Abstract
Objective
Depression is reported to be relatively common in idiopathic pulmonary fibrosis (IPF) patients. Thus far, however, whether or not depression independently determines the health-related quality of life (HRQOL) has not been evaluated exclusively in IPF patients. We designed this study to identify independent determinants of the St. George’s Respiratory Questionnaire (SGRQ) score among various factors, including a depression scale, in IPF patients.
Methods
We retrospectively analyzed consecutive subjects with IPF who completed a systematic evaluation including pulmonary function tests, PaO2 at rest, 6-minute walk test (6MWT), SGRQ, Baseline Dyspnea Index (BDI), and Hospital Anxiety and Depression Scale (HADS). All eligible patients in the present study had newly diagnosed IPF and had not received any prior treatments, such as antidepressants, pirfenidone, corticosteroids, immunosuppressants, or long-term oxygen therapy.
Results
The 121 patients with IPF included 99 men. On the SGRQ, mild to moderate disturbance was observed in the total and each component score. According to the HADS, 27 patients (22.3%) had borderline or definite depression. In a univariate regression analysis, the forced vital capacity (FVC), diffusion capacity of carbon monoxide (DLco), PaO2 at rest, BDI, HADS for Anxiety (HADS-A) and Depression (HADS-D), 6-minute walk distance (6MWD), and lowest SpO2 during the 6MWT were significantly correlated with the SGRQ total score. In a stepwise multiple regression model, BDI, 6MWD, and HADS-D were selected as independent determinants of the total SGRQ score. The total variance in this model was 59% (p<0.001).
Conclusion
We concluded that depression was a significant determinant of the HRQOL or health status in patients with IPF.
Keywords: idiopathic pulmonary fibrosis, health status, depression, dyspnea, 6-minute walk test
Introduction
Depression is 1.5-7 times likelier to develop in patients with chronic disease than in the general population (1-3). Evidence of the increased incidence of depressive symptoms in patients with chronic respiratory disease, including idiopathic pulmonary fibrosis (IPF), is growing (4). However, while several studies have shown that psychological factors affect the health-related quality of life (HRQOL) or health status in chronic obstructive pulmonary disease (COPD) patients (5), information is limited for IPF patients (6).
Patient-centered outcomes, including the HRQOL, are significant endpoints in research and clinical practice (7), as IPF is a chronic, progressive, and fatal lung disease for which there is still no proven etiology or cure. Indeed, the median survival time after a diagnosis of IPF is reported to be 2-3 years (8, 9), although recent studies have shown that pirfenidone and nintedanib reduce the disease progression (10-12).
By the quantification of patients’ perceptions, HRQOL tools obtain information unavailable through physiologic and radiographic tests. Therefore, the HRQOL or health status has been recognized as one of the more important outcomes in measuring the effectiveness of therapeutic interventions in patients with IPF (6, 13). A recent review shows that the psychometric properties of St. George’s Respiratory Questionnaire (SGRQ) are adequate for measuring the HRQOL in IPF patients (14). However, whether or not psychological factors such as depression independently affect the HRQOL in patients with IPF remains unclear.
The purpose of this study was to investigate the association between depressive symptoms and the HRQOL in IPF patients. We hypothesized that the level of depression was a determinant of the SGRQ score in IPF patients.
Materials and Methods
Subjects
Consecutive individuals with IPF who underwent an initial systematic evaluation at Tosei General Hospital between April 2009 and March 2013 were enrolled and analyzed retrospectively. The diagnosis of IPF was established based on the American Thoracic Society (ATS)/European Respiratory Society (ERS)/Japanese Respiratory Society (JRS)/The Latin America Thoracic Association (ALAT) statement (9). All patients included in the study had newly diagnosed IPF. Patients were excluded if they had malignancy or an unstable medical condition, such as significant coronary heart disease or musculoskeletal disease. Those who had received any prior treatment such as antidepressants, pirfenidone (PFD), corticosteroids, immunosuppressive agents, or long-term oxygen therapy (LTOT) at the time of enrollment were also excluded, as were patients who were unable to perform a 6-minute walk test (6MWT).
The study protocol was approved by the institutional review board (IRB) of Tosei General Hospital (IRB No 470). The IRB did not require the patients’ approval or informed consent for the retrospective chart reviews.
