Abstract
A close association of systemic inflammation with cardiovascular diseases and metabolic syndrome is recently a popular topic in medicine. Psoriasis is a chronic inflammatory skin disease with a prevalence of approximately 0.1-0.5% in Asians. It is characterized by widespread scaly erythematous macules that cause significant physical and psychological burdens for the affected individuals. The accelerated inflammation driven by the TNF-α/IL-23/IL-17A axis is now known to be the major mechanism in the development of psoriasis. Psoriasis is not a mere skin disease; it is significantly associated with cardiovascular diseases and metabolic syndrome, which suggests that the chronic skin inflammation extends the systemic inflammation beyond the skin. In this article, we review the epidemiological and pathological aspects of psoriasis and its comorbidities.
Keywords: psoriasis, cardiovascular disease, metabolic syndrome, tumor necrosis factor α, interleukin 23, interleukin 17
Introduction
The close association of systemic inflammation with cardiovascular diseases (CVD) and metabolic syndrome is a recent topic in medicine (1-3). Inflammatory skin diseases such as psoriasis are an integral part of diseases causing systemic inflammation (4). Psoriasis is a common immune-mediated chronic inflammatory skin disease that is characterized by the altered proliferation and differentiation of keratinocytes, vascular remodeling and inflammation in the skin (Figure). Psoriasis shows a diverse prevalence across populations worldwide: 2.5% in Europeans, 0.05-3% in Africans and 0.1-0.5% in Asians (5-8). Psoriasis patients comprise 4.43% of all Japanese dermatological patients (8). Men are likely to experience a higher severity of the disease than women are. Phenotypic heterogeneity of psoriasis has also been reported among ethnic populations. Small plaque psoriasis is specific to Asian populations, while severe psoriasis is more predominant in Western populations (5-8). Recent genome-wide association studies have identified numerous risk-associated variants within 44 susceptibility loci for psoriasis, including HLA-C*06:02, LCE3D, IL23R and CARD14. HLA-C*12:02 may be a susceptibility locus for late-onset psoriasis in the Japanese population (5, 9-11). These susceptibility genes are predominantly related to the innate and adaptive immune systems and skin barrier functions (5, 9).
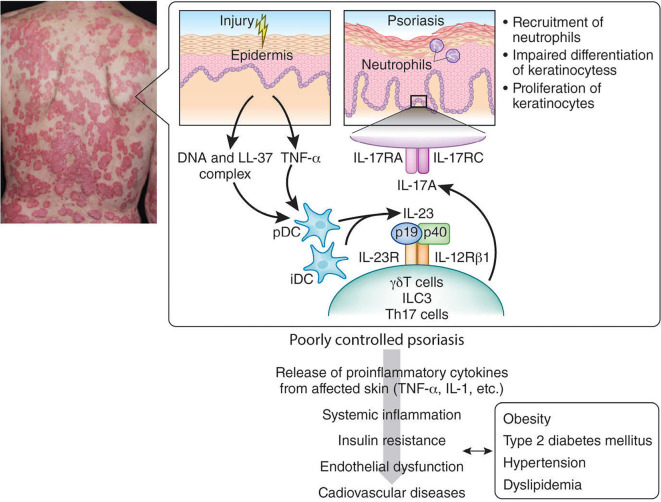
Figure.
Pathophysiology of psoriasis and its comorbidities (simplified model modified from references 5, 8 and 67). The initial trigger of psoriasis is thought to be the activation of plasmacytoid dendritic cells (DCs) by complexes of host DNA and the antimicrobial peptide LL-37 (cathelicidin) produced by keratinocytes after minor injuries. Plasmacytoids and recruited inflammatory DCs (pDCs and iDCs) produce TNF-α, IL-12 and IL-23. IL-23 is critically involved in the generation and activation of IL-17-producing effector cells. In humans and experimental psoriasis models, γδT cells (Vγ9Vδ2 T cells in human), innate lymphoid cells type 3 (ILC3) and Th17 cells are detected in the lesional skin and blood, and these cells readily produce IL-17A. IL-17A binds to the IL-17 receptor (IL-17R), which is composed of IL-17RA and IL-17RC. IL-17A upregulates the proliferation and downregulates the differentiation of keratinocytes and also enhances the recruitment of neutrophils and aids in the crosstalk between neutrophils and keratinocytes. Chronically released proinflammatory cytokines (e.g., TNF-α, IL-1) from poorly controlled psoriatic skin to the circulatory system potentiates and perpetuates the systemic inflammation and induces insulin resistance, endothelial dysfunction and cardiovascular diseases. This systemic inflammation also causes obesity, hypertension, dyslipidemia and type 2 diabetes mellitus. These systemic involvements interact with each other to increase the mortality for psoriatic patients from cardiovascular diseases.
