Abstract
Sepsis is a leading cause of mortality in intensive care units, and is more common in the geriatric population. The control of hyperinflammation has been suggested as a therapeutic approach in sepsis, but to date clinical trials utilizing this strategy have not lead to an effective treatment. In addition to hyperinflammation, patients with sepsis often experience a state of immunosuppression, which serves as an important determinant for increased morbidity and mortality. We previously used aged animals to demonstrate the effectiveness of combined treatment with human ghrelin (Ghr) and human growth hormone (GH) in improving organ injury and survival in septic animals. Here, we hypothesized that combined treatment with Ghr and GH could improve immune function in septic aged animals. Male 24-month-old rats were subjected to cecal ligation and puncture (CLP) for sepsis induction. Human Ghr (80 nmol/kg BW) plus GH (50 μg/kg BW) or vehicle (normal saline) was administrated subcutaneously at 5 h after CLP. The ex vivo production of TNF-α, IL-6 and IL-10 to LPS-stimulation, as well as TNF-α, IL-6, IL-10 and INF-γ production to anti-CD3/anti-CD28 antibody-stimulation, in splenocytes isolated 20 h after CLP, was significantly decreased compared to production of these cytokines in splenocytes from sham animals. The production of cytokines from splenocytes isolated from septic animals that received the combined treatment, however, was significantly higher than from those isolated from vehicle-treated septic animals. Combined treatment prevented the loss of splenic CD4+ and CD8+ T cells in septic aged rats, and reduced lymphocyte apoptosis. Combined treatment also inhibited an increase in the regulatory T cell (Treg) population and expression of the immune co-inhibitory molecule PD-1 in the spleens of septic aged rats. In contrast, expression of HLA-DR was increased after combined treatment with Ghr and GH. Based on these findings, we conclude that co-administration of Ghr and GH is a promising therapeutic tool for reversing immunosuppression caused by sepsis in the geriatric population.
Keywords: ghrelin, growth hormone, sepsis, immunosuppression, aging
Introduction
Sepsis is a clinical condition caused by a dysregulated immune response against invading pathogens, which may lead to multiple organ dysfunction and death [1]. Despite advances in treatment, sepsis remains the second leading cause of death among patients in non-coronary intensive care units [2, 3]. Traditionally, an early phase of hyperinflammation referred to as the “cytokine storm,” was thought to be responsible for triggering organ injury in sepsis. This hypothesis led to the development of anti-cytokine regimens that target pro-inflammatory cytokines, the therapeutic benefits of which have been demonstrated in animal models. Although such neutralizing strategies against commonly encountered cytokines have been adopted in several clinical trials for sepsis, benefits mirroring those seen in animal models have yet to be demonstrated in humans [4–6]. The failure of anti-cytokine therapies in humans is thought to be the result of a relatively short therapeutic window, which is more easily controlled in animal models [6].
Sepsis mortality is not only seen during the initial hyperinflammatory phase, but also at later time points during a period of prolonged immunosuppression [7, 8]. In fact, Hotchkiss et al. demonstrated that most deaths occur during this period, and reversal of this immune deficiency should generate a better outcome in sepsis [9]. The optimal approach to sepsis treatment remains a subject of heated debate. The concept of developing novel therapeutic targets that not only mitigate hyperinflammatory responses but also reverse prolonged immunosuppression is of great interest.
Sepsis-induced immunoparalysis has long been demonstrated in patients as the result of lymphocyte apoptosis and anergy, characterized by diminished proliferation and an inability to respond to antigens. Additional features of immunosuppression in septic patients include a loss of delayed hypersensitivity, an inability to clear infections, and a predisposition to secondary infections [10, 11]. Furthermore, splenocytes isolated postmortem from septic patients show diminished responses to lipopolysaccharides (LPS)- and anti-CD3/anti-CD28 antibody-stimulation, demonstrated by decreased release of various cytokines, compared to splenocytes isolated from non-septic control specimens [3].
It is well-established that immunosenescence occurs in the elderly population and is associated with a reduction in hematopoietic stem cell activity, leading to an alteration in the components and function of the innate and adaptive immune systems [3]. In fact, epidemiological studies have revealed that severe sepsis and septic shock are principally observed in elderly patients, and nearly 80% of septic deaths occur in patients older than 65 years of age [12]. As the human lifespan continues to increase, effective therapies that reduce morbidity and mortality in elderly patients with sepsis is urgently needed. Therefore, investigation into therapies that may reverse the immunosuppresive state in septic patients is a top priority [13].
Ghrelin (Ghr), an acylated small peptide predominantly produced by the stomach, binds to the growth hormone secretagogue receptor (GHSR)-1a to promote the release of growth hormone [14]. In addition to its endocrine function, Ghr has several non-endocrine activities, including an anti-inflammatory role, which is mediated by GHSR-1a distributed peripherally [15–17]. A growing body of literature describes the status of Ghr expression in rodents and humans during sepsis. While in rodent models of sepsis Ghr expression is downregulated, in humans a biphasic pattern of expression is seen. We previously reported decreased levels of plasma Ghr in rats during early as well as late phase sepsis [18]. Similarly, Ghr expression in the stomach and lungs was also significantly decreased in septic rats compared to sham-operated animals [16, 18]. Interestingly, in rodent models, although plasma levels of Ghr are significantly decreased in early- and late-stage sepsis, compared to septic young animals, Ghr levels in septic aged rats are even more reduced [18, 19].
In humans, bacterial endotoxin challenge results in a biphasic response in Ghr expression, with an increase early after challenge followed by a decline later on [20]. A recent case-control study involving critically ill patients with or without sepsis showed elevated serum concentrations of Ghr in critically ill patients compared to healthy controls, although there was no significant difference between critically ill patients with and without sepsis [21]. Furthermore, it has been demonstrated that high Ghr levels indicate lower mortality in sepsis patients admitted to intensive care units, and are also associated with a reduced need for mechanical ventilation in critically ill patients [21].
In young animals, the Ghr receptor is markedly upregulated in early sepsis and returns to levels similar to sham in later stages of sepsis. However, while Ghr levels are reduced in late sepsis, Ghr receptor levels and their responsiveness to Ghr remain intact. In fact, administration of Ghr has been shown to attenuate inflammation, maintain cardiovascular stability, reduce organ injury and increase survival in young adult septic animals [19]. On the other hand, Ghr receptor expression and plasma Ghr levels are significantly reduced in aged rats compared to young rats after sepsis [19]. Additionally, while Ghr is protective in young septic animals, administration of Ghr alone does not protect aged septic animals. Previous studies found that the combination of Ghr with low-dose growth hormone (GH) significantly increased Ghr receptor expression, markedly enhancing responsiveness to Ghr in aged septic animals. Co-administration of ghrelin and growth hormone reduced organ injury and increased survival in aged septic animals [22]. Since the integrity of immune function plays a crucial role in sepsis-related mortality, especially in the geriatric population, in the current study we further investigate the effect of combined Ghr and GH treatment in modulating immune activity in septic aged animals. We focus on the spleen, one of the largest lymphoid organs in mammals.
