Abstract
Background
This article aims to comprehensively describe indications, contraindications, technical aspects, diagnostic accuracy and complications of percutaneous lung biopsy.
Methods
Imaging-guided biopsy currently represents one of the predominant methods for obtaining tissue specimens in patients with lung nodules; in many cases treatment protocols are based on histological information; thus, biopsy is frequently performed, when technically feasible, or in case other techniques (such as bronchoscopy with lavage) are inconclusive.
Results
Although a coaxial system is suitable in any case, two categories of needles can be used: fine-needle aspiration biopsy (FNAB) and core-needle biopsy (CNB), with the latter demonstrated to have a slightly higher overall sensitivity, specificity and accuracy.
Conclusion
Percutaneous lung biopsy is a safe procedure even though a few complications are possible: pneumothorax, pulmonary haemorrhage and haemoptysis are common complications, while air embolism and seeding are rare, but potentially fatal complications.
Teaching points
• Imaging-guided biopsy is one of the main methods to obtain lung nodule specimens.
• CT has the highest accuracy for diagnosis as an imaging guide.
• Compared to FNAB, CNB has a higher accuracy for diagnosis.
• Pneumothorax and parenchymal pulmonary haemorrhage care the most frequent complications.
• Several clinical and technical variables can affect diagnostic accuracy and patient safety.
Keywords: Lung lesion, Percutaneous biopsy, Complication, Diagnostic accuracy, Fine-needle aspiration biopsy (FNAB), Core-needle biopsy (CNB)
Introduction
Chest tumours, in particular lung cancer, remain one of the most common causes of cancer-related death worldwide. With the diffusion of spiral CT, an increasing number of lung and mediastinal lesions is detected and histological diagnosis is often necessary to determine the most appropriate management of these lesions [1]. In this clinical scenario, imaging-guided biopsy is one of the main methods to obtain tissue specimens. Various imaging techniques including computed tomography (CT) fluoroscopy and ultrasound (US) can be used to guide chest biopsies, but CT is most frequently employed because of its high spatial and contrast resolution as well as its 3D imaging ability; in many cases CT is preferred based on the localisation of the nodule or other patient-related factors.
Clinical indication
Clinical indications of imaging-guided chest biopsy have significantly changed from the introduction of the technique as a result of technical advances in needle technology, imaging modalities, pathological analysis and immunohistochemistry techniques. The introduction of PET-CT further modified these indications, reducing the percentage of unnecessary biopsies [2]. At present, the growing list of indications (Table 1) includes histological diagnosis of undetermined and otherwise not characterisable pulmonary, mediastinal and chest wall lesions as well as biopsy or re-biopsy of known malignant lesions to obtain histological material for targeted therapy. (Fig. 1).
Table 1.
Indications for imaging-guided chest biopsy
| Indications for imaging-guided chest biopsy |
|---|
| 1. A new or enlarging solitary nodule or mass |
| 2. Multiple nodules in a patient without known neoplastic disease or in prolonged remission |
| 3. Focal parechymal infiltrates in which an infectious organism cannot be isolated |
| 4. Diagnosis of hilar masses following negative broncoscopy |
| 5. Undiagnosed mediastinal mass. |
| 6. Bipopsy or re-biopsy of malignancy for targeted therapy. |
Fig. 1.