Pathogenesis of Psoriasis
Like other forms of systemic inflammation, tumor necrosis factor (TNF)-α is a key pro-inflammatory cytokine in psoriasis because its inhibitor significantly improves coronary microvascular dysfunction and levels of highly sensitive C-reactive protein, as well as ameliorates psoriatic symptoms (12). In addition, recent therapeutic success with other biologics seems to have revealed the pivotal role of the TNF-α/interleukin (IL)-23/IL-17 axis in the pathogenesis of psoriasis (Figure). The initial trigger of psoriasis is thought to be the activation of plasmacytoid dendritic cells (DCs) being stimulated by complexes of host DNA and the antimicrobial peptide LL-37 (cathelicidin), which are produced by keratinocytes after minor injuries (8, 13-15). Activated plasmacytoid DCs and damaged keratinocytes produce interferon (IFN)-α and TNF-α, which results in the further production of TNF-α, IL-12 and IL-23 by plasmacytoids and recruited inflammatory DCs (8, 14, 15) (Figure). IL-12 promotes the differentiation of naïve CD4+ T cells into IFN-γ-producing T helper (Th) 1 cells. IL-23 is critically involved in the generation and activation of IL-17-producing effector cells (13, 15, 16) (Figure). In humans and experimental models of psoriasis, γδT cells (Vγ9Vδ2 T cells in human), innate lymphoid cells type 3 (ILC3) and Th17 cells are detected in blood and lesional skin. These cells readily produce IL-17A and IL-22 (8, 17, 18). IL-17A binds to the IL-17 receptor (IL-17R), which is composed of IL-17RA and IL-17RC (19) (Figure). IL-17A upregulates the proliferation of keratinocytes and downregulates its differentiation (20) and also enhances the recruitment of neutrophils and aids in the crosstalk between neutrophils and keratinocytes by upregulating the neutrophil-attractive chemokines CXCL8 and CXCL1 (21). IL-17A promotes the expression of TNF-α by keratinocytes (19), which indicates that the above-mentioned TNF-α/IL-23/IL-17 axis likely forms a vicious loop in the development of psoriasis lesions (Figure). The mechanistic hypothesis coincides with the fact that antibodies against TNF-α, IL-23(p19), IL-23(p40), IL-23R, IL-17A or IL-17RA exert remarkable clinical effects on psoriasis both in Caucasian and Asian ethnicities (8, 22-31). In contrast, the decisive pathogenic roles of IL-12, IFN-γ and IL-22 in psoriasis remain elusive, as clinical trials of the anti-IL-12 p35-p40 antibody (SMART), anti-IFN-γ antibody (HuZAF) and anti-IL-22 antibody (fezakinumab) have been discontinued (8).
High Clinical Burden in Psoriasis
Both the physical and psychological quality of life are significantly impaired in all populations of psoriasis sufferers (32-35), and patients' adherence to medication regimens and satisfaction with their results are very poor (36). The disease burden of psoriasis is further increased by its association with psoriatic arthritis, which is characterized by seronegative spondyloarthropathies, enthesitis and elevated C-reactive protein levels (5, 37-39). Approximately 15-30% of Caucasians with psoriasis eventually develop psoriatic arthritis (37). Although the prevalence is lower in Asian populations (Chinese 5.3-7.3%, Japanese 10.5% and Korean 11.2%), psoriatic arthritis significantly hampers patients' daily lives (37, 40). Additionally, psoriasis has significant comorbidity with CVD and metabolic syndrome.
Comorbidity with Cardiovascular Diseases
A recent meta-analysis and systematic review of 14 cohorts identified a CVD risk in individuals with severe psoriasis (defined as requiring systemic therapy or hospital admission): the risk ratio relative to the general population was 1.37 [95% confidence interval (CI): 1.17-1.60] for CVD mortality, 3.04 (95% CI: 0.65-14.35) for myocardial infarction and 1.59 (95% CI: 1.34-1.89) for stroke (41) (Table 1). The relative risks of CVD were highest in younger, more severe psoriasis patients [3.10 (95% CI: 1.98-4.86) for myocardial infarction at 30 years of age], and the absolute risks were greatest in older individuals with severe psoriasis (23.2 excess myocardial infarctions per 10,000 person-years at 60 years of age) (41). A German cross-sectional study that included 4,185 patients with psoriasis and a prospective cohort of German Health Insurance beneficiaries (n=1,811,098) showed that psoriasis was significantly associated with myocardial infarction (odds ratio: 2.26; 95% CI: 1.03-4.96) (42). In a longitudinal study, psoriasis slightly increased the incident risk for myocardial infarction (relative risk: 1.14; 95% CI: 1.06-1.22), with the highest risk increments found in systemically treated psoriasis, which accounted for 17 excess cases of myocardial infarction per 10,000 person-years (42). A significantly higher risk of myocardial infarction has been observed in psoriasis patients in the United Kingdom (43). For example, for a 30-year-old patient with mild or severe psoriasis, the adjusted relative risk of having a myocardial infarction is 1.29 (95% CI: 1.14-1.46) or 3.10 (95% CI: 1.98-4.86), respectively, compared with the control population (43). Furthermore, patients with severe psoriasis have an increased risk of CVD mortality that is independent of traditional CVD risk factors (44, 45). In a prospective cohort study that included 48,523 patients with psoriasis and 208,187 controls in the United Kingdom, 1,257 patients with psoriasis (2.59%) had a major CVD, compared with 4,784 controls (2.30%), during a median follow-up of 5.2 years (46). A multivariable analysis showed that the patients with psoriasis had significantly higher incidence of CVD than did the controls, with a hazard ratio (HR) 1.37 (95% CI: 1.29-1.45) for hypertension, HR 2.74 (95% CI: 2.41-3.12) for transient ischemic attack, HR 1.54 (95% CI: 1.36-1.73) for atrial fibrillation, HR 1.23 (95% CI: 1.05-1.44) for valvular heart disease, HR 1.32 (95% CI: 1.17-1.49) for thromboembolism and HR 1.57 (95% CI: 1.39-1.78) for congestive heart failure. The age- and gender-adjusted HRs of a major CVD for psoriasis were 1.10 (95% CI: 1.04-1.17) and 1.40 (95% CI: 1.07-1.84) for severe psoriasis (46).