Materials and Methods
Experimental animals and sepsis model
Male 24-month-old Fischer rats were obtained from Charles River Laboratories (Wilmington, MA). All animals were housed in a temperature-controlled room under a 12 h light-dark cycle and fed a standard laboratory rat chow diet. Rats were allowed to acclimate to the environment for at least 5 days before being used for experiments. All experiments were performed in accordance with the National Institutes of Health guidelines for the use of experimental animals, and the study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Feinstein Institute for Medical Research.
Sepsis in aged rats was induced by cecal ligation and puncture (CLP) as described previously [22]. Briefly, anesthesia was induced by isoflurane inhalation. The abdomen was then shaved and washed with 10% povidone iodine, and incised with a 2-cm midline laparotomy. The cecum was then exposed and 70% of its length was ligated using 4–0 silk suture distal to the ileocecal valve. The cecum was punctured twice with an 18G needle and returned to the abdominal cavity. The abdominal incision was closed in layers using 4-0 silk suture and the animals were immediately resuscitated with 30 ml/kg body weight (BW) normal saline subcutaneously. Sham-operated rats underwent the same surgical procedure except that the cecum was neither ligated nor punctured.
Administration of human ghrelin and human growth hormone
Human Ghr (Phoenix Pharmaceuticals, Belmont, CA) and human GH (ProSpec, Ness Ziona, Israel) were dissolved in normal saline. A mixture of human Ghr and GH (GG) was prepared by combining them to a concentration of 80 nmol/kg human Ghr and 50 μg/kg human GH. At 5 h after CLP, a single 500 μl bolus dose of GG or vehicle (normal saline) was injected subcutaneously.
Splenocyte isolation and stimulation
Spleens were harvested at 20 h after CLP and homogenized by gentle grinding between frosted glass slides, followed by passage through a 10 ml syringe several times and filtering through a 70 μm nylon mesh to obtain suspended splenocytes (BD Biosciences, San Jose, CA). Red blood cells were lysed using RBC lysing buffer (BD Biosciences). After centrifugation at 280g for 5 min, cell pellets were resuspended in RPMI-1640 medium (Life Technologies, Grand Island, NY) containing 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin,10 mM HEPES and 0.5 μM 2-mercaptaethanol. For the evaluation of immune responses, 2 × 106 cells/well were incubated in a 24-well plate and stimulated with LPS (100 ng/ml, Sigma, St. Louis, MO) for 5 h or anti-rat CD3/anti-rat CD28 antibodies (1 μg/ml each antibody, BioLegend, San Diego, CA) for 20 h.
Measurement of pro-inflammatory cytokines
Cytokine levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit specific for rat TNF-α, IL-6, IL-10 (BD Biosciences) and interferon gamma (INF-γ) (eBioscience, San Diego, CA). All measurements were performed according to manufacturer instructions.
Flow cytometry analysis
Isolated splenocytes were stained with PE/Cy7-labeled anti-rat CD4 (clone: RM4-5) or APC-labeled anti-rat CD8 (clone: 53-6.7) antibodies (Biolegend). Negative controls were incubated with isotype antibodies (Biolegend). To assess apoptotic cell death, cells were first stained with anti-CD4 or anti-CD8 antibodies and fixed with 2% paraformaldehyde; they were then permeabilized with 0.1% Triton X-100 and TUNEL staining was performed using an in situ cell death detection kit (Roche, Indianapolis, IN). In order to measure the immunosuppressive Treg population, cells were stained with PE/Cy7-labeled anti-rat CD4 (clone: RM4-5) antibody followed by intracellular staining with PE-labeled anti-rat FOXP3 (clone: 150D) antibody (Biolegend). All stained cells were analyzed on the LSRFortessa (BD Biosciences). Data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Immunohistochemical analysis
Spleen tissues were fixed in 10% buffered formalin solution and cut into paraffin sections using standard methods. Paraffin slides were dewaxed and rehydrated, followed by antigen retrieval as described previously [19]. Slides were incubated with mouse anti-rat CD4 and anti-rat CD8 (1:50 dilution, Biolegend), rabbit anti-rat programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1; 1:50 dilution, Abcam, Cambridge, MA), rabbit anti-rat cytotoxic T-lymphocyte-associated protein 4 (CTLA4; 1:50 dilution, FabGennix, Frisco, TX), and human leukocyte antigen-antigen D related (HLA-DR; 1:50 dilution, Proteintech, Chicago, IL) antibodies. Normal mouse and rabbit IgG (Vector Labs, Burlingame, CA) were used as negative controls. Slides were washed with Tris-buffered saline (TBS) after primary antibody incubation. Sections were then treated with biotinylated anti-mouse or anti-rabbit IgG followed by Vectastain ABC reagent and DAB substrate reaction (Vector Labs) to reveal positive staining. Slides were counterstained with hematoxylin. No immunostaining was observed on negative control slides.
Western Blot Analysis
Spleen tissues were homogenized and lysed in RIPA buffer (10 mM Tris–HCl pH 7.5, 120 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS) containing a protease inhibitor cocktail (Roche Diagonstics, Indianapolis, IN). Protein concentration was determined by the Bio Rad protein assay reagent. Protein lysates were electrophoresed on SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. The blots were then reacted overnight at 4°C with primary Abs for rabbit anti-rat PD-1 (Abcam) and rabbit anti-rat HLA-DR (Proteintech). After reacting the blots with green fluorochrome-labeled-anti-rabbit secondary Abs proteins were detected by Odyssey system (Li-Cor, Lincoln, NE), followed by the quantitative densitometry analysis using Image J software. The immunoblot was reprobed with anti-β-actin antibodies as loading control.
Statistical analysis
All data are expressed as mean ± SEM and compared by one-way ANOVA and student-Newman-Keuls (SNK) test. Differences in values were considered significant when P < 0.05.
Results
Combined treatment with human Ghr and GH improves the immune response of splenocytes isolated from septic aged rats to LPS
We first examined the innate immune status of aged rats at 20 h after CLP by measuring cytokine production in isolated splenocytes in response to LPS-stimulation. After 5 h of incubation with LPS, levels of TNF-α (Figure 1A), IL-6 (Figure 1B), and IL-10 (Figure 1C) in the medium of cultured splenocytes from vehicle-treated septic aged rats were barely detectable, while levels of these cytokines in the medium of cultured splenocytes from sham rats were 520.4, 196.8, and 292.2 pg/ml, respectively. When septic rats were treated with GG, their isolated splenocytes cultured ex-vivo released TNF-α, IL-6 and IL-10 at levels of 175.3, 78.3, and 188.3 pg/ml, respectively, in response to LPS-stimulation (Figure 1A–C). These results clearly demonstrate that GG treatment restores innate immune function in septic aged rats.
Figure 1.