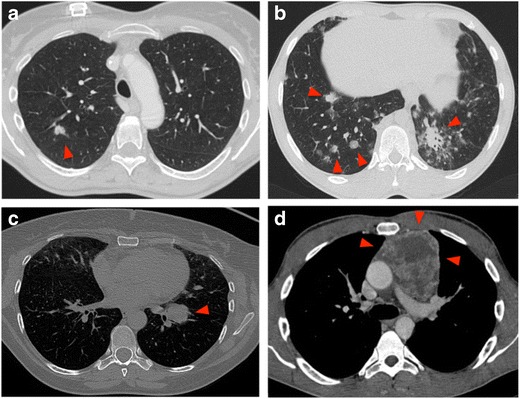
Indications for CT-guided chest biopsy. (a) Solitary pulmonary nodule. (b) Parenchymal infiltrates in which an infectious organism cannot be isolated. (c) Hilar mass following negative bronchoscopy. (d) Undiagnosed mediastinal mass
A new or enlarging solitary nodule or mass
The increasing clinical use of chest CT has resulted in an increasing incidental detection of small pulmonary nodules. In particular, pulmonary nodules are incidentally detected in around 8.5% of the general population [3] and in up to 51% of subjects with risk factors [4]. Therefore, a new or enlarging solitary nodule is the most common indication for imaging-guided chest biopsy. If a nodule is initially identified at conventional chest radiography, CT investigation is necessary to characterise the lesion, estimate the likelihood of malignancy [5] and identify lymphadenopathies or other accessible sites for biopsy (such as extra-thoracic metastases) [6, 7]. PET-CT examination may also be helpful in the management of pulmonary nodules larger than 1 cm, reducing the need of puncture of non-enhancing solid nodules [4–8] and with a sensitivity of 94% and a specificity of 83% for solid pulmonary nodules between 1 and 3 cm in diameter [9]. It must be taken in consideration that active inflammation (i.e. active tuberculosis, histoplasmosis, rheumatoid nodules) may cause false positives on PET-CT scans because of their high glucose metabolism [10]. On the other hand, low-grade malignant tumours, such as carcinoid [11] or low-grade adenocarcinoma [12], may produce false-negative results because of their low glucose metabolism. In these cases, careful follow-up with CT must be performed according to well-established guidelines to demonstrate regression/disappearance of the nodule after therapy or to address patients to biopsy if persistence or increase in size are evident [13].
Multiple nodules in a patient without known neoplastic disease or in prolonged remission
Several benign conditions can appear with slowly enlarging or multiple new pulmonary nodules, such as rheumatoid arthritis, sarcoidosis or infectious diseases, including tuberculosis and fungal infections, particularly in immunosuppressed patients [14–16]. In these cases appropriate clinical work-up and CT follow-up are sufficient to exclude malignancy. Nevertheless multiple nodular lesions may be the initial metastatic presentation in a patient with an unknown primary tumour or the sign of disease progression in a patient with known primary tumour in prolonged remission. In these cases imaging-guided biopsy may be necessary to confirm or exclude malignancy.
Focal parenchymal infiltrates in which an infectious organism cannot be isolated
When infectious organisms cannot be isolated from the culture of sputum, blood or lung lavage, lung biopsy can be necessary to obtain tissue samples for pathological examination. The biopsy sample can be cultured for diagnostic purposes to establish the most appropriate pharmacological treatment. Imaging-guided biopsy was demonstrated to have a sensitivity of 80% and a positive predictive value of 100% [17, 18].
Diagnosis of hilar masses following negative bronchoscopy
Transbronchial needle aspiration during bronchoscopy or under ultrasound guidance is still considered the reference technique for the diagnosis of hilar masses [19, 20]. However, when bronchoscopy cannot be performed or is inconclusive, hilar masses can be accurately diagnosed by imaging-guided biopsy.
Undiagnosed mediastinal mass
Mediastinal masses are most frequently located in the anterior mediastinum and include a variety of different entities, such as thymic malignancy, lymphomas, endocrine tumours and malignant germ cell tumours [21]. In the absence of typical clinical and imaging features, histological diagnosis is necessary [22]; imaging-guided biopsy should be always attempted in accessible lesions before diagnostic mediastinoscopy or in case of inconclusive results of mediastinoscopy.
Biopsy or re-biopsy of malignancy for targeted therapy
Advanced non-small-cell lung cancer in progression after first-line therapy may need imaging-guided re-biopsy to detect a possible new biological profile and to guide therapeutic decision-making in more than one-third of cases. [23].
Contraindications
Because of moderate risk of bleeding, abnormal clotting function or thrombocytopenia should be recognised and corrected before the procedure. Thus, oral anticoagulants and antiaggregant drugs should be reduced or stopped to reach an international normalised ratio (INR) of less than 1.5 and an activated partial thromboplastin time (aPTT) not more than 1.5 times the control value; platelet transfusion is recommended for counts <50,000/μl (Table 2) [24]. Suspected hydatid cyst or arterio-venous malformation should not be biopsied but can be identified on CT. Mechanical ventilation, which may lead to pneumothorax and favours air embolism, inability of a patient to co-operate during the procedure or to suspend respiration on request or control cough are the major contraindications. A unique functional lung is often considered another contraindication, while severe chronic obstructive pulmonary disease, pulmonary hypertension or cardiac insufficiency do not constitute absolute contraindications but increase the complication rate. [25].
Table 2.
