Abstract
A 71-year-old man with hypertension and diabetes mellitus presented with proteinuria. Laboratory data showed proteinuria of 3.1 g/g creatinine, serum albumin of 3.5 g/dL and serum creatinine of 1.03 mg/dL without autoantibodies. A renal biopsy revealed segmental granular IgG depositions on glomerular capillary walls. Electron microscopy showed segmentally subepithelial, intramembranous and mesangial deposits. Diffuse segmental membranous glomerulonephritis (MGN) was diagnosed with only IgG1 deposition and without M-type phospholipase A2 receptor or thrombospondin type-1 domain-containing 7A staining, suggesting secondary MGN with an unknown target antigen in immune deposits. Physicians should keep in mind the existence of segmental MGN to better understand the clinicopathological characteristics.
Keywords: adult, IgG subclass, membranous glomerulonephritis, renal pathology, secondary membranous glomerulonephritis
Introduction
Membranous glomerulonephritis (MGN) is characterized by globally distributed subepithelial deposits along the glomerular capillary walls (GCWs), but a small number of cases with segmentally distributed subepithelial deposits, called segmental MGN, have been reported, mostly in children (1-5). Certain pathological differences between global MGN and segmental MGN have been reported in the pediatric cases, suggesting that segmental MGN is a distinct clinical entity (4,5). However, there are few reports of segmental MGN in adults and no information as to whether it is primary MGN (including podocyte antigen-associated MGN) or secondary MGN, which is usually associated with systemic conditions such as systemic lupus erythematosus, infection, cancer or drug exposure (6).
We herein report an elderly man with diffusely distributed segmental membranous changes in the GCWs. We further examined the glomerular IgG subclass (7,8) and podocyte-related antigens (9,10) to differentiate idiopathic MGN and secondary MGN, and we briefly review the relevant literature.
Case Report
A 71-year-old man was admitted to our hospital because of persistent proteinuria. He had a history of hypertension for 10 years, diabetes mellitus for 4 years and old cerebellar infarction which occurred 3 years prior. Proteinuria had been negative 17 months prior and was first pointed out 13 months prior. His serum creatinine level had been around 1.0 mg/dL for several years. His proteinuria increased from 1+ to 3+ by a dipstick test without hematuria 3 months prior to admission. He was taking 2.5 mg amlodipine besylate once daily, 50 mg vildagliptin twice daily and 75 mg clopidogrel sulfate once daily. The patient had no history of taking other medications or supplements. His physical examination on admission showed height of 162.5 cm, body weight of 77 kg, blood pressure of 118/70 mmHg and no leg edema. Laboratory-test results showed urinary protein excretion of 3.1 g/g creatinine without hematuria and pyuria, serum albumin of 3.5 g/dL, serum creatinine of 1.03 mg/dL (corresponding to an estimated glomerular filtration rate [eGFR] of 54.1 mL/min/1.73 m2 as calculated by the revised serum creatinine-based Japanese equation (11)) and HbA1c of 7.0%. Results of serology showed IgG of 1,270 mg/dL, IgA of 188 mg/dL, IgM of 223 mg/dL and C-reactive protein of 2.5 mg/dL. Other serology such as anti-nuclear antigen, anti-dsDNA antibody, complements, immune complex, hepatitis B surface antigen, hepatitis C antibody, prostate-specific antigen, carcinoembryonic antigen, cancer antigen 19-9, squamous cell carcinoma and prostate-specific antigen were within reference ranges. Urine immunoelectrophoresis showed no monoclonal component. C-reactive protein was positive whereas there was no apparent focus of infection, inflammatory diseases or mass lesions by imaging tests, including chest X-ray, abdominal ultrasound and abdominal computed tomography. A fundoscopic examination revealed the existence of simple diabetic retinopathy.