Initial systematic evaluation and measurements
An initial systematic evaluation was routinely performed in an outpatient setting before a surgical lung biopsy and a diagnosis of IPF. All assessments, including questionnaires, the 6MWT, and the arterial blood gas test, were done within a month after the pulmonary function test. The questionnaires and 6MWT were administered on the same day.
Pulmonary function tests and artery blood gas tensions
All subjects underwent pulmonary function testing system (CHESTAC-55V; Chest, Tokyo, Japan), in accordance with the maneuver described by the ATS/ERS Task Force (15). The single-breath diffusion capacity of the lung for carbon monoxide (DLco) was also assessed (CHESTAC-55V). Based on the recommendations from JRS, the values for forced vital capacity (FVC) and DLco were expressed as the percent of the predicted values (16). According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD), patients with a post-bronchodilator FEV1/FVC ratio less than 70% are considered to have COPD as a comorbid condition (17). Artery blood gas tension was measured at rest.
Exercise capacity
To assess the functional exercise capacity, all patients who participated in the present study underwent the 6MWT in accordance with the ATS statement (18). The subjects were instructed to walk as far as possible for 6 minutes under the supervision of trained physiotherapists (19). The distance that individuals could walk was recorded as the 6-minute walk distance (6MWD) (20). Oxygen saturation was measured by pulse oximetry at rest and immediately after the test. All eligible subjects underwent the tests twice to minimize the training effects. The longest of the two 6MWD tests (performed the same day and separated by at least 30 minutes) was used in the data analysis.
Health status measurement
The health status (HRQOL) was evaluated by the SGRQ, which was originally developed for patients with COPD (21). The validity of the Japanese version of the SGRQ was previously evaluated (22). It provides a total score and three component scores, for ‘symptoms’, ‘activity’, and ‘impact’.
Each of the three components and the total are separately scored on a scale of 0 to 100, with higher scores corresponding to greater impairment of the HRQOL. Although the SGRQ was developed for patients with COPD, it has been validated and is frequently used to measure the HRQOL in patients with IPF (11). In a study of COPD, a total SGRQ score of 25 or higher is very uncommon in healthy persons (23).
Assessment of dyspnea in daily living
To evaluate dyspnea in daily living, we used the Japanese version of the Baseline Dyspnea Index (BDI) (22). The BDI has a scale of five grades for each of three categories: functional impairment, magnitude of task, and magnitude of effort. The possible score range for each category is 0 (‘severely impaired’) to 4 (‘not impaired’). Thus, the BDI yields a total score between 0 and 12 (24). The Japanese version of the BDI, for which the first two validation studies were published in 1998 (22, 25), was used in the present study.
Assessment of psychological status
The psychological evaluation was done with the Japanese version of the Hospital and Anxiety Depression Scale (HADS) (26). The HADS is a reliable, valid, responsive, and relatively convenient screening tool, allowing for the simultaneous and easy assessment of symptom severity for both anxiety and depression (27, 28), although psychiatric disorders can be diagnosed only on the basis of structured interviews adopting the Diagnostic and Statistical Manual of Mental Disorder, 5th edition (DSM-V) criteria (29). The HADS is composed of 14 items, 7 of which comprise the anxiety subscale and another 7 of which comprise the depression subscale. Each item has four potential responses (rating on a 0-3 scale). The scores for each subscale therefore yield a score ranging between 0 (no distress) and 21 (maximum distress), with higher scores corresponding to a more severe level of distress. The subscale scores are categorized as follows: 0-7, normal; 8-10, borderline abnormal; 11-21, abnormal. A previous study reported that HADS is a reliable screening instrument for determining the psychological status of outpatient populations (26). A minimally important difference of 1.5 has been established among patients with moderate to severe COPD (30). In a study of sarcoidosis, a significant correlation was observed between the HADS scores and SGRQ scores (31). The HADS scores were included as continuous variables in the regression analysis to increase the power.
Statistical analyses
All statistical analyses were performed using the SPSS software program, ver. 22 (SPSS, Chicago, IL, USA). The normal distribution was checked with the Shapiro-Wilk test. The Spearman’s correlation coefficients were assessed to identify the variables contributing to the HRQOL in a univariate analysis. Consequently, a stepwise multiple regression analysis was performed using the SGRQ score as a dependent variable to identify which factors significantly contribute to the health status. Any variables with a p value of less than 0.2 in the univariate analysis were enrolled into a multivariable model as potentially important determinants. To reduce the problem of multicollinearity, only one of the highly correlated variables (correlation coefficient ≥0.9) was entered in the multivariate model, if present. Gender was coded as 1 for men and 0 for women in the multiple regression analysis. A p value of less than 0.05 was considered to be statistically significant. All results are present as mean ± standard deviation, unless otherwise specified.