Abnormal values that should be corrected before the procedure
| Patient management before procedures with moderate risk of bleeding | |
|---|---|
| INR | Correct to <1.5 |
| aPTT | Should be corrected for values >1.5 × control |
| Plateles | Transfusion recommended for count <50.000/μl |
Imaging guidance techniques
CT, fluoroscopy and US may all be used to guide chest biopsies and operators should be familiar with the advantage and limitations of each one [26]. Parameters affecting the selection of the most appropriate imaging technique are lesions site, size and visibility as well as its relationship with critical anatomical structures to be avoided [5]. Whenever possible, chest biopsies should be performed under US guidance (Fig. 2) to exploit the advantages of real-time monitoring without radiation exposure to patients and operators [27]; however, US guidance is limited to superficial lesions adjacent to the chest wall and/or to lesions delineated by pleural effusion sufficient to create a suitable interface for ultrasound penetration. In the vast majority of cases, CT (or cone-beam CT) (Figs. 3 and 4) is the preferred guidance method for chest biopsies due to its optimal spatial and contrast resolution as well as its 3D imaging ability. Furthermore an intravenous contrast agent can be used to differentiate target lesions from atelectasis, necrosis and vascular structures. CT-guided chest biopsies can be performed either with the step-and-shoot approach or with CT-fluoroscopy (Fig. 5): while the step-and-shoot approach allows 3D multiplanar reconstruction and significantly reduces the radiation dose to the patient and operators, it does not allow real-time monitoring of lesion movement during respiration and is strongly influenced by the patient compliance. On the other hand, real-time monitoring of lesions movement with CT-fluoroscopy guidance allows reducing needle passes and procedure time; however, the radiation dose (to both the patients and operator) is significant [28]. To simplify the approach and to increase the speed and reliability of step-and-shoot CT-guided chest biopsies, several technical advances have been proposed, including augmented reality and use of robotic platforms [29].
Fig. 2.
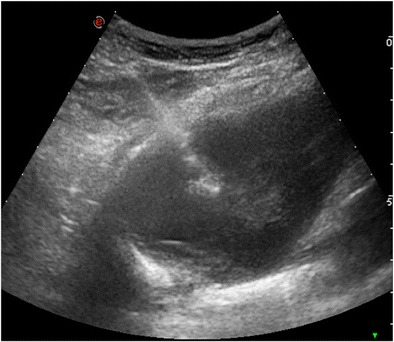
Ultrasound-guided chest biopsy of a pulmonary nodule in the right lower lobe
Fig. 3.
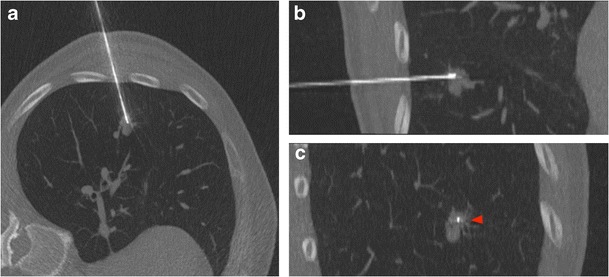
CT-guided chest biopsy of a pulmonary nodule in the left lower lobe. (a) Axial, (b) coronal and (c) sagittal intraprocedural CT scans showing the needle tip in the lesion (arrow)
Fig. 4.
Axial images during fluoroscopy-guided chest biopsy of a solitary subpleural nodule
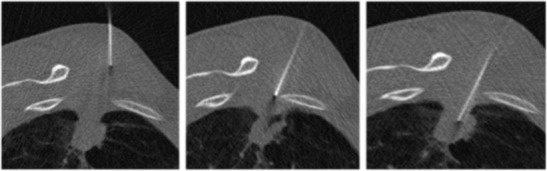
Fig. 5.
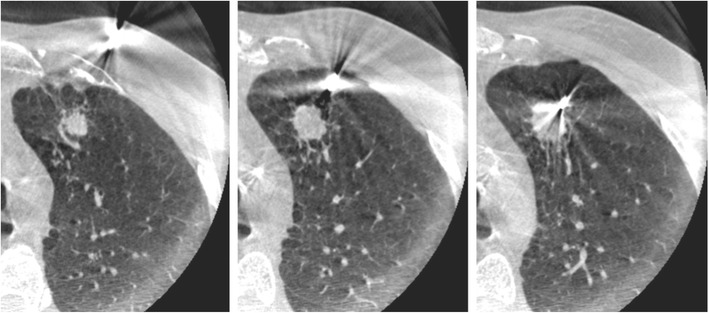
Progressive images of Cone-beam-guided chest biopsy of a pulmonary nodule in the left upper lobe
Furthermore, if a PET/CT is available, the needle should be led to the most enhancing area of the lesion to increase diagnostic accuracy.
