Abstract
We report a case of a 70-year-old man with acute acalculous cholecystitis caused by Giardia lamblia. Contrast-enhanced computed tomography (CT) showed distention of the gallbladder due to a pericholecystic abscess without gallstones. Magnetic resonance cholangiopancreatography and drip infusion cholecystocholangiography-CT demonstrated a stricture of the hilar bile duct and cystic duct obstruction. We conducted transpapillary bile duct brush cytology and a biopsy of the hilar bile duct stricture; numerous active trophozoites of Giardia lamblia were observed without malignant findings. We considered this bile duct lesion to be biliary giardiasis. Biliary giardiasis should be taken into consideration when diagnosing acute acalculous cholecystitis.
Keywords: Giardia lamblia, acute acalculous cholecystitis, biliary giardiasis, metronidazole
Introduction
Acute acalculous cholecystitis is an acute inflammation of the gallbladder in the absence of gallstones. It comprises from 2-15% of all cases of acute cholecystitis and occurs in critically ill patients (1,2). The causes of acute acalculous cholecystitis include severe infections, malignant diseases, trauma, surgery, and systemic diseases, and it is frequently associated with more serious morbidities and higher mortality rates than calculous cholecystitis (3,4).
Giardiasis is caused by the protozoa Giardia lamblia and is a travel-related infectious disease, sexually transmitted disease, and opportunistic infectious disease (5). The number of patients with giardiasis is comparatively high in developing countries; however, the condition occurs worldwide (6,7). G. lamblia attaches to the mucosal tissues of the duodenum and upper small intestine and causes persistent diarrhea or malabsorption, which is associated with body weight loss (8). Although many cases of intestinal giardiasis have been reported, few involved the biliary tract. Accordingly, the clinical manifestations of biliary giardiasis have not been fully clarified.
We describe a rare case of acute acalculous cholecystitis due to biliary infection by G. lamblia.
Case Report
A 70-year-old man was admitted to our hospital in September 2015 because of upper abdominal pain and a fever. His medical history included rheumatoid arthritis and pulmonary emphysema, and he was taking oral immunosuppressive agents, such as methotrexate and adalimumab. He used well water for drinking and had kept a Maltese dog as a pet for 30 years. Moreover, he had traveled to southeast Asian countries about 10 years earlier. On a physical examination, the patient had a body temperature of 38.3℃, pulse rate of 89 beats per minute, and blood pressure of 163/78 mmHg. His abdomen was soft and flat but revealed right-upper-quadrant tenderness with Murphy's sign. Laboratory data showed an elevated white blood cell count and serum C-reactive protein level at 12,600/mm3 (normal range, 3,600-9,600/mm3) and 12.9 mg/dL (normal range, ≤0.3 mg/dL), respectively. His serum total bilirubin, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and γ-glutamyl transpeptidase levels were slightly elevated to 0.69 mg/dL (normal range, 0.3-1.2 mg/dL), 50 U/L (normal range, 13-33 U/L), 13 U/L (normal range, 6-30 U/L), 594 U/L (normal range, 100-340 U/L), and 80 U/L (normal range, 10-47 U/L), respectively. Regarding tumor markers, his serum levels of carcinoembryonic antigen and carbohydrate antigen 19-9 were 1.8 U/mL (normal range, <5.0 ng/mL) and 474 U/mL (normal range, <37 U/mL), respectively.