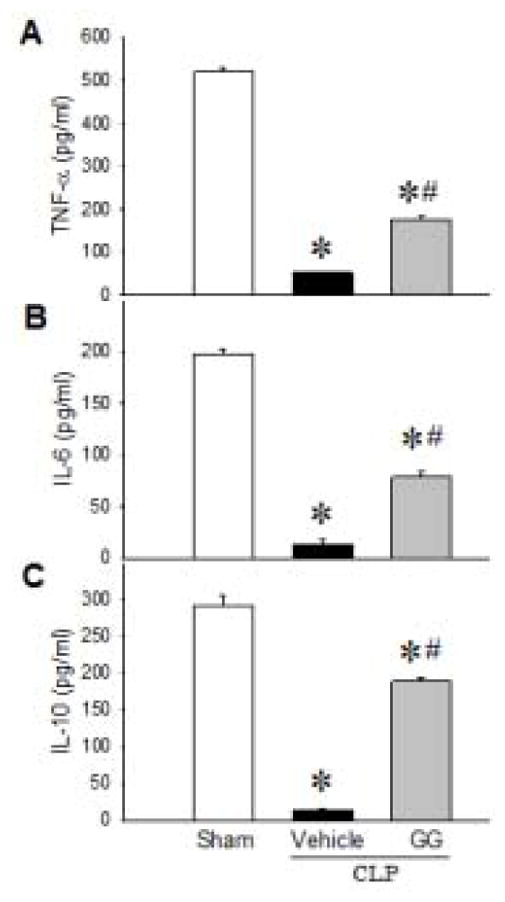
Cytokine secretion from splenocytes of septic aged rats after LPS-stimulation. Aged rats were subjected to sham operation or CLP with vehicle (normal saline) or GG (80 nmol/kg of Ghr and 50 μg/kg GH) treatment at 5 h after CLP. Splenocytes were isolated at 20 h after CLP and further stimulated with LPS (100 ng/ml) for 5 h. Levels of TNF-α (A), IL-6 (B) and IL-10 (C) in the culture supernatant were measured. Data are expressed as mean ± SEM (n = 5–8 per group). *P < 0.05 versus sham and #P < 0.05 versus vehicle-treated septic animals.
Combined treatment with human Ghr and GH improves the immune response of splenocytes isolated from septic aged rats to anti-CD3/anti-CD28 antibodies
We next examined the adaptive immune status of aged rats at 20 h after CLP by measuring cytokine production in isolated splenocytes in response to anti-CD3/anti-CD28 antibody-stimulation. The combination of anti-CD3 and anti-CD28 antibodies partially mimics in vivo T cell stimulation by antigen-presenting cells (APCs) [3, 23].
After 20 h of incubation with anti-CD3 and anti-CD28 antibodies, levels of TNF-α (Figure 2A), IL-6 (Figure 2B), IL-10 (Figure 2C), and INF-γ (Figure 2D) in the medium of cultured splenocytes from vehicle-treated septic aged rats were extremely low, while these levels in the medium of cultured splenocytes from sham rats were 84, 17, 534, and 179 pg/ml, respectively. In contrast, when septic rats were treated with GG, their cultured splenocytes responded to anti-CD3/anti-CD28 antibody-stimulation by releasing TNF-α, IL-6, IL-10, and INF-γ in the medium to levels of 49, 17, 392, and 48 pg/ml, respectively (Figure 2). These results demonstrate that GG treatment restores adaptive immune function in septic aged rats.
Figure 2.
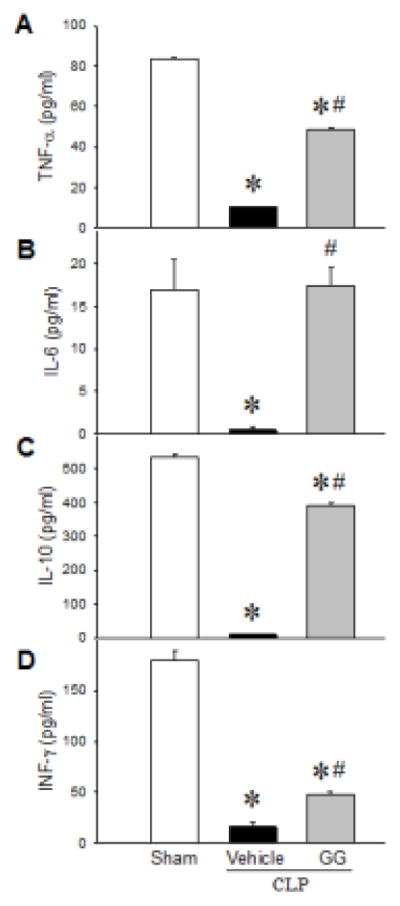
Cytokine secretion from splenocytes of septic aged rats after anti-CD3/anti-CD28-antibody stimulation. Aged rats were subjected to sham operation or CLP with vehicle (normal saline) or GG (80 nmol/kg of Ghr and 50 μg/kg GH) treatment at 5 h after CLP. Splenocytes were isolated at 20 h after CLP and further stimulated with anti-rat CD3 and anti-rat CD28 antibodies (1 μg/ml each) for 20 h. Levels of TNF-α (A), IL-6 (B), IL-10 (C) and INF-γ (D) in the cell culture supernatant were measured. Data are expressed as mean ± SEM (n = 5–8 per group). *P < 0.05 versus sham and #P < 0.05 versus vehicle-treated septic animals.
Combined treatment with human Ghr and GH decreases apoptotic T cells in the spleens of septic aged rats
It has previously been reported that sepsis induces T cell apoptosis [24]. Using immunohistochemistry, decreased CD4 T cell content in the spleens of septic patients has previously been demonstrated [24]. We first examined the populations of CD4+ and CD8+ T cells in the spleens of aged rats at 20 h after CLP using a similar immunohistochemical approach. As expected, the numbers of CD4+ and CD8+ T cells in the spleens of vehicle-treated septic rats were greatly reduced compared to the sham group (Figure 3A). After treatment with GG, the numbers of CD4+ and CD8+ T cells were found to be higher than those in the vehicle-treated septic rats (Figure 3A). Although the immunohistochemical approach is not ideal for generating quantitative data, it does nicely demonstrate the qualitative status of CD4 and CD8 T cell localization, especially in the germinal centers of the spleen.
Figure 3.
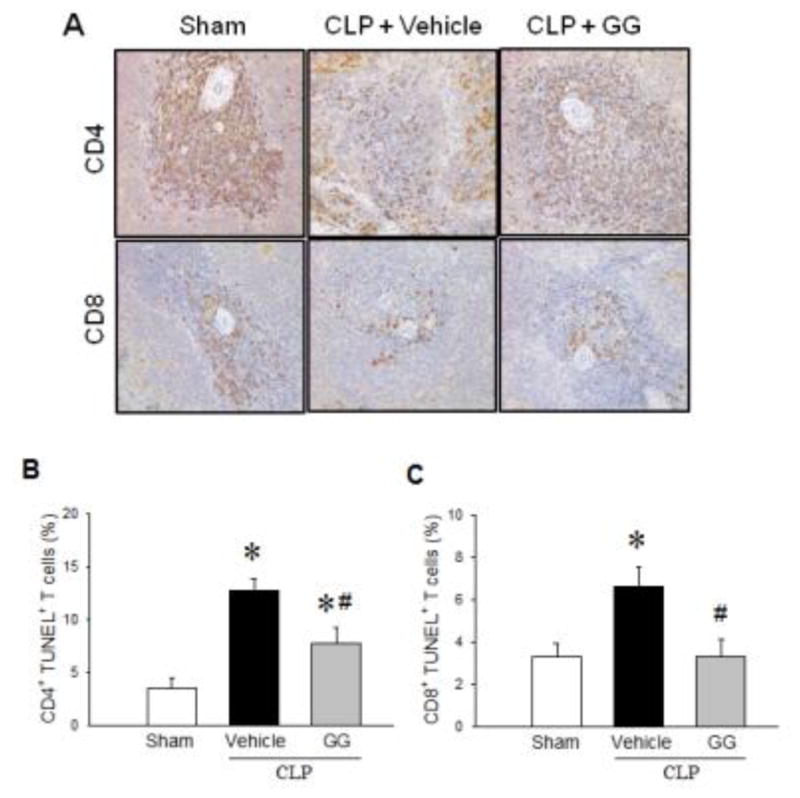
Apoptosis of CD4+ and CD8+ T cells in the spleens of septic aged rats. Aged rats were subjected to sham operation or CLP with vehicle (normal saline) or GG (80 nmol/kg of Ghr and 50 μg/kg GH) treatment at 5 h after CLP. Spleens were harvested at 20 h after CLP. Sections of splenic tissues were immunostained for CD4 and CD8 (A). In another group of animals, isolated splenocytes were stained with PE/Cy7-labeled anti-CD4 or APC-labeled anti-CD8 antibodies followed by FITC-labeled TUNEL reaction and analyzed by flow cytometry (B–C). Isotype-matched antibodies were used as negative controls. Data are expressed as mean ± SEM (n = 5–8 per group). *P < 0.05 versus sham and #P < 0.05 versus vehicle-treated septic animals.