Biopsy procedure
Patient positioning and instruction
The patient should be positioned prone, supine or lying on the side, based on the previously planned access site and needle trajectory. When needed, arms can be raised above the head to widen the intercostal spaces and obtain easier access. The procedure should be adequately explained to the patients with emphasis on the potential stinging sensation during the pleural puncture and breath-hold phases.
Access site
Whenever possible, the needle access site should be cephalic to the ribs to avoid intercostal vessel and nerves puncture (Fig. 6) [30]. In case of anterior parasternal access, internal mammary vessels should be carefully avoided [31]. Costal cartilage may traversed if needed but this may result in reduced needle mobility. The skin in the access site should be sterilised with standardised antiseptic solution and cutaneous and subcutaneous tissues should be infiltrated with lidocaine (lignocaine) up to a maximum dose of 20 ml of a 2% solution.
Fig. 6.
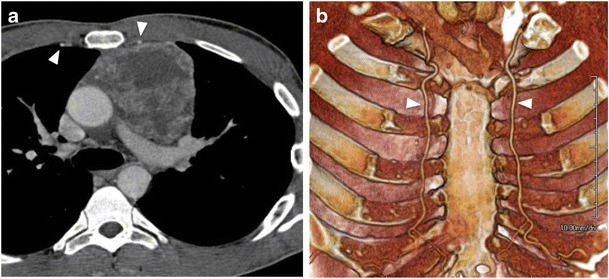
(a) Axial CT image and (b) 3D reconstruction showing internal mammary arteries (arrows), which must be avoided during the procedure
Breath-hold techniques and needle manipulation
The breath-hold technique stabilises the positions of the diaphragm, pleural planes, lung, fissures and, ultimately, target lesions; however, breath-hold capabilities can be extremely different from patient to patient, and even the same subject may not be able to reproduce breath-hold during the procedure because of stress or fatigue. Therefore, it can be easier to target larger tumours (>2/3 cm) instructing the patient to breath freely with shallow respiration; even though the target lesions moves slightly, its large size may be sufficient to require few adjustments. In the remaining cases, the patient can be instructed to maintain an inspiratory or expiratory apnoea to allow easier access to target lesions.
Needle types
Needle choice is based on the size and location of the lesion, intended needle trajectory, information expected from the pathologic sample, status of the lung parenchyma and operator preference. Some of the more commonly used fine-needle aspiration (FNA) devices include Chiba, Franseen, Westcott, MaxiCELL, Greene (Cook) and Turner (Cook) needles, which have circumferentially sharpened tips allowing for sampling. Core biopsy needles are designed to collect a small piece of tissue intended for surgical pathology analysis and can be designed as end-cutting or side-cutting devices. The most commonly used core biopsy needle is the Tru-Cut, which consists of an outer cutting cannula and an inner slotted stylet. The Temno core biopsy device is another similar commonly used automatic core biopsy needle. The Biopince full core is an automated end-cutting needle that produces a full cylindrical core specimen. The decision to perform core biopsy or both core biopsy and FNA is multifactorial and highly institution-/operator-dependent [28]. The number of passes needed per procedure has not been defined, but the decision to perform more than one puncture depends on procedure difficulty, risk of complications, quality of the first specimen obtained and the pathologist’s requests. The presence of an on-site pathologist may reduce the number of biopsies needed [5]. Also the use of a coaxial, a larger-bore needle that is kept in place to maintain access to the target lesion, may allow multiple tissue sampling, reducing repeated pleural or soft tissue punctures [32]. Several techniques have been proposed to seal the path of the needle after its removal to reduce the risk of pneumothorax and haemorrhage; the most successful option is to create a blood patch with autologous venous blood [33, 34].
Diagnostic accuracy
Ultrasound versus CT
In a recent meta-analysis, Di Bardino et al. compared the diagnostic accuracy of ultrasound and CT-guided thoracic biopsy. The overall pooled diagnostic accuracy of ultrasound-guided biopsy was 88.7% (446/503), with a sensitivity of 91.5% (366/400) and a specificity of around 100% for the diagnosis of malignancy. The overall pooled diagnostic accuracy of CT-guided biopsy was 92.1% (9567/10,383), with a sensitivity of 92.1% (7343/7975) and a specificity of around 100% for the diagnosis of malignancy (Tables 3 and 4) [35]. These data show a relative superiority of CT as guidance method, but data on CT-guided biopsies were more significant because of the higher number of cases in comparison with US-guided biopsies.