Table 1.
Comorbidity of Cardiovascular Diseases in Psoriasis.
| References | Subjects | Brief description |
|---|---|---|
| Ref.41 | Meta-analysis of 14 cohorts | Risk ratio relative to general population |
| Cardiovascular disease mortality; 1.37 (95% CI 1.17-1.60) | ||
| Myocardial infarction; 3.04 (95% CI 0.65-14.35) | ||
| Stroke; 1.59 (95% CI 1.34-1.89) | ||
| Ref.42 | German cross-sectional study (n=4,185) | Adjusted odds ratio |
| German Health Insurance beneficiaries (n=1,811,098) | Myocardial infarction; 2.26 (95% CI 1.03-4.96) | |
| Ref.43 | Control patients (n=556,995) | Adjusted relative risk in a 30-year old patient |
| Mild psoriasis (n=127,139) | Myocardial infarction in mild psoriasis; 1.29 (95% CI 1.14-1.46) | |
| Severe psoriasis (n=3,837) | Myocardial infarction in severe psoriasis; 3.10 (95% CI 1.98-4.86) | |
| Ref.44 | Control patients (n=14,330) | Adjusted relative risk in a 40-year old patient |
| Severe psoriasis (n=3,603) | Cardiovascular mortality in severe psoriasis; 2.69 (95% CI 1.45-4.99) | |
| Ref.46 | Prospective study for 5.2 years follow-up | Fully adjusted hazard ratio |
| Control patients (n=208,187) | Cardiovascular events; 1.02 (95% CI 0.95-1.08) | |
| Psoriasis (n=48,523) | ||
| Ref.47 | Hospital and clinic patients (n=113,065) | Adjusted odds ratio |
| Coronary heart disease; 1.27 (95% CI 1.01-1.58) | ||
| Hypertension; 7.78 (95% CI 7.25-8.36) | ||
| Ref.61 | Control patients (n=24,285) | Adjusted odds ratio |
| Psoriasis (n=12,502) | Hypertension; 1.37 (95% CI 1.29-1.46) |
A hospital-based study conducted by Shiba et al. in Japan analyzed the prevalence of coronary heart disease (n=5,167, 4.5%), hypertension (n=16,476, 14.5%), dyslipidemia (n=9,236, 8.1%), diabetes mellitus (n=11,555, 10.2%) and psoriasis (n=1,197, 1.1%) in 113,065 patients (47). Their multivariate analysis showed that psoriasis was an independent variable associated with coronary heart disease (adjusted odds ratio: 1.27; 95% CI: 1.01-1.58; p=0.0404), hypertension (7.78; 95% CI: 7.25-8.36; p<0.0001), dyslipidemia (2.35; 95% CI: 2.19-2.52; p<0.0001) and diabetes (2.86; 95% CI: 2.67-3.06; p<0.0001) (47).
Comorbidity with Metabolic Syndrome
The association of psoriasis with metabolic syndrome (obesity, glucose intolerance, hypertension and dyslipidemia) has also been well documented (48, 49). As Henseler and Christophers reported in an earlier study (50), obesity and type 2 diabetes mellitus are significantly associated with psoriasis in the United Kingdom (51), Israel (52, 53), Italy (54), Germany (55) and Denmark (56) (Table 2). These results have been consistent with those for psoriasis patients in Japan (57), Korea (58) and Thailand (59). Psoriasis is also comorbid with other components of metabolic syndrome, such as dyslipidemia (51, 52, 60) and hypertension (51, 52, 55, 61). Furthermore, a significant association of psoriasis with dyslipidemia (58) and hypertension (59) has been reported in Asian populations. Moreover, patients with severe psoriasis had a higher prevalence of diabetes (51) and obesity (51) than did those with mild psoriasis. In this context, it is intriguing that dipeptidyl peptidase-4 inhibitors, a new class of antidiabetic agents, have been shown to improve skin symptoms in some psoriasis patients (62). Because topical vitamin D3 application is effective in treating psoriasis (63), the vitamin D3 level and bone mineral density have been assessed (64, 65). In those studies, the serum level of vitamin 25(OH)D3 was inversely correlated with the severity of psoriasis (64). Patients with a low bone mineral density showed a statistically significant longer average duration of psoriasis than those with better bone density (65).
Table 2.