Results
During the study period, a total of 163 patients were diagnosed with IPF according to ATS/ERS/JRS/ALAT statements. Of these 163 patients, a systematic evaluation could not be completed in 10 due to cardiovascular disease or musculoskeletal problems. Thirty-two patients had received corticosteroid therapy, PFD, and/or LTOT prior to the systematic evaluation. These 42 patients were excluded from the analysis. Therefore, 121 eligible patients ultimately completed all the measurements and had no missing values. The mean age of the 121 subjects was 66.8±7.6 years, and most were men (81.8%). Fifty-seven patients underwent a diagnostic surgical lung biopsy (Fig. 1). The demographic details of the patients are summarized in Table 1. The present subjects showed mild to moderate deterioration of FVC and a reduced diffusion capacity. Only 5 (4.1%) of the 121 patients had COPD as a comorbid condition.
Figure 1.
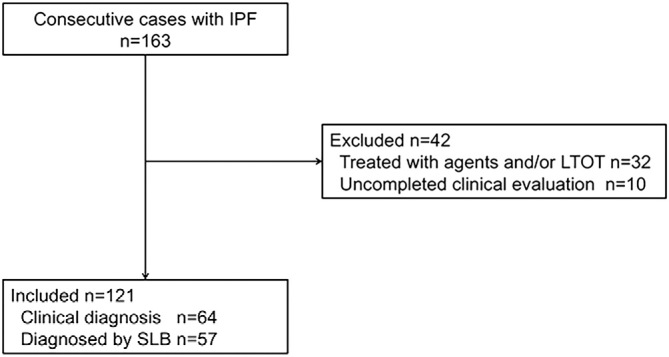
Screening and inclusion process for patients in the study.
Table 1.
Clinical Characteristics of 121 Patients with IPF.
| n=121 | |
|---|---|
| Male, n (%) | 99 (81.8) |
| Age (yrs), mean (SD) | 66.8 (7.6) |
| Smoking status, n (%) | |
| Current smoker | 9 (7.4) |
| Former smoker | 84 (69.4) |
| Never smoker | 28 (23.1) |
| BMI (kg/m2), mean (SD) | 23.5 (3.2) |
| FVC (%pred), mean (SD) | 81.1 (18.9) |
| FEV1/FVC, mean (SD) | 85.8 (7.3) |
| DLco (%pred), mean (SD) | 61.1 (19.9) |
| 6MWD (m), mean (SD) | 574 (131) |
| lowest SpO2 (%), mean (SD) | 83.5 (7.8) |
| PaO2 at rest (torr), mean (SD) | 81.2 (11.2) |
IPF: idiopathic pulmonary fibrosis, BMI: body mass index, FVC: forced vital capacity, FEV1: forced expiratory volume in 1 second, DLco: diffusion capacity for carbon monoxide, 6MWD: 6-min walk distance, SpO2: oxygen saturation values obtained from pulse oximetry, PaO2: partial pressure of oxygen in arterial blood
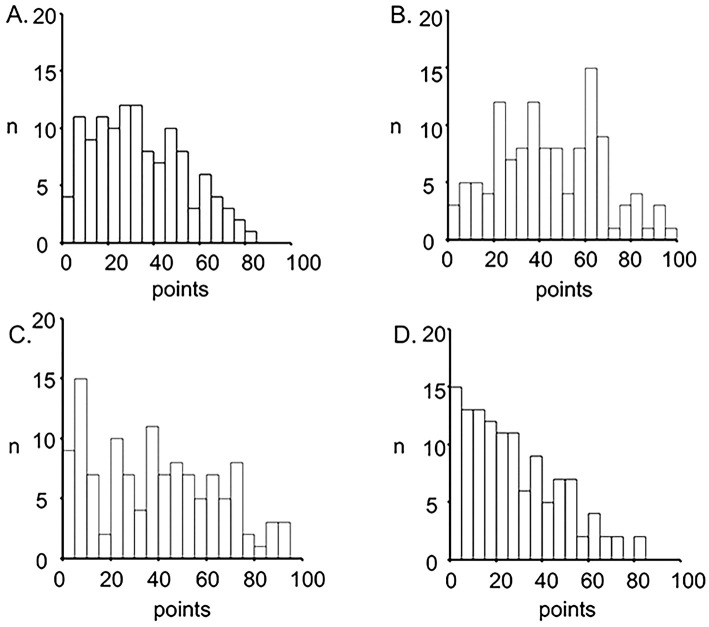
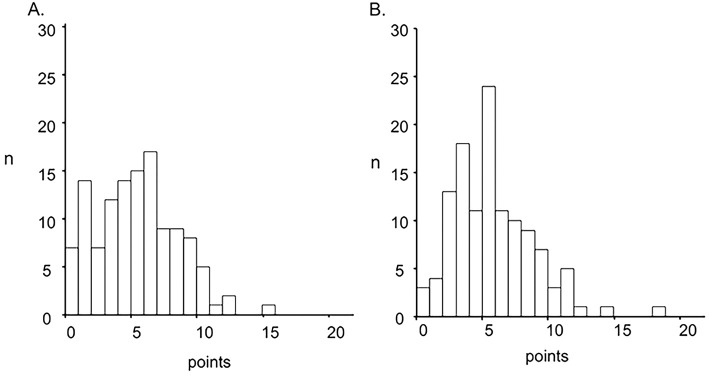