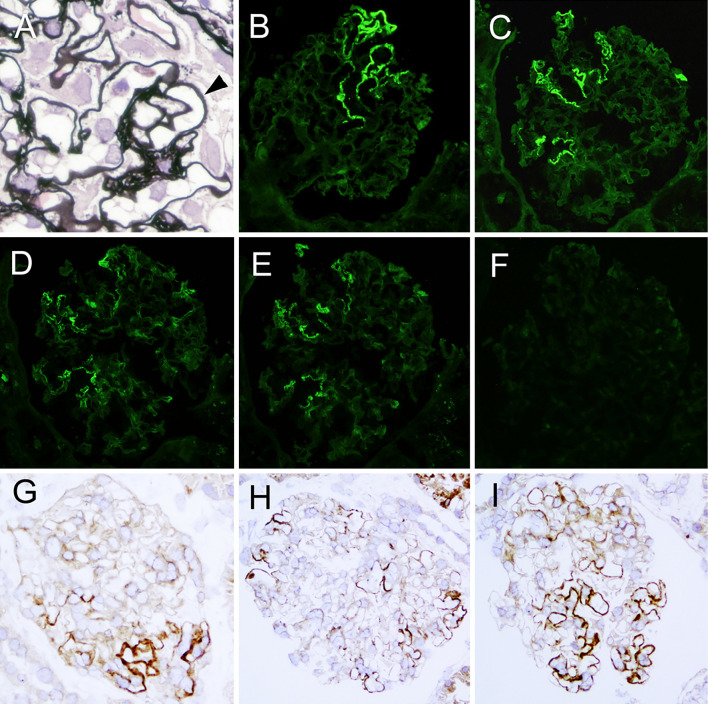
A renal biopsy showed 5 global sclerosis and 3 ischemic changes out of 28 obtained glomeruli. All global sclerotic glomeruli were in the cortical scar lesions. The remaining 20 glomeruli showed mild mesangial expansion, no mesangial hypercellularity and no exudative lesions (Fig. 1A). Segmental thickening, spike formation (Fig. 1A) and vacuolization along the very limited GCWs were found in each glomerulus. The small arteries showed approximately 20% tubulointerstitial damage and atherosclerotic changes. An immunofluorescence study showed negative staining for IgA, IgM, C1q and C3 but granular staining for IgG (Fig. 1B) and κ and λ light chains along segmental GCWs (Fig. 1D and E), suggesting the deposition of polyclonal IgG antibody. No immunoreactants were found in the tubulointerstitial areas. The glomerular IgG subclass consisted of IgG1 (Fig. 1C), but IgG2, IgG3 or IgG4 and glomerular M-type phospholipase A2 receptor (PLA2R) was negative (Fig. 1F). Immunohistochemistry for IgG showed segmental granular IgG staining along GCWs in not than half of the glomerular tuft (Fig. 1G-I), which was found in all of the glomeruli examined. There was no apparent trend in the distribution of IgG staining within a glomerulus. Antigen retrieval immunohistochemistry for PLA2R and thrombospondin type-1 domain-containing 7A (THSD7A) according to the method reported by Iwakura et al. (12) was negative in the glomeruli. When “diffuse” was defined as “a lesion involving most (≥50%) glomeruli” and “segmental” as “a lesion involving less than half of the glomerular tuft”, our case was diagnosed with diffuse segmental MGN. No apparent findings of diabetic nephropathy were found, but the decreased eGFR might have been caused by benign nephrosclerosis or ischemic renal damages.
Figure 1.
Light micrograph of a part of the glomerulus, showing spikes (arrow head) of the basement membrane in the segmental capillary loops (A). Immunofluorescent findings show segmental positive granular IgG (B), IgG1 (C), κ light chain (D) and λ light chain (E) staining along the glomerular capillary walls and negative M-type phospholipase A2 receptor staining (F). Indirect immunohistochemistry for IgG shows granular staining along the segmental glomerular capillary walls with a variety of distribution (G-I). A: periodic acid-methenamin silver. Original magnification 600×, B-I: Original magnification 200×. G-I; Polyclonal anti-human IgG (DAKO Japan, Japan) was used.