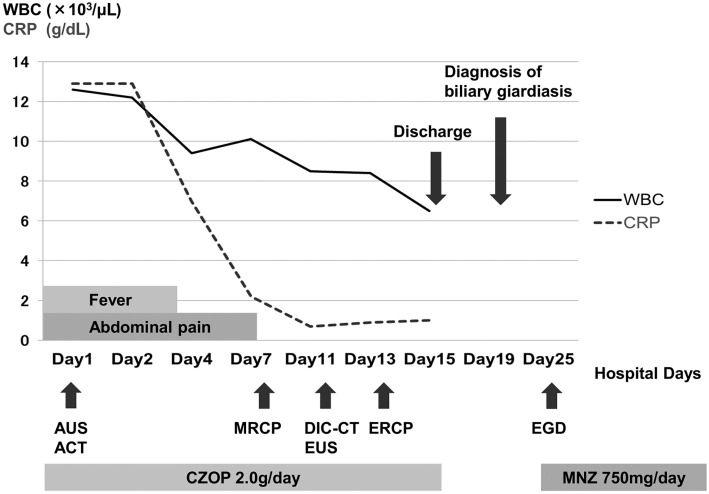
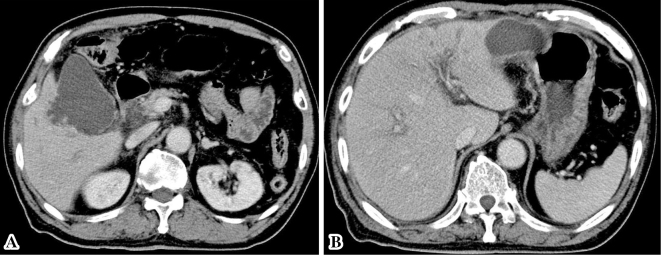
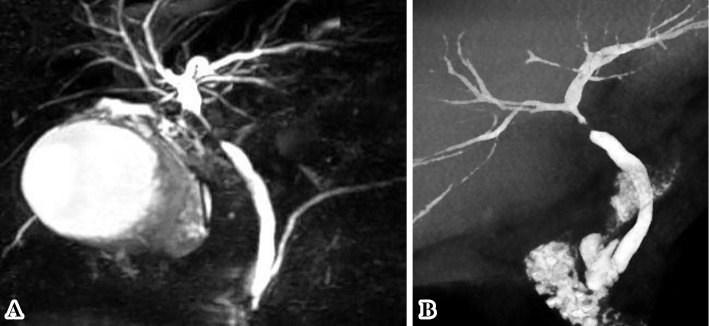
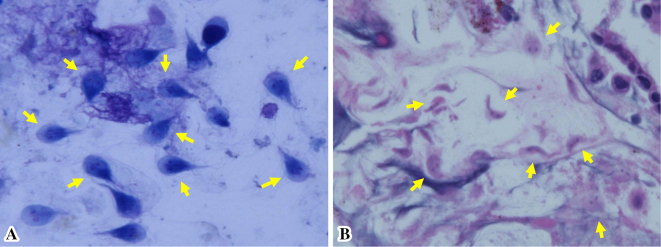
Fig. 1 provides a timeline of the patient's clinical course as described in this article. Abdominal ultrasonography and contrast-enhanced computed tomography (CT) showed distention of the gallbladder with peripheral cholecystic and perihepatic fluid collections without gallstones (Fig. 2). Pericholecystic fluid collection was irregularly rounded with peripheral rim enhancement and a hypodense lesion in segment 5 of the liver, which communicated with the gallbladder. Based on these findings, we diagnosed the patient with acute cholecystitis with pericholecystic abscess. We considered performing percutaneous trans-hepatic gallbladder drainage (PTGBD). However, there was a possibility of acute acalculous cholecystitis due to malignant biliary disease at the time of hospital admission. PTGBD was considered at risk for abdominal dissemination via a PTGBD tube; therefore, we started conservative therapy without gallbladder drainage. The patient received empirical antibiotic therapy with cefozopran 2.0 g per day intravenously from the date of hospital admission for 14 days and experienced clinical improvement with amelioration of his abdominal pain and overall condition on Day 7. Magnetic resonance cholangiopancreatography (MRCP) on Day 7 and drip infusion cholecystocholangiography (DIC)-CT on Day 11 demonstrated a stricture of the hilar bile duct and cystic duct obstruction (Fig. 3). As gallstones were not noted on these imaging examinations, we performed endoscopic ultrasonography (EUS) on Day 11 to rule out cholangiocarcinoma. However, we could not detect the hilar bile duct, and a detailed observation of the target lesion was impossible by EUS. Thus, endoscopic retrograde cholangiopancreatography (ERCP) was necessary to rule out malignant biliary disease. ERC on Day 13 revealed a slightly stenotic lesion of the hilar bile duct with a smooth luminal surface (Fig. 4A), and transpapillary intraductal ultrasonography (IDUS) of the bile duct indicated a continuously thickened wall from the upper to the lower bile duct with a smooth circular symmetric outer margin, a smooth inner margin, and a homogenous internal echo pattern (Fig. 4B). We conducted transpapillary bile duct brush cytology and a biopsy of the hilar bile duct stricture for histopathological evaluation, which revealed numerous active trophozoites of G. lamblia but no malignant findings (Fig. 5). After ruling out malignant biliary disease based on both the ERC and IDUS findings, we did not perform endoscopic nasobiliary drainage for further cytological examinations. We tried to identify G. lamblia from fecal samples and the duodenum in order to prove that the pathogenesis of biliary giardiasis involved access to the bile duct via the ampulla of Vater. Although upper gastrointestinal endoscopy revealed no abnormal duodenal findings, we performed a random duodenal biopsy, and G. lamblia was detected in both a stool sample and biopsy specimens of the duodenum. Therefore, we considered this bile duct lesion to be biliary giardiasis and ultimately made a diagnosis of acute acalculous cholecystitis caused by G. lamblia.