Comorbidity of Metabolic Syndrome in Psoriasis.
| References | Subjects | Brief description |
|---|---|---|
| Ref.42 | German cross-sectional study (n=4,185) | Adjusted odds ratio |
| German Health Insurance beneficiaries (n=1,811,098) | Diabetes mellitus; 2.36 (95% CI 1.26-4.41) | |
| Ref.45 | Control patients (n=14,330) | Adjusted hazard ratio |
| Psoriasis (n=3,603) | Mortality from diabetes mellitus; 2.86 (95% CI 1.08-7.59) | |
| Ref.46 | Prospective study for 5.2 years follow-up | Adjusted hazard ratio |
| Control patients (n=208,187) | Diabetes mellitus; 1.18 (95% CI 1.06-1.31) | |
| Psoriasis (n=48,523) | ||
| Ref.47 | Hospital and clinic patients (n=113,065) | Adjusted odds ratio |
| Diabetes mellitus; 2.86 (95% CI 2.67-3.06) | ||
| Dyslipidemia; 2.35 (95% CI 2.19-2.52) | ||
| Ref.51 | Control patients (n=14,065) | Adjusted odds ratio in severe psoriasis |
| Severe psoriasis (n=3,854) | Diabetes mellitus; 1.62 (95% CI 1.3-2.01) | |
| Obesity; 1.79 (95% CI 1.55-2.05) | ||
| Ref.52 | Control patients (n=6,643) | Adjusted odds ratio |
| Psoriasis (n=340) | Diabetes mellitus; 1.5 (95% CI 1.2-2.0) | |
| Dyslipidemia; 1.2 (95% CI 1.0-1.6) | ||
| Obesity; 1.3 (95% CI 1.0-1.7) | ||
| Ref.53 | Control patients (n=74,987) | Adjusted odds ratio |
| Psoriasis (n=16,851) | Diabetes mellitus; 1.58 (95% CI 1.49-1.68) | |
| Ref.56 | Danish population based twin study (n=34,781 twins) | Odds ratio |
| Diabetes mellitus; 1.53 (95% CI 1.03-2.27) | ||
| Obesity; 1.81 (95% CI 1.28-2.55) | ||
| Ref.57 | Control patients (n=16,028) | Odds ratio |
| Psoriasis (n=429) | Obesity; 2.25 (95% CI 1.57-3.22) | |
| Ref.60 | Control patients (n=22,996) | Adjusted odds ratio |
| Psoriasis (n=10,669) | Dyslipidemia; 1.19 (95% CI 1.12-1.26) |
Pathophysiology of Comorbidity
As mentioned above, psoriasis is a chronic inflammatory skin disease driven by the TNF-α/IL-23/IL-17 axis (1, 4, 10). This notion is well supported by the high clinical efficacy of biologics against the TNF-α/IL-23/IL-17 pathway (5, 8, 15, 66). The release of excessive amounts of proinflammatory cytokines such as TNF-α and IL-1 may cause chronic low-grade systemic inflammation (67, 68) (Figure). The chronic state of inflammation appears to be a central mechanism underlying the pathophysiology of insulin resistance, visceral adiposity, hypertension and dyslipidemia. All of these conditions increase the risk for the development of type 2 diabetes and cardiovascular disease (67-69) (Figure). Consistent with this hypothesis, subclinical systemic and vascular inflammation have been detected by 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with psoriasis (70). In a six-month prospective study, carotid arterial stiffness in patients with psoriasis was significantly improved by anti-TNF-α therapy (71). In addition, other markers for systemic inflammation such as asymmetric dimethylarginine, red blood cell distribution width, the neutrophil-to-lymphocyte ratio, and the platelet-to-lymphocyte ratio, have proven to be upregulated in psoriatic patients (72-74).
In parallel with the above notions, the blockade of TNF-α by etanercept (anti-TNF-α treatment) improves the glucose tolerance in obese diabetic Zucker rats (75). Anti-TNF-α treatment with etanercept also suppresses cerebral damage caused by middle cerebral artery occlusion/reperfusion in both normal and diabetic rats (76). Furthermore, the inhibition of TNF-α production by a thalidomide analog decreases triglyceride levels and increases high-density lipoprotein levels in rats fed hypercholesterolemic diets (77).
Conclusion
Although the pathomechanisms of psoriasis are not fully understood, increasing research attention is being paid to the cutaneous-to-systemic expansion of the inflammatory process, which is now identified as the “psoriatic march” or “inflammatory skin march” (67, 68). This systemic inflammatory expansion is also seen in patients with severe eczema who have an increased risk for CVD (78). Another important fact related to “inflammatory skin march” is the significant association of psoriasis with other immune-mediated or autoimmune diseases such as inflammatory bowel disease (79, 80), multiple sclerosis (81) and autoimmune hepatitis (82). In addition, frequent comorbidity is noted in psoriasis and autoimmune bullous diseases (83-88). Further immunological studies into the genuine master switch underlying the comorbidity are warranted.