A summary of all questionnaire scores, including the number of subjects with the minimum and maximum possible scores, is shown in Table 2. Patients showed mild to moderate disturbances in the total score and each component of the SGRQ; the severity of disturbance in the impact component was relatively mild. The total score, impact, and the activity component had non-normal distributions, whereas the symptom component had a normal distribution (Fig. 2). Seventy-six patients (62.8%) had an SGRQ total score ≥25. Minor ceiling effects [lowest possible score (best HRQOL)] were observed in three components. Fig. 3 shows a distribution histogram of the HADS scale. Borderline or definite anxiety and depression (HADS scale ≥8) were detected in 26 (21.5%) and 27 (22.3%) patients, respectively.
Table 2.
Results of Dyspnea Scale, Psychological Assessment, and the SGRQ Score (n=121).
| No. of subjects with | ||||||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | n | (%) | minimum score | maximum score | |
| BDI (0-12) | 9.1 (2.3) | 2-12 | 0 | 18 | ||
| HADS-A (0-21) | 5.0 (3.1) | 0-18 | 7 | 0 | ||
| Normal (0-7) | 95 | (78.5) | ||||
| Borderline (8-10) | 22 | (18.2) | ||||
| Definite (11-21) | 4 | (3.3) | ||||
| HADS-D (0-21) | 5.4 (3.1) | 0-15 | 3 | 0 | ||
| Normal (0-7) | 94 | (77.7) | ||||
| Borderline (8-10) | 19 | (15.7) | ||||
| Definite (11-21) | 8 | (6.6) | ||||
| SGRQ total (0-100) | 33.9 (20.1) | 0.7-80.6 | 0 | 0 | ||
| total score<25 | 45 | (37.2) | ||||
| total score ≥25 | 76 | (62.8) | ||||
| SGRQ symptom (0-100) | 44.5 (22.7) | 0-100 | 2 | 1 | ||
| SGRQ activity (0-100) | 38.4 (25.9) | 0-93.3 | 9 | 0 | ||
| SGRQ impact (0-100) | 27.3 (20.1) | 0-81.8 | 5 | 0 | ||
SGRQ: St. George’s Respiratory Questionnaire, BDI: Baseline Dyspnea Index, HADS: Hospital Anxiety and Depression Scale, HADS-A: anxiety of HADS, HADS-D: depression of HADS
Figure 2.
Frequency distribution histograms of each score for the SGRQ/A. Total score of the SGRQ, B. Symptom component of the SGRQ, C. Activity component of the SGRQ, D. Impact component of the SGRQ. SGRQ: St. George’s Respiratory Questionnaire
Figure 3.