Figure 1.
Timeline of the patient’s clinical course. AUS: abdominal ultrasonography, ACT: abdominal computed tomography, MRCP: magnetic resonance cholangiopancreatography, DIC-CT: drip infusion cholecystocholangiography, EUS: endoscopic ultrasonography, ERCP: endoscopic retrograde cholangiopancreatography, EGD: esophagogastroduodenoscopy, CZOP: cefozopran, MNZ: metronidazole
Figure 2.
Contrast-enhanced abdominal computed tomography (CT) on admission revealed distention of the gallbladder with peripheral cholecystic (A) and perihepatic (B) fluid collections without gallstones.
Figure 3.
Magnetic resonance cholangiopancreatography (MRCP) (A) and drip infusion cholecystocholangiography (DIC) -CT (B) findings before metronidazole treatment. The images demonstrated a stricture of the hilar bile duct and cystic duct obstruction.
Figure 4.
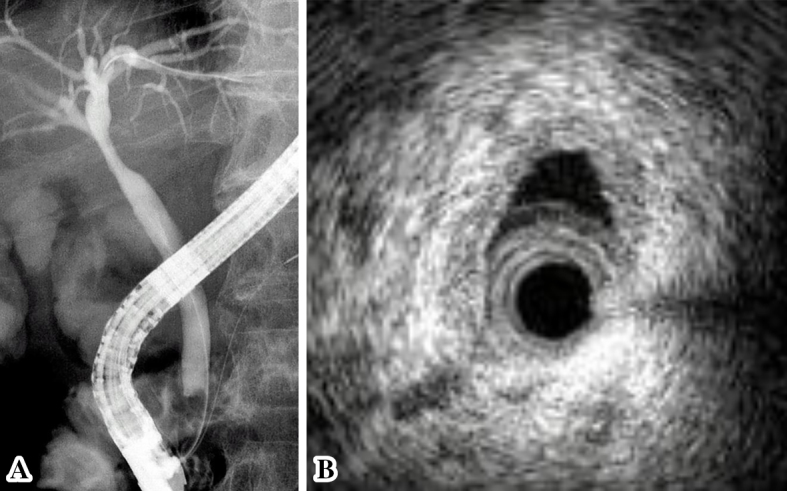
(A) Endoscopic retrograde cholangiography revealed a slightly stenotic lesion of the hilar bile duct, and we conducted transpapillary bile duct brush cytology and a biopsy of the hilar bile duct stricture. (B) Transpapillary intraductal ultrasonography of the bile duct indicated a continuously thick wall from the upper to the lower bile duct with a smooth circular symmetric outer margin, a smooth inner margin, and a homogenous internal echo pattern.
Figure 5.
A histopathological evaluation due to the findings on transpapillary bile duct brush cytology (A) and the biopsy (B). (A) Numerous active trophozoites of Giardia lamblia (arrows) (Giemsa staining×100). (B) Numerous active trophozoites of Giardia lamblia (arrows) in the bile duct wall but no malignant findings (Hematoxylin and Eosin staining ×100).
The patient was discharged from our hospital on Day 15. After the diagnosis of biliary giardiasis on Day 19, the patient was treated with metronidazole 250 mg 3 times per day on Day 25 for 7 days. Three months after initiation of metronidazole therapy, follow-up MRCP and DIC-CT were conducted to validate the efficacy of metronidazole therapy for biliary tract and disprove malignant disease. MRCP and DIC-CT demonstrated dramatic improvement in the hilar bile duct stricture, and a cystic duct was clearly detected (Fig. 6). Furthermore, the pericholecystic abscess had disappeared, and the gallbladder exhibited a normal appearance. The patient is currently undergoing outpatient follow-up, and no relapse has occurred to date.