Finally, a disfigured appearance because of psoriasis may affect the psychological stability of patients (89). The cosmetic disfigurement associated with psoriasis increases the risk of depression, anxiety, feeling stigmatized and self-harm ideation, which profoundly impairs their satisfaction and adherence to medical care (36, 89, 90). In addition, internal physicians may hesitate to perform electrocardiogram, echocardiogram and even blood examinations, especially in severe cases with extensive skin eruption. These undesirable factors may delay the correct diagnosis and treatment of comorbid CVD and metabolic syndromes. As anti-TNF-α treatment is feasible for managing both skin lesions and comorbidities in psoriasis (12, 66), the early diagnosis and appropriate intervention by internal physicians in cooperation with dermatologists is required in order to increase the physical and mental quality of life in psoriatic patients.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Libby P, Nahrendorf M, Swirski FK. Leukocytes link local and systemic inflammation in ischemic cardiovascular disease: an expanded “cardiovascular continuum”. J Am Coll Cardiol 67: 1091-1103, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Souza Bastos A, Graves DT, de Melo Loureiro AP, et al. . Diabetes and increased lipid peroxidation are associated with systemic inflammation even in well-controlled patients. J Diabetes Complications 30: 1593-1599, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herder C, Færch K, Carstensen-Kirberg M, et al. . Biomarkers of subclinical inflammation and increases in glycaemia, insulin resistance and beta-cell function in non-diabetic individuals: the Whitehall II study. Eur J Endocrinol 175: 367-377, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Joshi AA, Lerman JB, Aberra TM, et al. . GlycA is a novel biomarker of inflammation and subclinical cardiovascular disease in psoriasis. Circ Res pii: CIRCRESAHA.116.309637, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boehncke WH, Schön MP. Psoriasis. Lancet 386: 983-994, 2015. [DOI] [PubMed] [Google Scholar]
- 6. Yin X, Low HQ, Wang L, et al. . Genome-wide meta-analysis identifies multiple novel associations and ethnic heterogeneity of psoriasis susceptibility. Nat Commun 6: 6916, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim J, Oh CH, Jeon J, et al. . Molecular phenotyping small (Asian) versus large (Western) plaque psoriasis shows common activation of IL-17 pathway genes but different regulatory gene sets. J Invest Dermatol 136: 161-172, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Furue M, Kadono T. Psoriasis: behind the scenes. J Dermatol 43: 4-8, 2016. [DOI] [PubMed] [Google Scholar]
- 9. Baurecht H, Hotze M, Brand S, et al. . Genome-wide comparative analysis of atopic dermatitis and psoriasis gives insight into opposing genetic mechanisms. Am J Hum Genet 96: 104-120, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mabuchi T, Ota T, Manabe Y, et al. . HLA-C*12:02 is a susceptibility factor in late-onset type of psoriasis in Japanese. J Dermatol 41: 697-704, 2014. [DOI] [PubMed] [Google Scholar]
- 11. Feng C, Wang T, Li SJ, Fan YM, Shi G, Zhu KJ. CARD14 gene polymorphism c.C2458T (p.Arg820Trp) is associated with clinical features of psoriasis vulgaris in a Chinese cohort. J Dermatol 43: 294-297, 2016. [DOI] [PubMed] [Google Scholar]
- 12. Piaserico S, Osto E, Famoso G, et al. . Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis 251: 25-30, 2016. [DOI] [PubMed] [Google Scholar]
- 13. Teng MW, Bowman EP, McElwee JJ, et al. . IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med 21: 719-729, 2015. [DOI] [PubMed] [Google Scholar]
- 14. Lande R, Gregorio J, Facchinetti V, et al. . Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449: 564-569, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol 32: 227-255, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGeachy MJ, McSorley SJ. Microbial-induced Th17: superhero or supervillain? J Immunol 189: 3285-3291, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramírez-Valle F, Gray EE, Cyster JG. Inflammation induces dermal Vγ4+ γδT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proc Natl Acad Sci USA 112: 8046-8051, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teunissen MB, Yeremenko NG, Baeten DL, et al. . The IL-17A-producing CD8+ T-cell population in psoriatic lesional skin comprises mucosa-associated invariant T cells and conventional T cells. J Invest Dermatol 134: 2898-2907, 2014. [DOI] [PubMed] [Google Scholar]
- 19. Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity 34: 149-162, 2011. [DOI] [PubMed] [Google Scholar]