Frequency distribution histograms of HADS/A. HADS-A, B. HADS-D. Score definition, normal: 0-7; borderline abnormality: 8-10; abnormal: 11-21. HADS: Hospital Anxiety and Depression Scale, HADS-A: HADS anxiety subscale, HADS-D: HADS depression subscale
Table 3 shows the Spearman’s correlation coefficients between the SGRQ scores and various measurement variables. The pulmonary function (FVC and DLco) was significantly correlated with each score for the SGRQ. The BDI score showed the strongest correlation with the total score and each component for the SGRQ. Significant correlations were found between the 6MWD and the SGRQ (total score and three components), as well as between the lowest SpO2 during the 6MWT and the SGRQ (total score and three components). The HADS-D had significant correlations with the total and impact score for the SGRQ, while the HADS-A had significant correlations with the total, symptom, and impact score for the SGRQ.
Table 3.
Results of Univariate Analysis for Factors Related to the SGRQ Score (n=121).
| Total | Symptom | Activity | Impact | |||||
|---|---|---|---|---|---|---|---|---|
| rho | p | rho | p | rho | p | rho | p | |
| Age | 0.125 | 0.172 | 0.035 | 0.701 | 0.151 | 0.098 | 0.103 | 0.262 |
| Gender | -0.133 | 0.146 | -0.154 | 0.091 | -0.003 | 0.973 | -0.166 | 0.068 |
| BMI | -0.068 | 0.460 | -0.069 | 0.451 | 0.041 | 0.652 | -0.127 | 0.166 |
| FVC | -0.352 | <0.001 | -0.237 | 0.009 | -0.393 | <0.001 | -0.270 | 0.003 |
| DLco | -0.449 | <0.001 | -0.434 | <0.001 | -0.496 | <0.001 | -0.316 | <0.001 |
| 6MWD | -0.533 | <0.001 | -0.337 | <0.001 | -0.575 | <0.001 | -0.431 | <0.001 |
| lowest SpO2 | -0.341 | <0.001 | -0.319 | <0.001 | -0.351 | <0.001 | -0.284 | 0.002 |
| PaO2 at rest | -0.240 | 0.008 | -0.135 | 0.140 | -0.297 | 0.001 | -0.192 | 0.035 |
| BDI | -0.746 | <0.001 | -0.600 | <0.001 | -0.746 | <0.001 | -0.614 | <0.001 |
| HADS-A | 0.212 | 0.020 | 0.196 | 0.031 | 0.134 | 0.142 | 0.245 | 0.007 |
| HADS-D | 0.227 | 0.012 | 0.134 | 0.142 | 0.190 | 0.037 | 0.239 | 0.008 |
Data are Spearman’s correlation coefficients. See Table 1 and Table 2 legends for expansion of abbreviations.
A stepwise multiple regression analysis was done with gender, BDI, HADS-D, HADS-A, FVC, DLco, 6MWD, and lowest SpO2 during the 6MWT as possible independent predictors for the total score and each component of the SGRQ. In this analysis, the BDI, 6MWD, and HADS-D were selected as independent determinants of the total SGRQ score (Table 4). The total variance explained in this model was 59% (p<0.001).
Table 4.
Results of Stepwise Regression Analysis for the SGRQ Score (n=121).
| Total | Symptom | Activity | Impact | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SD | Standardized β | p | β | SD | Standardized β | p | β | SD | Standardized β | p | β | SD | Standardized β | p | ||||
| Age, yrs | NS | NS | NS | NS | |||||||||||||||
| Gender, Male | NS | -0.985 | 4.265 | -0.168 | 0.023 | NS | NS | ||||||||||||
| FVC,%pred | NS | NS | NS | NS | |||||||||||||||
| DLco,%pred | NS | NS | NS | NS | |||||||||||||||
| BDI | -5.414 | 0.650 | -0.619 | <0.001 | -0.582 | 0.718 | -0.590 | <0.001 | -5.570 | 0.898 | -0.495 | <0.001 | -5.324 | 0.599 | -0.610 | <0.001 | |||
| HADS-A | NS | NS | NS | NS | |||||||||||||||
| HADS-D | 1.077 | 0.387 | 0.165 | 0.006 | NS | 1.126 | 0.503 | 0.134 | 0.027 | 1.408 | 0.448 | 0.216 | 0.002 | ||||||
| 6MWD,m | -0.025 | 0.011 | -0.167 | 0.027 | NS | -0.050 | 0.015 | -0.257 | 0.001 | NS | |||||||||
| lowest SpO2, % | NS | NS | -0.480 | 0.212 | -0.145 | 0.025 | NS | ||||||||||||
| PaO2, torr | NS | NS | NS | NS | |||||||||||||||
| Cumulative R2 | 0.590 | p<0.001 | 0.364 | p<0.001 | 0.555 | p<0.001 | 0.442 | p<0.001 | |||||||||||
NS: not significant. See Table 1 and Table 2 legends for expansion of abbreviations.