We further confirmed apoptotic cell death of CD4+ and CD8+ splenocytes by TUNEL staining, followed by flow cytometric analysis. The percentages of CD4+TUNEL+ and CD8+TUNEL+ T cells in vehicle-treated septic rats were increased 3.6- and 2.0-fold, respectively, compared to those in the sham group (Figures 3B, C). In contrast, in the GG treatment group, percentages of CD4+TUNEL+ and CD8+TUNEL+ T cells were reduced by 38% and 50%, respectively, compared to those in the vehicle-treated septic rats (Figures 3B, C). These results clearly show that GG treatment prevents apoptotic loss of CD4+ and CD8+ T cells in the spleen of septic aged rats.
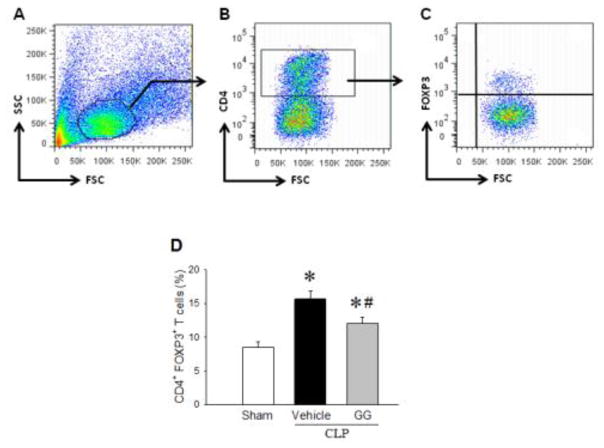
Combined treatment with human Ghr and GH reduces the immune inhibitory Treg population in the spleens of septic aged rats
Treg cells are important inducers of immunosuppression or exhaustion [3, 25]. To determine the Treg population, splenocytes isolated from aged rats at 20 h after CLP were stained with PE/Cy7-labeled anti-CD4 and PE-labeled anti-FOXP3 antibodies, followed by flow cytometric analysis (Figures 4A–C). The frequency of Treg cells in the spleen was increased from 8.5% in the sham to 15.7% in the vehicle-treated septic rats (Figure 4D). In contrast, GG treatment in septic rats significantly reduced the percentage of Treg cells by 23% compared to vehicle treatment (Figure 4D).
Figure 4.
Treg population in the spleens of septic aged rats. Aged rats were subjected sham operation or CLP with vehicle (normal saline) or GG (80 nmol/kg of Ghr and 50 μg/kg GH) treatment at 5 h after CLP. Spleens were harvested at 20 h after CLP. Isolated splenocytes were stained for PE/Cy7-labeled anti-CD4 antibody (A and B), followed by staining with PE-labeled anti-FOXP3 antibody (C). The percentage of Treg cells (CD4+ FOXP3+) was assessed by flow cytometry (D). Data are expressed as mean ± SEM (n = 5–8 per group). *P < 0.05 versus sham and #P < 0.05 versus vehicle-treated septic animals.
Combined treatment with human Ghr and GH downregulates the expression of immune inhibitory molecules in the spleens of septic aged rats
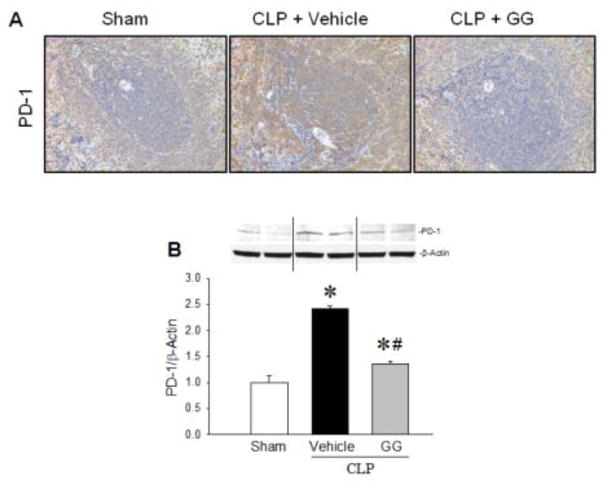
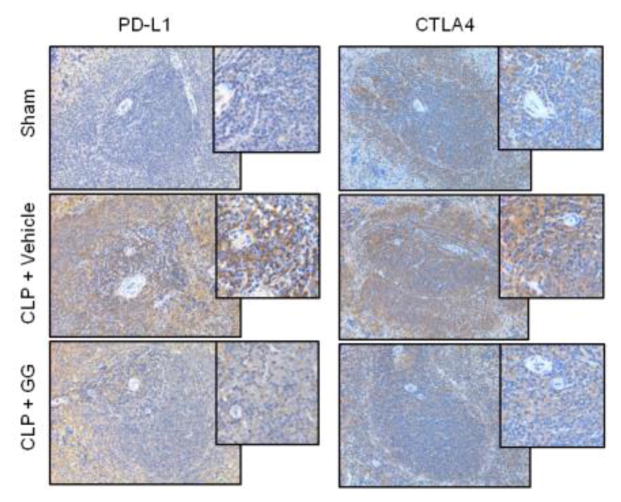
After observing the effect of GG treatment on the immune response in septic aged rats, we examined the expression of several molecules involved in the induction of immunosuppresion in the spleen by immunohistochemistry. PD-1 is an immune checkpoint receptor found primarily on adaptive immune cells [26]. When PD-1 engages its ligand PD-L1, it transmits a signal to inhibit immune responses [27, 28]. Immunostaining demonstrated an increase in the expression of PD-1 in the spleens of vehicle-treated aged rats 20 h after CLP compared to sham-operated rats; however, after administration of GG, expression of PD-1 was decreased in the spleen compared to the vehicle-treated septic rats (Figure 5A). Western blot analysis of the splenic tissues further indicated that protein levels of PD-1 in the vehicle-treated septic rats were significantly increased by 41.8% compared to sham-operated animals (Figure 5B). In contrast, GG treatment significantly reduced the expression of PD-1 by 43.5% compared to vehicle-treated animals (Figure 5B). Similarly, the immunostaining demonstrated an increase in the positive staining of PD-L1 cells in the spleens of vehicle-treated aged rats 20 h after CLP compared to sham-operated rats. Although immunostaining results may have limitations to generate quantitative values, a trend towards increased positive staining in the spleen of vehicle-treated aged septic animal is clearly demonstrated. On the other hand, after administration of GG, expression of PD-L1 was decreased qualitatively in the spleen compared to the vehicle-treated septic rats (Figure 6). Of note, since PD-1 serves as the receptor for PD-L1, significant inhibition in the expression of PD-1 after combined treatment with GG could reverse the immunosuppression phenomenon of T cells during sepsis, even if the expression of PD-L1 does not change dramatically. CTLA4 is another immune checkpoint receptor expressed mainly on the T cell surface [26, 29]. It signals T cells to inhibit their activation [26, 30]. CTLA4 positive staining was also increased in the spleens of vehicle-treated septic aged rats compared to sham-operated rats (Figure 6). After administration of GG, the staining of CTLA4 was decreased in the spleen compared to the vehicle-treated septic rats (Figure 6).