Figure 6.
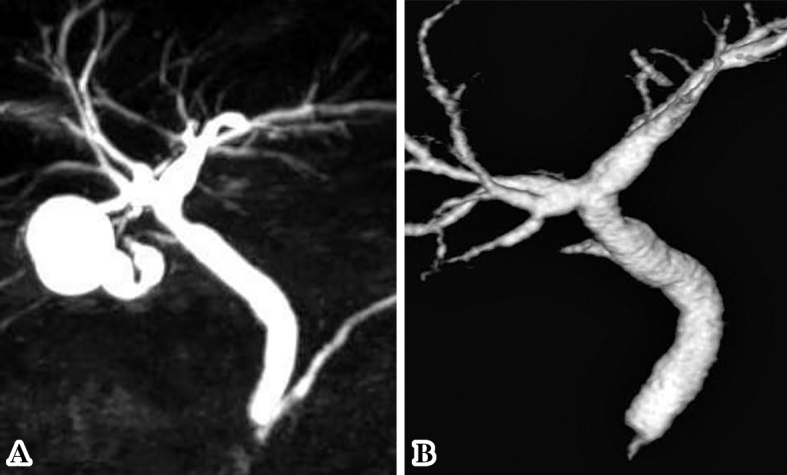
MRCP (A) and DIC-CT (B) findings after metronidazole treatment. The images demonstrated dramatic improvement in the hilar bile duct stricture, and a cystic duct was detected.
Discussion
We describe a case of acute acalculous cholecystitis due to G. lamblia. Giardiasis is a diarrheal disease caused by G. lamblia, which is a common intestinal protozoan parasite of humans. The prevalence of giardiasis is 2-5% in developed countries and 20-30% in developing and underdeveloped countries, largely due to unsanitary conditions (8,9). G. lamblia infects approximately 2% of adults and 6-8% of children in developed countries worldwide (5). Accordingly, giardiasis is a global disease. The lifecycle of G. lamblia comprises an active form known as a trophozoite and an inactive form called a cyst. Trophozoites can be found in feces but rarely survive for a prolonged period outside the host. In contrast, cysts are the infectious and resistant form and can survive for several months outside the host. Giardiasis can be spread by ingestion of G. lamblia in feces from an infected person or animal, drinking water from sources contaminated with G. lamblia, such as untreated water from lakes or wells, and contact with a person suffering from giardiasis (5,8). Hence, travel to countries in which giardiasis is common is associated with a risk of infection. In the present case, the patient frequently drank water from wells in a mountainous area and had a pet dog. Moreover, he had traveled to southeast Asian countries about 10 years earlier. Therefore, the ingestion of contaminated water or food containing G. lamblia was likely the source of his infection. Immunodeficiency, hypochlorhydria, and achlorhydria increase the susceptibility to giardiasis (10) and may be associated with persistent infection. The patient did not have a history of proton pump inhibitor or histamine H2 receptor antagonist intake; however, he had been taking methotrexate and adalimumab regularly for rheumatoid arthritis, which might have led to the development of giardiasis.
A definitive diagnosis requires the microscopic identification of cysts or trophozoites of G. lamblia (11). However, Giardia infection is rarely diagnosed because organisms are excreted only intermittently. Therefore, multiple stool specimens collected over several days are usually required to detect G. lamblia. Gardner et al. reported that one stool sample facilitates the detection of 60-80% of infections, two stool samples 80-90%, and three stool samples enable detection of >90% of infections (5,12,13). In patients who cannot be diagnosed by an examination of the stool, endoscopy with duodenal fluid sampling and a biopsy should be performed (5). In this case, G. lamblia was detected in duodenal and biliary biopsy specimens, which facilitated the diagnosis of giardiasis.