For the symptom score of the SGRQ, gender and the BDI score were significant parameters. The BDI score, 6MWD, lowest SpO2, and HAD-D score were selected as independent predictors of the activity score of the SGRQ. For the impact score, the BDI and HADS-D were selected as independent parameters. The FVC, DLco, PaO2 at rest, and HADS-A were excluded from the model in the three domains of the SGRQ (Table 4). In the model of the total SGRQ score, BDI exclusion changed 12.7% of the standardized β for the HADS-D. In contrast, HADS-D exclusion changed only 1.5% of the standardized β for BDI, and 6MWD exclusion resulted in changes of 16.5% and 7.3% in the standardized β for BDI and HADS-D, respectively.
Discussion
The present study is the first report showing that this depression scale independently predicted the SGRQ in the initial evaluation of IPF patients. In the stepwise multiple regression analysis, the HADS-D scale was an independent contributor to the total score, activity, and the impact component of the SGRQ. This finding suggests that the effective management of depression may improve the HRQOL in IPF patients. Thus far, few studies have examined this significant comorbidity and attempted to evaluate whether its treatment can improve the HRQOL and functional status in patients with IPF. The screening and management of depression should be considered clinically important in IPF patients, even if their pulmonary function impairment is mild to moderate.
Although physiologic variables (FVC, DLco, and PaO2 at rest) had statistically significant correlations with the SGRQ score in the univariate regression analysis, they were not selected as independent variables in the multiple regression analysis. The depression scale, dyspnea, and exercise capacity more accurately predicted the SGRQ total score than the physiologic variables. Indeed, although pharmacological intervention has been shown to prevent a decline in the lung function, its efficacy in preventing the deterioration of the HRQOL is inconsistent in IPF subjects. In two phase 3 trials of INPULSIS (INPULSIS-1 and -2), nintedanib significantly reduced a decline in the FVC, which was consistent with slowing the progression of the disease, compared with a placebo. However, no consistent effect of nintedanib on the change in the total score of the SGRQ was found in either trial (11).
The present study showed that even under conditions of mild-to-moderate pulmonary function impairment, anxiety and depressive symptoms were present in IPF patients who were newly diagnosed with IPF prior to any intervention. Of the 121 cases, 26 (22%) showed borderline or definite depression. Our result is in line with the findings of previous studies showing that depression is very common in individuals with ILD, including IPF, affecting 23-27% of individuals with ILD (32). Depression should be routinely screened in patients with IPF, and appropriate supportive management, including antidepressants, psychological counseling, and pulmonary rehabilitation, should be offered. Recent studies have shown that pulmonary rehabilitation improves the 6MWD, HRQOL, dyspnea, and depression score in these patients (6, 33).
The data in the present study showed that the association between depression and the SGRQ in IPF was weaker than that in COPD (5). This discrepancy may be due to differences in the severity of respiratory disease in the study population. In the present study, we included only patients who were newly diagnosed with IPF and had not been treated previously. As a result, patients with more progressive and severe disease were excluded from the present study. In contrast, COPD studies include patients with severe and very severe disease (5).
Interestingly, the HADS-A scale was not selected as a significant factor contributing to the total and all component scores of the SGRQ in a multiple regression analysis, although the HADS-A scale was significantly correlated with the SGRQ in a univariate regression analysis. This discrepancy may be due to the association between dyspnea and anxiety in the present study, although the mechanism underlying this reasoning is unclear.
In the stepwise multiple regression model, the BDI and 6MWD were selected as independent factors contributing to the SGRQ total score. This result is in line with the findings of previous studies (34-36). The consistency between the present and previous findings strengthens the evidence of an association between the HRQOL and these variables (dyspnea and 6MWD).
The multiple regression analysis also showed that the most influential factor was BDI, based on the β value. A recent study showed that dyspnea is associated with depression in IPF subjects (32). Variables such as dyspnea, depression, and the health status are correlated with each other, and determining causality is difficult. In the present study, we performed a multiple regression analysis to control for confounding factors. Although a causal relationship could not be concluded from our findings, ‘depression symptoms’ was selected as an independent predictor for the health status, in addition to dyspnea and exercise impairment. The present study is the first to show that the depression scale is an independent predictor of the SGRQ score in IPF patients.