Figure 5.
Expression of PD-1 in the spleens of septic aged rats. Aged rats were subjected to sham operation or CLP with vehicle (normal saline) or GG (80 nmol/kg of Ghr and 50 μg/kg of GH) treatment at 5 h after CLP. Spleens were harvested at 20 h after CLP. Paraffin sections of splenic tissues were immunostained for PD-1 (A). Positively-staining cells appear brown. Representative images of stained spleen sections are shown. Original magnification 200×. PD-1 levels in spleen tissues were measured by Western blotting (B). Data are expressed as mean ± SEM (n = 4–5 per group). *P < 0.05 versus sham and #P < 0.05 versus vehicle-treated septic animals.
Figure 6.
Expression of PD-L1 and CTLA4 in the spleens of septic aged rats. Aged rats were subjected to sham or CLP operation and treated with vehicle (normal saline) or GG (80 nmol/kg of Ghr and 50 μg/kg of GH) 5 h after CLP. Spleens were harvested at 20 h after CLP. Paraffin sections of splenic tissues were immunostained for PD-L1 and CTLA4. Positively-staining cells appear brown. Representative images of stained spleen sections are shown. Original magnification 200× and 400× (Insert).
Combined treatment with human Ghr and GH restores the expression of major histocompatibility complex molecule in the spleens of septic aged rats
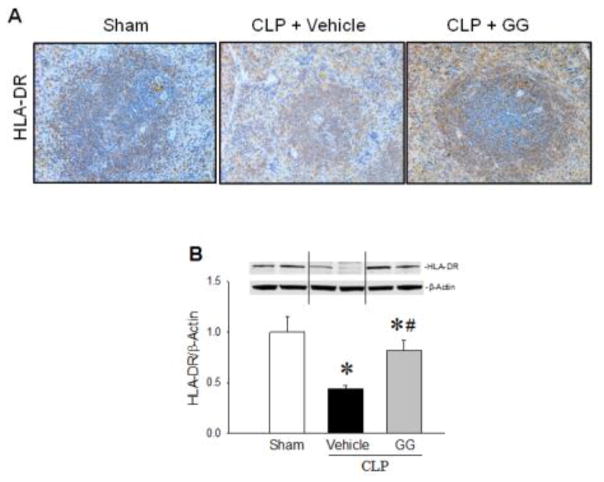
HLA-DR is a major histocompatibility complex (MHC) molecule critical for the initiation of immune responses by APCs [31]. As shown in Figure 7A, immunostaining of HLA-DR was markedly decreased in the spleens of vehicle-treated aged rats at 20 h after CLP compared to sham-operated animals, while it was increased in the GG-treated septic animals, compared to the vehicle-treated septic rats. Western blot analysis of the splenic tissues further indicated that protein levels of HLA-DR in the vehicle-treated septic rats were reduced by 56%, compared to sham-operated animals (Figure 7B). GG treatment significantly restored the expression of HLA-DR to levels comparable to those of sham-operated animals (Figure 7B).
Figure 7.
Expression of HLA-DR in the spleens of septic aged rats. Aged rats subjected to sham operation or CLP with vehicle (normal saline) or GG (80 nmol/kg of Ghr and 50 μg/kg of GH) treatment at 5 h after CLP. Spleens were harvested at 20 h after CLP. Sections of splenic tissues were immunostained for HLA-DR. Positively-staining cells appear brown. Representative images of stained spleen sections are shown (A). In addition, HLA-DR levels in spleen tissues were measured by Western blotting (B). Data are expressed as mean ± SEM (n = 4–5 per group). * P < 0.05 versus sham and #P < 0.05 versus vehicle-treated septic animals.
Discussion
Strong evidence from human subjects indicates that immune dysfunction and subsequent development of immunosuppression contribute to impaired sepsis recovery and sepsis-related mortality [3]. Age-related alterations in immunity secondary to immunosenescence result in a higher risk for the development of immunosuppression in the elderly population after sepsis [32]. Using the clinically-relevant CLP model of polymicrobial sepsis [33]. we have previously reported that combined treatment with human Ghr and GH improves 10-day survival in septic aged rats from 29% to 64% [22]. In this study, we further demonstrated that combined treatment with human Ghr and GH restores the responsiveness of isolated splenocytes to LPS- and anti-CD3/anti-CD28 antibody-stimulation; prevents the loss of splenic CD4+ and CD8+ T cells by apoptosis; reduces the splenic Treg population; inhibits the expression of splenic PD-1; and increases the expression of splenic HLA-DR in septic aged rats. These findings are summarized in Figure 8. These results clearly demonstrate the development of immunosuppression in septic aged rats. Combined treatment with human Ghr and GH has a profound impact on restoring immune function in these animals.
Figure 8.
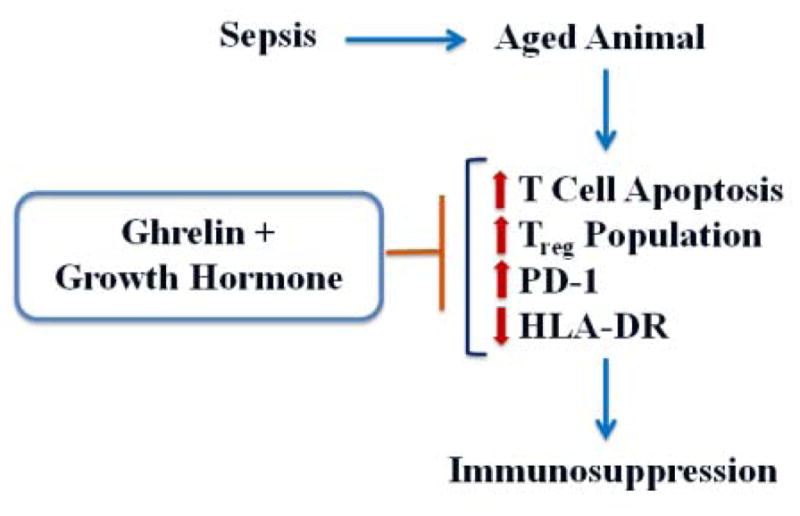
Summary of findings. Immune function is severely impaired in aged animals after sepsis, which is associated with increased T cell apoptosis, elevation in the Treg population, upregulation of the immune inhibitory signal, and downregulation of the immune initiating molecule HLA-DR. Combined treatment with human Ghr and GH reverses these changes in septic aged animals to restore immune function.