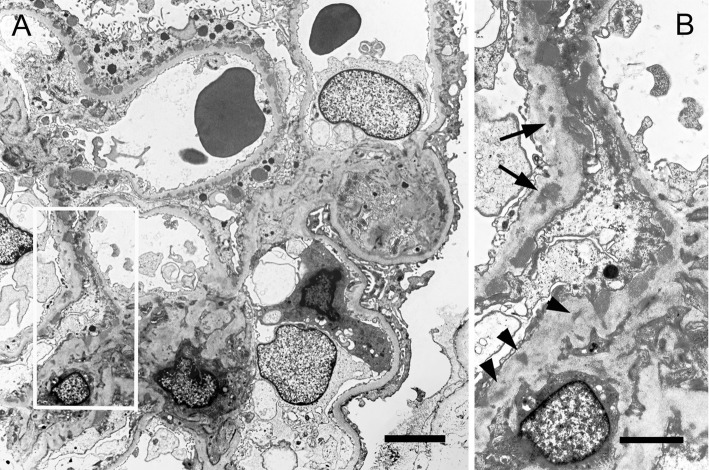
Electron microscopy revealed glomerular capillary loops without electron dense deposits (EDD) and with subepithelial EDDs (histological stage I to III according to Ehrenreich and Churg) and a small amount of intramembranous EDDs (Fig. 2). The amount and size of subepithelial EDDs were largely different among capillary loops (Fig. 2A). There was extensive effacement of the foot processes of the visceral epithelial cells over the subepithelial EDDs (Fig. 2A). Endothelial cells were unremarkable and did not contain tubuloreticular inclusions. EDDs were also contained in some mesangial areas (Fig. 2). The glomerular basement membrane (GBM) thickness on electron micrographs was measured at the 10 spots of the GCWs without EDDs, where the plane of the GBM was perpendicular to that of the section, showing a thickness of 348 nm with a standard deviation of 10 nm. This confirmed the lack of a thickened GBM indicating diabetic nephropathy. The electron microscopic findings confirmed segmental MGN.
Figure 2.
Electron-microscopic findings. Normal capillary loop (right lower part) and capillary loops with subepithelial electron dense deposits (EDDs) can be seen (A). The area of the square in Fig. 2A shows not only subepithelial EDDs but also intramembranous EDDs (arrows) and mesangial EDDs (arrow heads). A; Bar=5 μm, B; Bar=2 μm.
The patient was followed up with the angiotensin receptor blocker 100 mg irbesartan once daily together with 5 mg cilnidipine once daily in place of amlodipine besylate. An examination of the prostate and an endoscopic examination of the upper and lower gastrointestinal tracts did not show any findings of malignancy. Ten months after the renal biopsy, the patient's status had not progressively deteriorated, with proteinuria of 3.60 g/g creatinine, serum albumin of 3.7 g/dL and serum creatinine of 1.13 mg/dL, and no findings relevant to secondary MGN.
Discussion
Glomerulonephritis with segmental subepithelial immune deposits conventionally, called segmental MGN, is uncommon. Most cases have been reported in children (1-5), and some of the reported clinicopathological characteristics have been as follows: 1) mostly non-nephrotic-range proteinuria (2,3), 2) a good prognosis (3-5), 3) association with mesangial hypercellularity (5), 4) association with mesangial deposition (3-5), 5) frequent observation of C1q deposition (4,5) and 6) complement activation of the classical pathway associated with glomerular IgG1 and IgG3 staining, in contrast to global MGN, which has complement activation of both the alternative and lectin pathways associated with glomerular IgG2 and IgG4 staining (5). The pathological characteristics in reported cases indicate secondary MGN. Our elderly case also showed non-nephrotic-range proteinuria at the biopsy, but the follow-up period was too short to evaluate the prognosis. He did not have apparent mesangial hypercellularity or C1q deposition, but he showed mesangial deposits and glomerular IgG1 staining without IgG4, sharing some common characteristics with segmental MGN in children.
Primary MGN is mostly caused by in situ immune complex formation by autoantibodies against podocyte antigens, with positive glomerular PLA2R or THDS7A staining often associated with predominant IgG4 staining (13). We found that glomerular PLA2R and THSD7A staining were negative in our case. PLA2R or THDS7A antigen positivity is highly suggestive of a primary form of MN; however, antigen negativity is less helpful (12). Huang et al. reported that IgG4 was dominant or ‘codominant' in 76% of primary MN, and IgG1 was the most expressed IgG subclass in 60% of secondary MN (14). Therefore, it is quite unlikely that segmental MGN in our case is primary MGN, based on the immunopathological findings.
It is highly conceivable that the mechanisms of immune complex formation in secondary MGN may result from an accumulation of circulating immune complexes and/or in situ immune complex formation by planted proteins as antigens, not by anti-podocyte antibodies; as such, not only subepithelial EDDs but also mesangial EDDs have sometimes been found to be associated (15). Autoantigen-autoantibody type immune complex-mediated lupus nephritis Class V shows intramembranous, subendothelial and mesangial EDDs in addition to both global and segmental subepithelial EDDs. If podocyte antigens are only target antigens in the pathogenesis of primary MGN, anti-podocyte antibodies can theoretically form only subepithelial immune deposits. Therefore, the existence of intramembranous and mesangial EDDs may have been the most crucial finding when diagnosing secondary MGN in our case.