Gender was selected as an independent determinant of the symptom component score, but not total score, activity or impact component score. A previous study reported that male gender is associated with less dyspnea, a worse physical HRQOL, and a better emotional QOL in IPF patients (37). As gender may also be a confounding factor, we included it in the multiple regression analysis. As a result, male gender was found to be independently associated with a better symptom domain score, but not with the total score, on the SGRQ.
In the univariate analysis, the lowest SpO2 during 6MWD was significantly correlated with the total score and the scores of the three components in the SGRQ. In the multiple regression analysis, the lowest SpO2 during the 6MWT was selected as an independent predictor of the activity component, but not the total score, on the SGRQ-possibly because of an association between desaturation during exercise and the dyspnea scale. Although desaturation in 6MWT is reported to be a significant prognostic indicator in patients with IPF (38, 39), no studies have shown that the lowest SpO2 during the 6MWT determines the total and domain scores on the SGRQ.
The etiology of the association between depression and IPF is still not fully understood. Physiological dysregulation [activation of the sympathetic nervous system and hypothalamic-pituitary-adrenal (HPA) axis] induced by chronic psychological stress may weaken the immune function and vulnerability to respiratory infection (40-42). In COPD patients, biological changes (e.g., HPA axis) induced by depression are speculated to be a potential pathway underlying the relationship between depression and COPD exacerbation (43), which is associated with a poor HRQOL. Thus far, however, the exact mechanisms linking IPF with depression and the long-term impact of depression on IPF have not been identified.
Several limitations associated with the present study warrant mention. First, the cross-sectional design of the study does not provide evidence for causality in the relationship between psychological factors and the HRQOL. Second, the present patients showed mild-to-moderate lung impairment. It is therefore unclear whether or not the present results can be applied to individuals with a more severe disease status. Third, although stepwise multiple regression analyses were conducted to compare the relative contributions, over one-third of the contributory factors are still unknown. Finally, although the HADS has been widely used in clinical practice and research, it is a screening instrument rather than a diagnostic test (44). The diagnosis of anxiety or depressive disorder should be confirmed using a structured clinical interview and the DSM-V (29).
In conclusion, our results suggest that depression contributes to the HRQOL or health status in patients with IPF. Depression status should be included in the assessment of patients with IPF.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
This study was partially supported by a grant to the Diffuse Lung Disease Research Group from the Ministry of Health, Labor and Welfare, Japan and the NPO Respiratory Disease Conference.
References
- 1.Zheng D, Macera CA, Croft JB, Giles WH, Davis D, Scott WK. Major depression and all-cause mortality among white adults in the United States. Ann Epidemiol 7: 213-218, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 370: 851-858, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 31: 58-69, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Lee AS, Mira-Avendano I, Ryu JH, Daniels CE. The burden of idiopathic pulmonary fibrosis: an unmet public health need. Respir Med 108: 955-967, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Blakemore A, Dickens C, Guthrie E, et al. Depression and anxiety predict health-related quality of life in chronic obstructive pulmonary disease: systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 9: 501-512, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swigris JJ, Brown KK, Make BJ, Wamboldt FS. Pulmonary rehabilitation in idiopathic pulmonary fibrosis: a call for continued investigation. Respir Med 102: 1675-1680, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belkin A, Swigris JJ. Health-related quality of life in idiopathic pulmonary fibrosis: where are we now? Curr Opin Pulm Med 19: 474-479, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Natsuizaka M, Chiba H, Kuronuma K, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med 190: 773-779, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Raghu G, Collard HR, Egan JJ, et al. ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788-824, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taniguchi H, Ebina M, Kondoh Y, et al. ; Pirfenidone Clinical Study Group in J Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 35: 821-829, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Richeldi L, du Bois RM, Raghu G, et al. ; INPULSIS Trial Investigators Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370: 2071-2082, 2014. [DOI] [PubMed] [Google Scholar]
- 12.King TE Jr, Bradford WZ, Castro-Bernardini S, et al. Group AS. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083-2092, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Swigris JJ, Kuschner WG, Jacobs SS, Wilson SR, Gould MK. Health-related quality of life in patients with idiopathic pulmonary fibrosis: a systematic review. Thorax 60: 588-594, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swigris JJ, Esser D, Conoscenti CS, Brown KK. The psychometric properties of the St George's Respiratory Questionnaire (SGRQ) in patients with idiopathic pulmonary fibrosis: a literature review. Health Qual Life Outcomes 12: 124, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, et al. Force AET. Standardisation of spirometry. Eur Respiratory J 26: 319-338, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Guideline of respiratory function tests--spirometry, flow-volume curve, diffusion capacity of the lung. Nihon Kokyuki Gakkai zasshi (The Journal of the Japanese Respiratory Society) 42 Suppl: 1-56, 2004(in Japanese). [PubMed] [Google Scholar]
- 17. Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) website, 2016 .