Giardia infection has various clinical intestinal manifestations, including diarrhea, abdominal cramps, and upset stomach or nausea (5,10,14,15). Active trophozoites attach to the mucous membrane of the duodenum and upper jejunum, the alkaline pH of which favors their growth, which is responsible for the manifestations of giardiasis. These symptoms can last for 1 to 2 weeks or longer and may lead to weight loss. Concerning biliary giardiasis, few cases have been reported. The literature in the MEDLINE database was searched for English-language papers from the year of database inception to August 2016. The search strategies included terms for Giardia lamblia, biliary, cholangitis, cholecystitis, and biliary giardiasis. The literature search in the database generated only 6 articles with an abstract available after reviewing the titles and abstracts for eligibility (16-21). In the present case, the pathogenesis of biliary giardiasis involved access to the bile duct by the active trophozoite via the ampulla of Vater, followed by attachment to the bile duct wall. An imaging examinations showed no gallbladder stones but did reveal a biliary stenosis at the hilar bile duct with acute cholecystitis. The distended gallbladder might have partially compressed the hilar bile duct. Therefore, one cause of biliary stricture is thought to be a Mirrizzi syndrome, in a broad sense. However, we suspected that cholangiocarcinoma may have been the cause of cholecystitis; therefore, ERCP was performed to detect malignant disease. Bile duct brush cytology and a biopsy of the hilar bile duct stricture revealed numerous active G. lamblia trophozoites, which resulted in a diagnosis of biliary giardiasis. On comparing Fig. 3 (MRCP) and Fig. 4A (ERC), we found that these cholangiography results were slightly different from each other. A severe stenotic lesion was observed in the hilar bile duct with MRCP despite the presence of mild hilar biliary stricture with ERC. The bile duct wall of biliary giardiasis is flexible and relatively easy to expand using the pressure of contrast medium during the ERC procedure. This elasticity of the thickened bile duct wall may have been the cause of the discrepancy between the MRCP and ERC findings. The cystic duct was occluded by cholangitis with giardiasis at the time of admission, which was considered to be the direct cause of acute cholecystitis. The cystic duct obstruction and hilar bile duct stricture were ameliorated after recovery from cholangitis due to metronidazole treatment.
The most common antibiotics used for the treatment of giardiasis are the 5-nitroimidazoles, which include metronidazole, tinidazole, secnidazole, and ornidazole, with metronidazole being the most common (5,22,23). The efficacy rate of monotherapy with a single course of treatment varies from 60% to 100%, with an average of over 80% (5,7). Metronidazole is typically administered at a dose of 250 mg 3 times per day for 5-7 days for adults and has few side effects. The patient was administered metronidazole after amelioration of acute cholecystitis, and the abnormal lesions in the biliary tract showed almost complete improvement. Accordingly, metronidazole is considered to be efficacious as a first-line treatment for biliary giardiasis. However, several cases of treatment failure with nitroimidazoles for giardiasis have also been reported (7,24,25). The treatment of such cases of refractory biliary giardiasis should be reconsidered.
In summary, we report a case of acute acalculous cholecystitis with bile duct stricture due to G. lamblia. Acute acalculous cholecystitis is generally associated with major cardiac and abdominal surgery, malignant disease, severe trauma, burns, prolonged fasting, and long-term total parenteral nutrition. However, biliary giardiasis should be considered as a cause as well. Because it is impossible to diagnose biliary giardiasis from only imaging findings, the first step to diagnose it is to ask patients detailed questions. Therefore, taking a thorough medical history, including inquiries into their habits, pets, travel history, and medications, is fundamental and essential for diagnosing biliary giardiasis. The accumulation of further similar cases is necessary to clarify the pathophysiology of biliary giardiasis.