Our case showed diffuse distribution of glomeruli with segmental granular IgG deposition. In contrast, most reported cases with segmental MGN have stated that focal distribution of affected glomeruli or only segmental MGN; however, most cases have shown segmental membranous changes in the tissues on light microscopy, immunofluorescence studies and electron microscopy, even in tissue from re-biopsied kidney (1-5), suggesting that the segmental glomerular lesions might be diffusely distributed, as in our case. The mechanisms of the development of segmental subepithelial EDDs are largely unknown. Since subepithelial EDD shows histological stages I to III with significant changes in the glomerular basement membrane, the evolutionary phase does not indicate histologically early MGN. Some cases with segmental MGN in adults have been reported to be associated with other glomerular lesions, such as minimal change, hereditary glomerulonephritis, diabetic nephropathy and crescentic glomerulonephritis (2,16). Intraglomerular hemodynamic changes or alteration of the glomerular basement membrane itself caused by existing glomerular lesions might contribute to the segmental distribution of circulating immune complexes and/or planted antigens along the GCWs. Our case had diabetes and hypertension; however, glomeruli with segmental IgG deposition showed neither diabetic nephropathy nor apparent nephrosclerotic changes.
In conclusion, segmental MGN in our elderly case was concluded to be a pathologically secondary form of MGN. However, the patient had no clear underlying disease for secondary MGN. Careful follow-up is necessary while bearing in mind that underlying disease such as systemic lupus erythematosus, infectious disease or cancer may become clinically evident. Segmental MGN might have been ignored in some cases and, as a result, no further examinations were performed. Therefore, nephrologists and renal pathologists should be aware of the existence of segmental MGN in adults to better understand the clinical characteristics and pathophysiology of the unknown target antigen in immune deposits.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We would like to thank Hiromi Yamaguchi for her technical assistance.
References
- 1.Gaffney EF, Alexander RW, Donnelly WH. Segmental membranous glomerulonephritis. Arch Pathol Lab Med 106: 409-412, 1982. [PubMed] [Google Scholar]
- 2.Bertani T, Appel GB, D'Agati V, Nash MA, Pirani CL. Focal segmental membranous glomerulonephropathy associated with other glomerular diseases. Am J Kidney Dis 2: 439-448, 1983. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto S, Inaba S, Yoshida R, et al. Clinicopathological characteristics of the focal and segmental form of idiopathic membranous nephropathy: comparison with the typical form of this disease. Acta Paediatr Jpn 39: 349-353, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Obana M, Nakanishi K, Sako M, et al. Segmental membranous glomerulonephritis in children: comparison with global membranous glomerulonephritis. Clin J Am Soc Nephrol 1: 723-729, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Segawa Y, Hisano S, Matsushita M, et al. IgG subclasses and complement pathway in segmental and global membranous nephropathy. Pediatr Nephrol 25: 1091-1099, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Ronco P, Debiec H. Antigen identification in membranous nephropathy moves toward targeted monitoring and new therapy. J Am Soc Nephrol 21: 564-569, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Imai H, Hamai K, Komatsuda A, Ohtani H, Miura AB. IgG subclasses in patients with membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis. Kidney Int 51: 270-276, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Lönnbro-Widgren J, Ebefors K, Mölne J, Nyström J, Haraldsson B. Glomerular IgG subclasses in idiopathic and malignancy-associated membranous nephropathy. Clin Kidney J 8: 433-439, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck LH Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11-21, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomas NM, Beck LH Jr, Meyer-Schwesinger C, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371: 2277-2287, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982-992, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Iwakura T, Ohashi N, Kato A, Baba S, Yasuda H. Prevalence of enhanced granular expression of thrombospondin Type-1 domain-containing 7A in the glomeruli of Japanese patients with idiopathic membranous nephropathy. PLoS One 10: e0138841, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ronco P, Debiec H. Pathogenesis of membranous nephropathy: recent advances and future challenges. Nat Rev Nephrol 8: 203-213, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Huang CC, Lehman A, Albawardi A, et al. IgG subclass staining in renal biopsies with membranous glomerulonephritis indicates subclass switch during disease progression. Mod Pathol 26: 799-805, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Sinico RA, Mezzina N, Trezzi B, Ghiggeri GM, Radice A. Immunology of membranous nephropathy: from animal models to humans. Clin Exp Immunol 183: 157-165, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasr SH, Said SM, Valeri AM, et al. Membranous glomerulonephritis with ANCA-associated necrotizing and crescentic glomerulonephritis. Clin J Am Soc Nephrol 4: 299-308, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]