- 20. Ha HL, Wang H, Pisitkun P, et al. . IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms. Proc Natl Acad Sci USA 111: E3422-E3431, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reich K, Papp KA, Matheson RT, et al. . Evidence that a neutrophil-keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis. Exp Dermatol 24: 529-535, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Torii H, Terui T, Matsukawa M, et al. . Safety profiles and efficacy of infliximab therapy in Japanese patients with plaque psoriasis with or without psoriatic arthritis, pustular psoriasis or psoriatic erythroderma: results from the prospective post-marketing surveillance. J Dermatol 43: 767-778, 2016. [DOI] [PubMed] [Google Scholar]
- 23. Asahina A, Torii H, Ohtsuki M, et al. . Safety and efficacy of adalimumab treatment in Japanese patients with psoriasis: results of SALSA study. J Dermatol 43: 1257-1266, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asahina A, Ohtsuki M, Etoh T, et al. . Adalimumab treatment optimization for psoriasis: results of a long-term phase 2/3 Japanese study. J Dermatol 42: 1042-1052, 2015. [DOI] [PubMed] [Google Scholar]
- 25. Kawakami H, Matsumoto Y, Abe N, et al. . Perioperative management of tumor necrosis factor-alpha blocker-treated psoriatic patients: case reports and review. J Dermatol 43: 190-193, 2016. [DOI] [PubMed] [Google Scholar]
- 26. Kawakami H, Maeda T, Abe N, et al. . Efficacy of adalimumab and methotrexate combination therapy on generalized pustular psoriasis patients unresponsive to infliximab monotherapy due to anti-infliximab antibody development. J Dermatol 42: 94-95, 2015. [DOI] [PubMed] [Google Scholar]
- 27. Takahashi H, Iinuma S, Tsuji H, Honma M, Iizuka H. Biologics are more potent than other treatment modalities for improvement of quality of life in psoriasis patients. J Dermatol 41: 686-689, 2014. [DOI] [PubMed] [Google Scholar]
- 28. Kusakari Y, Yamasaki K, Takahashi T, et al. . Successful adalimumab treatment of a psoriasis vulgaris patient with hemodialysis for renal failure: a case report and a review of the previous reports on biologic treatments for psoriasis patients with hemodialysis for renal failure. J Dermatol 42: 727-730, 2015. [DOI] [PubMed] [Google Scholar]
- 29. Umezawa Y, Hayashi M, Kikuchi S, et al. . Ustekinumab treatment in patients with psoriasis undergoing hemodialysis. J Dermatol 42: 731-734, 2015. [DOI] [PubMed] [Google Scholar]
- 30. Nimmannitya K, Tateishi C, Mizukami Y, et al. . Successful treatment with ustekinumab of psoriasis vulgaris in a patient undergoing hemodialysis. J Dermatol 43: 92-94, 2016. [DOI] [PubMed] [Google Scholar]
- 31. Ohtsuki M, Morita A, Abe M, et al. . Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol 41: 1039-1046, 2014. [DOI] [PubMed] [Google Scholar]
- 32. Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 41: 401-407, 1999. [DOI] [PubMed] [Google Scholar]
- 33. Do YK, Lakhani N, Malhotra R, Halstater B, Theng C, Østbye T. Association between psoriasis and leisure-time physical activity: findings from the National Health and Nutrition Examination Survey. J Dermatol 42: 148-153, 2015. [DOI] [PubMed] [Google Scholar]
- 34. Ng CY, Yang YW, Liu SH, et al. . SF-36 healty survey on psoriasis quality-of-life: a study of 414 Taiwanese patients. J Dermatol 42: 159-165, 2015. [DOI] [PubMed] [Google Scholar]
- 35. Atakan N, Yazici AC, Özarmağan G, et al. . TUR-PSO: a cross-sectional, study investigating quality of life and treatment status of psoriasis patients in Turkey. J Dermatol 43: 298-304, 2016. [DOI] [PubMed] [Google Scholar]
- 36. Saeki H, Imafuku S, Abe M, et al. . Poor adherence to medication as assessed by the Morisky Medication Adherence Scale-8 and low satisfaction with treatment in 237 psoriasis patients. J Dermatol 42: 367-372, 2015. [DOI] [PubMed] [Google Scholar]
- 37. Stuart PE, Nair RP, Tsoi LC, et al. . Genome-wide association analysis of psoriatic arthritis and cutaneous psoriasis reveals differences in their genetic architecture. Am J Hum Genet 97: 816-836, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Asahina A, Umezawa Y, Yanaba K, Nakagawa H. Serum C-reactive protein levels in Japanese patients with psoriasis and psoriatic arthritis: long-term differential effects of biologics. J Dermatol 43: 779-784, 2016. [DOI] [PubMed] [Google Scholar]
- 39. Takata T, Takahashi A, Taniguchi Y, Terada Y, Sano S. Detection of asymptomatic enthesitis in psoriasis patients: an onset of psoriatic arthritis? J Dermatol 43: 650-654, 2016. [DOI] [PubMed] [Google Scholar]
- 40. Yamamoto T, Ohtsuki M, Sano S, et al. . Epidemiological analysis of psoriatic arthritis patients in Japan. J Dermatol 43: 1193-1196, 2016. [DOI] [PubMed] [Google Scholar]
- 41. Samarasekera EJ, Neilson JM, Warren RB, Parnham J, Smith CH. Incidence of cardiovascular disease in individuals with psoriasis: a systematic review and meta-analysis. J Invest Dermatol 133: 2340-2346, 2013. [DOI] [PubMed] [Google Scholar]