Currently there are two models that describe the dynamics between hyperinflammation and immunosuppression during sepsis. Traditional thinking maintains that sepsis is characterized by an initial hyperinflammatory phase, followed by a more protracted immunosuppressive phase [34, 35]. On the other hand, more recent studies from trauma and burn patients suggest that both pro-inflammatory and anti-inflammatory responses occur early and simultaneously in sepsis [35]. In early sepsis, the pro-inflammatory response predominates; as sepsis progresses, the anti-inflammatory response prevails [34]. Early deaths in sepsis may be the result of uncontrolled inflammation, while later deaths may be due to development of immunosuppression and susceptibility to secondary infections [36]. Early diagnosis of sepsis remains a clinical challenge and thus limits the ability to prevent early deaths. Inhibiting immunosuppression appears key to controlling sepsis-related mortality.
We first used ex vivo assays to demonstrate the effectiveness of GG treatment in enhancing cytokine production in splenocytes from septic aged rats after LPS stimulation. LPS, or bacterial endotoxin, can induce an innate immune response through toll-like receptor (TLR) activation and the release of pro-inflammatory cytokines from innate immune cells, including macrophages, dendritic cells, monocytes and natural killer cells [3, 37]. The spleen is the site of many innate and adaptive immune processes and serves a critical role in immune function [38]. Monocytes from septic patients exhibit a diminished capacity to release pro-inflammatory cytokines after LPS challenge [39]. We also demonstrated that GG treatment increases cytokine production in splenocytes from septic aged rats after anti-CD3/anti-CD28 antibody-stimulation. Decreased splenocyte responsiveness to these two stimulations in vehicle-treated septic aged rats clearly demonstrates that the immune cells of these aged animals are in the suppressive stage. Likewise, immune cells in septic patients have also been reported to suffer from anergy and therefore fail to proliferate or secrete cytokines in response to their specific antigens [3, 39]. Furthermore, defective T cell proliferation and cytokine secretion were shown to be correlated with higher mortality in septic patients [40], which corresponds to our previous survival study in septic aged rats [22].
Multiple cellular mechanisms of sepsis-induced immunosuppression have been reported, including apoptotic depletion of innate and adaptive immune cells, activation of immune inhibitory Treg cells and myeloid-derived suppressor cells, and upregulation of immune inhibitory signals [39, 41, 42]. We have observed a marked reduction of CD4+ and CD8+ T cells in the spleens of septic aged rats, accompanied by an increase in the apoptotic T cell population. Autopsies from patients who died of sepsis reveal a profound, progressive apoptosis in the adaptive immune system [3, 24, 43]. We have demonstrated that GG treatment prevents the loss of these immune cells in septic aged rats. Contrary to the apoptosis of CD4+ and CD8+ T cells, we observed an increase in splenic Treg cells in septic aged rats. Treg cells are a subpopulation of T cells that suppresses the responses of other T cell subsets [43]. In septic patients, an increase in the Treg population has also been reported [44]. Such an increase might be due to the loss of effector T cells rather than an absolute increase in Treg numbers in sepsis [44]. In addition, it has been suggested that Treg cells are more resistant to sepsis-induced apoptosis, thereby preventing the recovery of the immune system from anergy [44].
We have demonstrated an upregulation of immune co-inhibitory signals in septic aged rats, including PD-1, PD-L1 and CTLA4. PD-1 and its ligand, PD-L1, negatively control immune responses and are thought to contribute to T cell exhaustion in sepsis [13, 45]. PD-1 is a cell surface receptor expressed on T and B lymphocytes and myeloid cells [26, 46], while PD-L1 is expressed on T cells, epithelial cells, endothelial cells, monocytes, macrophages, and dendritic cells [47]. Patients with severe sepsis show increased levels of PD-1 and PD-L1 on monocyte and T lymphocyte populations, which is associated with increased secondary nosocomial infections and mortality [45]. In addition, CTLA4 is a regulatory T cell molecule and it has been shown to be elevated in sepsis [3, 29, 30].
In contrast, we have shown that the expression of HLA-DR is markedly decreased in septic aged rats. HLA-DR is normally expressesed on APCs, such as dendritic cells, monocytes and phagocytes [31]. HLA-DR triggers an immune response after binding pathogens [31]. The reduction of HLA-DR expression in monocytes has been reported in septic patients [48], which correlates with an increased risk of infectious complications and death, and serves as a surrogate marker of monocyte anergy [39, 49]. By demonstrating the effect of GG treatment on the expression of regulatory immune molecules in septic aged rats, we provide further evidence to demonstrate that this treatment can modulate sepsis-induced immunosuppression to protect these animals from septic death.
The effect of Ghr on immune function may be mediated through activation of the central vagal pathway or direct activity on immune cells. Ghr has been shown to have anti-inflammatory activity to attenuate the severity of sepsis in animals through stimulation of the vagus nerve [19, 50]. Lymphoid organs like the spleen can receive extensive autonomic innervations [51], supporting the notion that involvement of the central nervous system is critical in mediating the observed effects of Ghr. In addition, Ghr can pass through the blood brain barrier and bind to GHSR-1a expressed in the hypothalamus and brain stem [19, 52]. GHSR-1a can be detected on human lymphocytes, T cell subpopulations and monocytes [53, 54]. Ghr can redirect leptin-induced T cell differentiation from a predominantly Th1 to a Th2 profile, leading to the production of anti-inflammatory cytokines, an attenuated Th1-type response in Th1-driven autoimmune disease, and enhanced proliferation of murine T cells [17, 53, 55]. This evidence suggests that Ghr may directly regulate immune cells in septic aged rats. The detailed downstream pathway of Ghr in modulating immune function in sepsis warrants further investigation.
Immunotherapy has recently been suggested as a potential therapeutic approach in sepsis via inhibition of sepsis-induced immunosuppression [10, 39, 56]. In pre-clinical studies, immuno-adjuvant therapy with anti-PD-1, anti-PD-L1 and anti-CTLA-4 antibodies reversed sepsis-induced immunosuppression and improved survival in fungal and bacterial sepsis [57, 58]. Likewise, a recent case report of a patient with bacterial sepsis that was unresponsive to antibiotics demonstrated that IFN-γ treatment improved outcomes by increasing HLA-DR expression on monocytes and shifting from a Th2 to a predominatly Th1/Th17 phenotype, with clinical resolution of sepsis [59]. Here, we demonstrate that combined Ghr and GH treatment can modulate immune function in septic aged rats, which provides promising evidence of its therapeutic potential. Human Ghr has been administered to human subjects in more than 100 clinical studies covering various disease conditions and has demonstrated a very good safety profile [60]. Human GH has been approved by the Food and Drug Administration for the treatment of short stature due to GH deficiency in the pediatric population, chronic renal insufficiency, short bowel syndrome in adults, and AIDS-associated cachexia. Although administration of high-dose GH has been associated with increased mortality in patients with prolonged critical illness [61], a very low-dose GH was used in our combined treatment as a Ghr-sensitizing agent, with beneficial effects demonstrated in septic aged animals [19, 22].
