-psych-010814-015104. [DOI] [PubMed]
- Werker JF, Tees RC. Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behavior and Development. 1984;7:49–63. [Google Scholar]
- Werker JF, Tees RC. Speech perception as a window for understanding plasticity and commitment in language systems of the brain. Developmental Psychobiology. 2005;46(3):233–51. doi: 10.1002/dev.20060. http://doi.org/10.1002/dev.20060. [DOI] [PubMed] [Google Scholar]
- Wetherford MJ, Cohen LB. Developmental Changes in Infant Visual Preferences for Novelty and Familiarity. Child Development. 1973;44(3):416–424. http://doi.org/10.2307/1127994. [PubMed] [Google Scholar]
- Yeung HH, Werker JF. Lip movements affect infants’ audiovisual speech perception. Psychological Science. 2013;24(5):603–12. doi: 10.1177/0956797612458802. http://doi.org/10.1177/0956797612458802. [DOI] [PubMed] [Google Scholar]