- 42. Koch M, Baurecht H, Ried JS, et al. . Psoriasis and cardiometabolic traits: modest association but distinct genetic architectures. J Invest Dermatol 135: 1283-1293, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 296: 1735-1741, 2006. [DOI] [PubMed] [Google Scholar]
- 44. Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J 31: 1000-1006, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol 163: 586-592, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parisi R, Rutter MK, Lunt M, et al. . Psoriasis and the risk of major cardiovascular events: cohort study using the clinical practice research datalink. J Invest Dermatol 135: 2189-2197, 2015. [DOI] [PubMed] [Google Scholar]
- 47. Shiba M, Kato T, Funasako M, et al. . Association between psoriasis vulgaris and coronary heart disease in a hospital-based population in Japan. PLoS One 11: e0149316, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Takahashi H, Iizuka H. Psoriasis and metabolic syndrome. J Dermatol 39: 212-218, 2012. [DOI] [PubMed] [Google Scholar]
- 49. Puig L, Kirby B, Mallbris L, Strohal R. Psoriasis beyond the skin: a review of the literature on cardiometabolic and psychological co-morbidities of psoriasis. Eur J Dermatol 24: 305-311, 2014. [DOI] [PubMed] [Google Scholar]
- 50. Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol 32: 982-986, 1995. [DOI] [PubMed] [Google Scholar]
- 51. Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol 55: 829-835, 2006. [DOI] [PubMed] [Google Scholar]
- 52. Cohen AD, Gilutz H, Henkin Y, et al. . Psoriasis and the metabolic syndrome. Acta Derm Venereol 87: 506-509, 2007. [DOI] [PubMed] [Google Scholar]
- 53. Cohen AD, Dreiher J, Shapiro Y, et al. . Psoriasis and diabetes: a population-based cross-sectional study. J Eur Acad Dermatol Venereol 22: 585-589, 2008. [DOI] [PubMed] [Google Scholar]
- 54. Gisondi P, Tessari G, Conti A, et al. . Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based case-control study. Br J Dermatol 157: 68-73, 2007. [DOI] [PubMed] [Google Scholar]
- 55. Schwandt A, Bergis D, Dapp A, et al. . Psoriasis and diabetes: a multicenter study in 222078 type 2 diabetes patients reveals high levels of depression. J Diabetes Res 2015: 792968, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lønnberg AS, Skov L, Skytthe A, Kyvik KO, Pedersen OB, Thomsen SF. Association of psoriasis with the risk for type 2 diabetes mellitus and obesity. JAMA Dermatol 152: 761-767, 2016. [DOI] [PubMed] [Google Scholar]
- 57. Naito R, Imafuku S. Distinguishing features of body mass index and psoriasis in men and women in Japan: a hospital-based case-control study. J Dermatol 43: 1406-1411, 2016. [DOI] [PubMed] [Google Scholar]
- 58. Choi WJ, Park EJ, Kwon IH, Kim KH, Kim KJ. Association between psoriasis and cardiovascular risk factors in Korean patients. Ann Dermatol 22: 300-306, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kokpol C, Aekplakorn W, Rajatanavin N. Prevalence and characteristics of metabolic syndrome in South-East Asian psoriatic patients: a case-control study. J Dermatol 41: 898-902, 2014. [DOI] [PubMed] [Google Scholar]
- 60. Dreiher J, Weitzman D, Davidovici B, Shapiro J, Cohen AD. Psoriasis and dyslipidaemia: a population-based study. Acta Derm Venereol 88: 561-565, 2008. [DOI] [PubMed] [Google Scholar]
- 61. Cohen AD, Weitzman D, Dreiher J. Psoriasis and hypertension: a case-control study. Acta Derm Venereol 90: 23-26, 2010. [DOI] [PubMed] [Google Scholar]
- 62. Nagai H, Fujiwara S, Takahashi Y, Nishigori C. Ameliorating effect of the novel dipeptidyl peptidase-4 inhibitor teneligliptin on psoriasis: a report of two cases. J Dermatol 42: 1094-1097, 2015. [DOI] [PubMed] [Google Scholar]
- 63. Mattozzi C, Paolino G, Richetta AG, Calvieri S. Psoriasis, vitamin D and the importance of the cutaneous barrier's integrity: an update. J Dermatol 43: 507-514, 2016. [DOI] [PubMed] [Google Scholar]
- 64. Kincse G, Bhattoa PH, Herédi E, et al. . Vitamin D3 levels and bone mineral density in patients with psoriasis and/or psoriatic arthritis. J Dermatol 42: 679-684, 2015. [DOI] [PubMed] [Google Scholar]
- 65. D'Epiro S, Marocco C, Salvi M, et al. . Psoriasis and bone mineral density: implications for long-term patients. J Dermatol 41: 783-787, 2014. [DOI] [PubMed] [Google Scholar]
- 66. Imafuku S, Honma M, Okubo Y, et al. . Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol 43: 1011-1017, 2016. [DOI] [PubMed] [Google Scholar]
- 67. Boehncke WH, Boehncke S, Tobin AM, Kirby B. The ‘psoriatic march': a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol 20: 303-307, 2011. [DOI] [PubMed] [Google Scholar]
- 68. Yamanaka K, Mizutani H. “Inflammatory skin march”: IL-1-mediated skin inflammation, atopic dermatitis, and psoriasis to cardiovascular events. J Allergy Clin Immunol 136: 823-824, 2015. [DOI] [PubMed] [Google Scholar]