In the present study, we have chosen our model of polymicrobial sepsis in aged rats based on our previous publications in sepsis models utilizing young and aged rats [16, 22, 62]. According to several relevant studies, our current model of sepsis is shown to be associated with an early, hyperdynamic phase (2–10 h after CLP), which is followed by a late, hypodynamic phase (16 h after CLP and later) [16, 63]. Our present sepsis model in aged rats has an expected mortality of 10% at day 1 and 50% at day 2, as demonstrated in our previous study in which the same model was used [22]. Since these rats start to die at day one after sepsis induction, and our current data demonstrates considerable death of CD4 and CD8 T cells in the spleen during this period, we may assume that the 20 h time point is appropriate to capture animals in the immunosuppressive stage. Furthermore, future study using a later time point after CLP when more animals will be likely to begin dying due to immunosuppression would be of great interest to show the effect of combined treatment of human Ghr and GH to improve the status of systemic T cells in aged septic rats.
In conclusion, immune function is severely impaired in septic aged rats. Co-administration of human Ghr and GH restores immune activity, which is associated with a reduction in organ injury and improvement in survival. Combined treatment with human Ghr and GH is a promising immunomodulatory therapy for elderly patients with sepsis.
Supplementary Material
Highlights.
Development of immunosuppression in sepsis contributes to morbidity and mortality.
Treatment with Ghr and GH prevents the loss of splenic T cells in septic aged rats.
Ghr and GH reduce splenic Treg and co-inhibitory factors in septic aged rats
Ghr and GH treatment reverses sepsis-induced immunosuppression.
Acknowledgments
We thank Dr. Alexandra C. Bolognese for critically reviewing and editing our manuscript.
Grant support
This study was supported by the National Institutes of Health (NIH) grant R35GM118337 to PW.
Footnotes
Authorship
MZ, W-LY, PW conceived the project. MZ, MA, W-LY designed the experiments. MZ, GM performed the experiments. MZ analyzed the data and wrote the initial manuscript. MZ, MA revised the manuscript. MZ, MA, PW reviewed and edited the manuscript. PW generated the idea and supervised the whole project. All authors read and approved the final manuscript.
Conflict of interest
PW is the inventor of the United States Patent No. US 8,324,151 B2: “Treatment of sepsis and septic shock using ghrelin and growth hormone.” TheraSource, LLC holds the exclusive option to license the technology from the Feinstein Institute for Medical Research. PW is a cofounder of TheraSource, LLC. Other authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gustot T. Multiple organ failure in sepsis: prognosis and role of systemic inflammatory response. Curr Opin Crit Care. 2011;17:153–159. doi: 10.1097/MCC.0b013e328344b446. [DOI] [PubMed] [Google Scholar]
- 2.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 3.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, 2nd, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinarello CA. Anti-cytokine therapies in response to systemic infection. J Investig Dermatol Symp Proc. 2001;6:244–250. doi: 10.1046/j.0022-202x.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- 5.Minnich DJ, Moldawer LL. Anti-cytokine and anti-inflammatory therapies for the treatment of severe sepsis: progress and pitfalls. Proc Nutr Soc. 2004;63:437–441. doi: 10.1079/pns2004378. [DOI] [PubMed] [Google Scholar]
- 6.Dinarello CA, Abraham E. Does blocking cytokines in sepsis work? Am J Respir Crit Care Med. 2002;166:1156–1157. doi: 10.1164/rccm.2208006. [DOI] [PubMed] [Google Scholar]
- 7.Vincent JL, Abraham E, Annane D, Bernard G, Rivers E, Van den Berghe G. Reducing mortality in sepsis: new directions. Crit Care. 2002;6(Suppl 3):S1–18. doi: 10.1186/cc1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien JM, Abraham E. New approaches to the treatment of sepsis. Clin Chest Med. 2003;24:521–548. doi: 10.1016/s0272-5231(03)00102-3. [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 12.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 13.Bermejo-Martin JF, Andaluz-Ojeda D, Almansa R, Gandía F, Gómez-Herreras JI, Gomez-Sanchez E, Heredia-Rodriguez M, Eiros JM, Kelvin DJ, Tamayo E. Defining immunological dysfunction in sepsis: A requisite tool for precision medicine. J Infect. 2016;72:525–536. doi: 10.1016/j.jinf.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Arvat E, Di Vito L, Broglio F, Papotti M, Muccioli G, Dieguez C, Casanueva FF, Deghenghi R, Camanni F, Ghigo E. Preliminary evidence that Ghrelin, the natural GH secretagogue (GHS)-receptor ligand, strongly stimulates GH secretion in humans. J Endocrinol Invest. 2000;23:493–495. doi: 10.1007/BF03343763. [DOI] [PubMed] [Google Scholar]
- 15.Wu JT, Kral JG. Ghrelin: integrative neuroendocrine peptide in health and disease. Ann Surg. 2004;239:464–474. doi: 10.1097/01.sla.0000118561.54919.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu R, Dong W, Zhou M, Zhang F, Marini CP, Ravikumar TS, Wang P. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am J Respir Crit Care Med. 2007;176:805–813. doi: 10.1164/rccm.200604-511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130:1707–1720. doi: 10.1053/j.gastro.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 18.Wu R, Zhou M, Cui X, Simms HH, Wang P. Upregulation of cardiovascular ghrelin receptor occurs in the hyperdynamic phase of sepsis. Am J Physiol Heart Circ Physiol. 2004;287:H1296–H1302. doi: 10.1152/ajpheart.00852.2003. [DOI] [PubMed] [Google Scholar]
- 19.Wu R, Zhou M, Dong W, Ji Y, Miksa M, Marini CP, Ravikumar TS, Wang P. Ghrelin hyporesponsiveness contributes to age-related hyperinflammation in septic shock. Ann Surg. 2009;250:126–133. doi: 10.1097/SLA.0b013e3181ad85d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vila G, Maier C, Riedl M, Nowotny P, Ludvik B, Luger A, Clodi M. Bacterial endotoxin induces biphasic changes in plasma ghrelin in healthy humans. J Clin Endocrinol Metab. 2007;92:3930–3934. doi: 10.1210/jc.2007-1194. [DOI] [PubMed] [Google Scholar]
- 21.Koch A, Sanson E, Helm A, Voigt S, Trautwein C, Tacke F. Regulation and prognostic relevance of serum ghrelin concentrations in critical illness and sepsis. Crit Care. 2010;14:R94. doi: 10.1186/cc9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang WL, Ma G, Zhou M, Aziz M, Yen HT, Mavropoulos S, Ojamaa K, Wang P. Combined Administration of Human Ghrelin and Human Growth Hormone Attenuates Organ Injury and Improves Survival in Aged Septic Rats. Mol Med. 2016;22:124–135. doi: 10.2119/molmed.2015.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trickett A, Kwan YL. T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods. 2003;275:251–255. doi: 10.1016/s0022-1759(03)00010-3. [DOI] [PubMed] [Google Scholar]