- 18.Laboratories ATSCoPSfCPF ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166: 111-117, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Eaton T, Young P, Milne D, Wells AU. Six-minute walk, maximal exercise tests: reproducibility in fibrotic interstitial pneumonia. Am J Respir Crit Care Med 171: 1150-1157, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Lama VN, Flaherty KR, Toews GB, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med 168: 1084-1090, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 145: 1321-1327, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T. Comparison of discriminative properties among disease-specific questionnaires for measuring health-related quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 157: 785-790, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Miravitlles M, Soriano JB, Garcia-Rio F, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax 64: 863-868, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 85: 751-758, 1984. [DOI] [PubMed] [Google Scholar]
- 25.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T. Analysis of clinical methods used to evaluate dyspnea in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 158: 1185-1189, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Matsudaira T, Igarashi H, Kikuchi H, et al. Factor structure of the Hospital Anxiety and Depression Scale in Japanese psychiatric outpatient and student populations. Health Qual Life Outcomes 7: 42, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 67: 361-370, 1983. [DOI] [PubMed] [Google Scholar]
- 28.Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes 1: 29, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.In: Diagnostic and Statistical Manual of Mental Disorder. 5th ed American Psychiatric Association, 2013. [Google Scholar]
- 30.Puhan MA, Frey M, Buchi S, Schunemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes 6: 46, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Boer S, Kolbe J, Wilsher ML. The relationships among dyspnoea, health-related quality of life and psychological factors in sarcoidosis. Respirology 19: 1019-1024, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Ryerson CJ, Berkeley J, Carrieri-Kohlman VL, Pantilat SZ, Landefeld CS, Collard HR. Depression and functional status are strongly associated with dyspnea in interstitial lung disease. Chest 139: 609-616, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Ryerson CJ, Cayou C, Topp F, et al. Pulmonary rehabilitation improves long-term outcomes in interstitial lung disease: a prospective cohort study. Respir Med 108: 203-210, 2014. [DOI] [PubMed] [Google Scholar]
- 34.Nishiyama O, Taniguchi H, Kondoh Y, et al. Health-related quality of life in patients with idiopathic pulmonary fibrosis. What is the main contributing factor? Respir Med 99: 408-414, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Chang JA, Curtis JR, Patrick DL, Raghu G. Assessment of health-related quality of life in patients with interstitial lung disease. Chest 116: 1175-1182, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Verma G, Marras T, Chowdhury N, Singer L. Health-related quality of life and 6 min walk distance in patients with idiopathic pulmonary fibrosis. Can Respir J 18: 283-287, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han MK, Swigris J, Liu L, et al. Gender influences Health-Related Quality of Life in IPF. Respir Med 104: 724-730, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holland AE, Hill CJ, Glaspole I, Goh N, Dowman L, McDonald CF. Impaired chronotropic response to 6-min walk test and reduced survival in interstitial lung disease. Respir Med 107: 1066-1072, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Nishiyama O, Taniguchi H, Kondoh Y, et al. A simple assessment of dyspnoea as a prognostic indicator in idiopathic pulmonary fibrosis. Eur Respiratory J 36: 1067-1072, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Esler M. The sympathetic system and hypertension. Am J Hypertens 13: 99S-105S, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Cohen S, Tyrrell DA, Smith AP. Negative life events, perceived stress, negative affect, and susceptibility to the common cold. J Pers Soc Psychol 64: 131-140, 1993. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen A, Zachariae R, Bovbjerg DH. Influence of psychological stress on upper respiratory infection: a meta-analysis of prospective studies. Psychosom Med 72: 823-832, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Laurin C, Moullec G, Bacon SL, Lavoie KL. Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med 185: 918-923, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 52: 69-77, 2002. [DOI] [PubMed] [Google Scholar]