- 69. Welty FK, Alfaddagh A, Elajami TK. Targeting inflammation in metabolic syndrome. Transl Res 167: 257-280, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Youn SW, Kang SY, Kim SA, Park GY, Lee WW. Subclinical systemic and vascular inflammation detected by 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with mild psoriasis. J Dermatol 42: 559-566, 2015. [DOI] [PubMed] [Google Scholar]
- 71. Pina T, Corrales A, Lopez-Mejias R, et al. . Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol 43: 1267-1272, 2016. [DOI] [PubMed] [Google Scholar]
- 72. Pina T, Genre F, Lopez-Mejias R, et al. . Asymmetric dimethylarginine but not osteoprotegerin correlates with disease severity in patients with moderate-to-severe psoriasis undergoing anti-tumor necrosis factor-α therapy. J Dermatol 43: 389-394, 2016. [DOI] [PubMed] [Google Scholar]
- 73. Kim DS, Shin D, Jee H, et al. . Red blood cell distribution width is increased in patients with psoriasis vulgaris: a retrospective study on 261 patients. J Dermatol 42: 567-571, 2015. [DOI] [PubMed] [Google Scholar]
- 74. Kim DS, Shin D, Lee MS, et al. . Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. J Dermatol 43: 305-310, 2016. [DOI] [PubMed] [Google Scholar]
- 75. Grauballe MB, Østergaard JA, Schou S, Flyvbjerg A, Holmstrup P. Effects of TNF-α blocking on experimental periodontitis and type 2 diabetes in obese diabetic Zucker rats. J Clin Periodontol 42: 807-816, 2015. [DOI] [PubMed] [Google Scholar]
- 76. Iwata N, Takayama H, Xuan M, et al. . Effects of etanercept against transient cerebral ischemia in diabetic rats. Biomed Res Int 2015: 189292, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fumian MM, da Motta NA, Maia R, et al. . LASSBio-1425, an analog of thalidomide, decreases triglyceride and increases HDL cholesterol levels by inhibition of TNF-α production. Int J Cardiol 202: 497-499, 2016. [DOI] [PubMed] [Google Scholar]
- 78. Silverberg JI, Greenland P. Eczema and cardiovascular risk factors in 2 US adult population studies. J Allergy Clin Immunol 135: 721-728, 2015. [DOI] [PubMed] [Google Scholar]
- 79. Egeberg A, Mallbris L, Warren RB, et al. . Association between psoriasis and inflammatory bowel disease: a Danish nationwide cohort study. Br J Dermatol 175: 487-492, 2016. [DOI] [PubMed] [Google Scholar]
- 80. Nakamura K, Asano Y, Shibata S, et al. . Case of psoriasis vulgaris developing ulcerative colitis during adalimumab treatment. J Dermatol 42: 1029-1030, 2015. [DOI] [PubMed] [Google Scholar]
- 81. Egeberg A, Mallbris L, Gislason GH, Skov L, Hansen PR. Risk of multiple sclerosis in patients with psoriasis: a Danish nationwide cohort study. J Invest Dermatol 136: 93-98, 2016. [DOI] [PubMed] [Google Scholar]
- 82. Jensen P, Egeberg A, Gislason G, Hansen PR, Thyssen JP, Skov L. Increased risk of autoimmune hepatitis in patients with psoriasis: a Danish nationwide cohort study. J Invest Dermatol 136: 1515-1517, 2016. [DOI] [PubMed] [Google Scholar]
- 83. Takahashi H, Sato K, Takagi A, et al. . Subepidermal autoimmune blistering lesion in a case of psoriasis successfully treated with cyclosporin. J Dermatol 42: 1125-1126, 2015. [DOI] [PubMed] [Google Scholar]
- 84. Maki N, Demitsu T, Umemoto N, et al. . Possible paraneoplastic syndrome case of bullous pemphigoid with immunoglobulin G anti-BP180 C-terminal domain antibodies associated with psoriasis and primary macroglobulinemia. J Dermatol 43: 571-574, 2016. [DOI] [PubMed] [Google Scholar]
- 85. Okahashi K, Oiso N, Ishii N, et al. . Bullous pemphigoid associated with psoriasis: a possible example of an inverse intramolecular epitope-spreading phenomenon. J Dermatol 42: 758-759, 2015. [DOI] [PubMed] [Google Scholar]
- 86. Ishida S, Takahashi K, Kanaoka M, et al. . Case of subepidermal autoimmune bullous disease with psoriasis vulgaris reacting to both BP180 C-terminal domain and laminin gamma-1. J Dermatol 42: 391-393, 2015. [DOI] [PubMed] [Google Scholar]
- 87. Imanishi A, Tateishi C, Imanishi H, et al. . Pemphigoid with antibodies to laminin γ1, BP180 and BP230, associated with psoriasis vulgaris: successful disease control with cyclosporin. J Dermatol 42: 394-397, 2015. [DOI] [PubMed] [Google Scholar]
- 88. Ansai S, Hashizume S, Kawana S, Tateishi C, Koga H, Hashimoto T. Case of anti-laminin gamma-1 pemphigoid with antibody against C-terminal domain of BP180 in a patient with psoriasis vulgaris. J Dermatol 41: 1031-1033, 2014. [DOI] [PubMed] [Google Scholar]
- 89. Tomas-Aragones L, Marron SE. Body image and body dysmorphic concerns. Acta Derm Venereol 96: 47-50, 2016. [DOI] [PubMed] [Google Scholar]
- 90. Offidani E, Del Basso D, Prignago F, Tomba E. Discriminating the presence of psychological distress in patients suffering from psoriasis: an application of the clinimetric approach in dermatology. Acta Derm Venereol 96: 69-73, 2016. [DOI] [PubMed] [Google Scholar]