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Ganpathi IS, Diddapur RK, Eugene H, Karim M. Acute acalculous cholecystitis: challenging the myths. HPB (Oxford) 9: 131-134, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poddighe D, Cagnoli G, Mastricci N, Bruni P. Acute acalculous cholecystitis associated with severe EBV hepatitis in an immunocompetent child. BMJ Case Rep 2014: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Branco L, Vieira M, Couto C, Coelho MD, Laranjeira C. Acute acalculous cholecystitis by Epstein-Barr virus infection: a rare association. Infect Dis Rep 7: 6184, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Savoca PE, Longo WE, Zucker KA, McMillen MM, Modlin IM. The increasing prevalence of acalculous cholecystitis in outpatients. Results of a 7-year study. Ann Surg 211: 433-437, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardner TB, Hill DR. Treatment of giardiasis. Clin Microbiol Rev 14: 114-128, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Escobedo AA, Cimerman S. Giardiasis: a pharmacotherapy review. Expert Opin Pharmacother 8: 1885-1902, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Lopez-Velez R, Batlle C, Jimenez C, Navarro M, Norman F, Perez-Molina J. Short course combination therapy for giardiasis after nitroimidazole failure. Am J Trop Med Hyg 83: 171-173, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Solaymani-Mohammadi S, Genkinger JM, Loffredo CA, Singer SM. A meta-analysis of the effectiveness of albendazole compared with metronidazole as treatments for infections with Giardia duodenalis. PLoS Negl Trop Dis 4: e682, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farthing MJ. Diarrhoeal disease: current concepts and future challenges. Pathogenesis of giardiasis. Trans R Soc Trop Med Hyg 3 87 Suppl: 17-21, 1993. [DOI] [PubMed] [Google Scholar]
- 10. Wolfe MS. Giardiasis. Clin Microbiol Rev 5: 93-100, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnston SP, Ballard MM, Beach MJ, Causer L, Wilkins PP. Evaluation of three commercial assays for detection of Giardia and Cryptosporidium organisms in fecal specimens. J Clin Microbiol 41: 623-626, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hiatt RA, Markell EK, Ng E. How many stool examinations are necessary to detect pathogenic intestinal protozoa? Am J Trop Med Hyg 53: 36-39, 1995. [PubMed] [Google Scholar]
- 13. Goka AK, Rolston DD, Mathan VI, Farthing MJ. The relative merits of faecal and duodenal juice microscopy in the diagnosis of giardiasis. Trans R Soc Trop Med Hyg 84: 66-67, 1990. [DOI] [PubMed] [Google Scholar]
- 14. Farthing MJ. Giardia comes of age: progress in epidemiology, immunology and chemotherapy. J Antimicrob Chemother 30: 563-566, 1992. [DOI] [PubMed] [Google Scholar]
- 15. Sullivan PB, Marsh MN, Phillips MB, et al. Prevalence and treatment of giardiasis in chronic diarrhoea and malnutrition. Arch Dis Child 66: 304-306, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soto JM, Dreiling DA. Giardia lamblia. A case presentation of chronic cholecystitis and duodenitis. Am J Gastroenterol 67: 265-269, 1977. [PubMed] [Google Scholar]
- 17. McGowan JM, Nussbaum CC, Burroughs EW. Cholecystitis due to Giardia lamblia in a left-sided gallbladder. Ann Surg 128: 1032-1037, 1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhatia V, Garg PK, Agarwal V, Sharma M, Ray S. Inflammatory papillary stenosis due to Giardia lamblia in a patient with hyper-immunoglobulin M immunodeficiency syndrome. Gastrointest Endosc 66: 181-182; discussion 182, 2007. [DOI] [PubMed] [Google Scholar]
- 19. Aronson NE, Cheney C, Rholl V, Burris D, Hadro N. Biliary giardiasis in a patient with human immunodeficiency virus. J Clin Gastroenterol 33: 167-170, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Perez-Martin G, Gomez-Cerezo J, Codoceo R, et al. Bilirubinate granules: main pathologic bile component in patients with idiopathic acute pancreatitis. Am J Gastroenterol 93: 360-362, 1998. [DOI] [PubMed] [Google Scholar]
- 21. el Sheikh Mohamed AR, al Karawi MA, Yasawy MI. Modern techniques in the diagnosis and treatment of gastrointestinal and biliary tree parasites. Hepatogastroenterology 38: 180-188, 1991. [PubMed] [Google Scholar]
- 22. Pasupuleti V, Escobedo AA, Deshpande A, Thota P, Roman Y, Hernandez AV. Efficacy of 5-nitroimidazoles for the treatment of giardiasis: a systematic review of randomized controlled trials. PLoS Negl Trop Dis 8: e2733, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zaat JO, Mank TG, Assendelft WJ. A systematic review on the treatment of giardiasis. Trop Med Int Health 2: 63-82, 1997. [DOI] [PubMed] [Google Scholar]
- 24. Nash TE, Ohl CA, Thomas E, Subramanian G, Keiser P, Moore TA. Treatment of patients with refractory giardiasis. Clin Infect Dis 33: 22-28, 2001. [DOI] [PubMed] [Google Scholar]
- 25. Munoz Gutierrez J, Aldasoro E, Requena A, et al. Refractory giardiasis in Spanish travellers. Travel Med Infect Dis 11: 126-129, 2013. [DOI] [PubMed] [Google Scholar]