- 24.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 25.Penaloza-MacMaster P, Kamphorst AO, Wieland A, Araki K, Iyer SS, West EE, O'Mara L, Yang S, Konieczny BT, Sharpe AH, Freeman GJ, Rudensky AY, Ahmed R. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J Exp Med. 2014;211:1905–1918. doi: 10.1084/jem.20132577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Osta H, Shahid K, Mills GM, Peddi P. Immune checkpoint inhibitors: the new frontier in non-small-cell lung cancer treatment. Onco Targets Ther. 2016;9:5101–5116. doi: 10.2147/OTT.S111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR, Chaudhary D. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 28.Velu V, Shetty RD, Larsson M, Shankar EM. Role of PD-1 co-inhibitory pathway in HIV infection and potential therapeutic options. Retrovirology. 2015;12:14. doi: 10.1186/s12977-015-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue S, Bo L, Bian J, Unsinger J, Chang K, Hotchkiss RS. Dose-dependent effect of anti-CTLA-4 on survival in sepsis. Shock. 2011;36:38–44. doi: 10.1097/SHK.0b013e3182168cce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santarpia M, González-Cao M, Viteri S, Karachaliou N, Altavilla G, Rosell R. Programmed cell death protein-1/programmed cell death ligand-1 pathway inhibition and predictive biomarkers: understanding transforming growth factor-beta role. Transl Lung Cancer Res. 2015;4:728–742. doi: 10.3978/j.issn.2218-6751.2015.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsushima GK, Itoh-Lindstrom Y, Ting JP. Activation of the HLA-DRA gene in primary human T lymphocytes: novel usage of TATA and the X and Y promoter elements. Mol Cell Biol. 1992;12:5610–5619. doi: 10.1128/mcb.12.12.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rimmelé T, Payen D, Cantaluppi V, Marshall J, Gomez H, Gomez A, Murray P, Kellum JA, Workgroup AX. Immune cell phenotype and function in sepsis. Shock. 2016;45:282–291. doi: 10.1097/SHK.0000000000000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG. Inflammation, P. Host Response to Injury Large-Scale Collaborative Research, A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imler JL, Hoffmann JA. Toll receptors in innate immunity. Trends Cell Biol. 2001;11:304–311. doi: 10.1016/s0962-8924(01)02004-9. [DOI] [PubMed] [Google Scholar]
- 38.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 39.Delano MJ, Ward PA. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest. 2016;126:23–31. doi: 10.1172/JCI82224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heidecke CD, Hensler T, Weighardt H, Zantl N, Wagner H, Siewert JR, Holzmann B. Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. Am J Surg. 1999;178:288–292. doi: 10.1016/s0002-9610(99)00183-x. [DOI] [PubMed] [Google Scholar]
- 41.Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 42.Ni Choileain N, MacConmara M, Zang Y, Murphy TJ, Mannick JA, Lederer JA. Enhanced regulatory T cell activity is an element of the host response to injury. J Immunol. 2006;176:225–236. doi: 10.4049/jimmunol.176.1.225. [DOI] [PubMed] [Google Scholar]
- 43.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 44.Venet F, Chung CS, Kherouf H, Geeraert A, Malcus C, Poitevin F, Bohe J, Lepape A, Ayala A, Monneret G. Increased circulating regulatory T cells (CD4(+)CD25 (+)CD127 (-)) contribute to lymphocyte anergy in septic shock patients. Intensive Care Med. 2009;35:678–686. doi: 10.1007/s00134-008-1337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Li J, Lou J, Zhou Y, Bo L, Zhu J, Zhu K, Wan X, Cai Z, Deng X. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit Care. 2011;15:R70. doi: 10.1186/cc10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad Sci. 2011;1217:45–59. doi: 10.1111/j.1749-6632.2010.05919.x. [DOI] [PubMed] [Google Scholar]
- 47.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lukaszewicz AC, Grienay M, Resche-Rigon M, Pirracchio R, Faivre V, Boval B, Payen D. Monocytic HLA-DR expression in intensive care patients: interest for prognosis and secondary infection prediction. Crit Care Med. 2009;37:2746–2752. doi: 10.1097/CCM.0b013e3181ab858a. [DOI] [PubMed] [Google Scholar]
- 49.Venet F, Tissot S, Debard AL, Faudot C, Crampé C, Pachot A, Ayala A, Monneret G. Decreased monocyte human leukocyte antigen-DR expression after severe burn injury: Correlation with severity and secondary septic shock. Crit Care Med. 2007;35:1910–1917. doi: 10.1097/01.CCM.0000275271.77350.B6. [DOI] [PubMed] [Google Scholar]
- 50.Wu R, Dong W, Cui X, Zhou M, Simms HH, Ravikumar TS, Wang P. Ghrelin down-regulates proinflammatory cytokines in sepsis through activation of the vagus nerve. Ann Surg. 2007;245:480–486. doi: 10.1097/01.sla.0000251614.42290.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buijs RM, van der Vliet J, Garidou ML, Huitinga I, Escobar C. Spleen vagal denervation inhibits the production of antibodies to circulating antigens. PLoS One. 2008;3:e3152. doi: 10.1371/journal.pone.0003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 53.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW, Jr, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bishop NC, Hayashida H, Clark M, Coombs C, Miller S, Stensel DJ. Effect of acute and regular exercise on growth hormone secretagogue receptor-1a expression in human lymphocytes, T cell subpopulation and monocytes. Brain Behav Immun. 2014;39:172–179. doi: 10.1016/j.bbi.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 55.Lee JH, Patel K, Tae HJ, Lustig A, Kim JW, Mattson MP, Taub DD. Ghrelin augments murine T-cell proliferation by activation of the phosphatidylinositol-3-kinase, extracellular signal-regulated kinase and protein kinase C signaling pathways. FEBS Lett. 2014;588:4708–4719. doi: 10.1016/j.febslet.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patil NK, Bohannon JK, Sherwood ER. Immunotherapy: A promising approach to reverse sepsis-induced immunosuppression. Pharmacol Res. 2016;111:688–702. doi: 10.1016/j.phrs.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang KC, Burnham CA, Compton SM, Rasche DP, Mazuski RJ, McDonough JS, Unsinger J, Korman AJ, Green JM, Hotchkiss RS. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care. 2013;17:R85. doi: 10.1186/cc12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nalos M, Santner-Nanan B, Parnell G, Tang B, McLean AS, Nanan R. Immune effects of interferon gamma in persistent staphylococcal sepsis. Am J Respir Crit Care Med. 2012;185:110–112. doi: 10.1164/ajrccm.185.1.110. [DOI] [PubMed] [Google Scholar]
- 60.Narula T, deBoisblanc BP. Ghrelin in Critical Illness. Am J Respir Cell Mol Biol. 2015;53:437–442. doi: 10.1165/rcmb.2014-0226TR. [DOI] [PubMed] [Google Scholar]
- 61.Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341:785–792. doi: 10.1056/NEJM199909093411102. [DOI] [PubMed] [Google Scholar]
- 62.Wu R, Chaung WW, Dong W, Ji Y, Barrera R, Nicastro J, Molmenti EP, Coppa GF, Wang P. Ghrelin maintains the cardiovascular stability in severe sepsis. J Surg Res. 2012;178:370–377. doi: 10.1016/j.jss.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang P, Chaudry IH. Mechanism of hepatocellular dysfunction during hyperdynamic sepsis. Am J Physiol. 1996;270:R927–R938. doi: 10.1152/ajpregu.1996.270.5.R927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.