Table 3.
Test characteristics for different imaging guidance techniques (ultrasound vs. CT), biopsy techniques (FNAB vs. core biopsy) and lesion location (pamediastinal vs. peripheral)
| Accuracy | Sensitivity | Specificity | ||
|---|---|---|---|---|
| Imaging guidance techniqueE | Ultrasound-guide biopsy | 88.7% | 91.5% | 100% |
| CT-guide biopsy | 92.1% | 92.1% | 100% | |
| Biopsy technique | FNAB | 64–97% | 82–99% | 86–100% |
| Core biopsy | 93% | 89% | 97% | |
| Lesion location | Paramediastinal | 95.4% | 95.6% | 100% |
| Peripheral | 94.7% | 94.2% | 100% |
Table 4.
Safety profile of imaging-guided biopsy
| PTX | Haemorrhage | |
|---|---|---|
| CT-guided biopsy | 20.5% | 2.8% |
| Ultrasound-guided biopsy | 4.4% | – |
| Fluoroscopy-guided biopsy | 11.1% | – |
FNAB versus core biopsy
There is a wide variation in reporting diagnostic accuracies of fine-needle aspiration biopsy (FNAB) between different institutions, ranging from 64% to 97%. The false-negative rate of FNAB varies, occurring in 6 to 54% of biopsies. The sensitivity, specificity and accuracy of FNAB for pulmonary lesions are 82 to 99%, 86 to 100% and 64 to 97%, respectively [28]. Core biopsy has been shown to have slightly higher overall sensitivity, specificity and accuracy, with respective values of 89%, 97% and 93% (Table 3). Several recent papers advocate the use of 18- and 20-gauge cutting needles as well as coaxial techniques to improve the diagnostic yield, reporting diagnostic accuracies of 74–95% for the diagnosis of malignancy. Charig and Phillips reported similar accuracy of FNAB and core biopsy when an on-site pathologist was available; in a similar setting Capalbo et al. observed a greater diagnostic accuracy of FNAB compared to core needle biopsy (94.83% vs. 81.82. %) [36]. Nowadays, the test of genetic mutations is important to plan targeted therapies in patient with lung cancers. With regard to this point, Schneider et al. observed a statistically significantly higher number of samples sufficient for molecular testing in core biopsy than FNAB (67% vs. 46%; P = 0.007) [37].
Central versus peripheral lesions
Various studies evaluated the effects of lesion location on the diagnostic accuracy of imaging-guided biopsy in chest tumours. In a paper from Layfield et al. [38], the sensitivity in diagnosing lung tumours decreased from 100% in peripheral lesions to 82% in central lesions; however, this study was conducted in 1996 with basic CT equipment, as demonstrated by the striking differences in sensitivity between fluoroscopy guidance (97%) and CT guidance (80%). Yamagamy et al. [39] demonstrated that some peripherally located lesions are not accessible at all with conventional CT guidance because of their relationship with rib arcs, thus requiring needle positioning under CT-fluoroscopy guidance. Finally, Wang et al. [40] recently compared the rates of complications and diagnostic accuracy of CT-guided biopsy in peripheral versus paramediastinal lesions, demonstrating how this technique may be safe and reliable even for deeply located tumours; in particular, their paper reports diagnostic accuracy of 95.4% in paramediastinal lesions and 94.7% in peripheral lesions, with a sensitivity of 95.6% and 94.2% respectively (Table 3).
Post-procedural care and complications
Once the biopsy has been performed, a CT scan of the chest is obtained to identify any immediate post-procedural complications. According to some authors, the patients should be rolled over onto the punctured side to reduce the risk of delayed PNX; anyway, this is a controversial opinion, since other authors have reported no benefits of putting patients in the “biopsy down position” [41]. Following measures include observation and monitoring of vital signs for at least 4 h. Chest films are usually acquired after 4 h to detect possible asymptomatic PNX. If the clinical suspicion of a PNX arises, chest radiography must be obtained immediately. In low-risk patients, many interventional radiology services reasonably perform lung biopsies on an outpatient basis, with discharge at 4 h and readmission only if symptoms develop [42].
Pneumothorax
PNX is the most common complication after imaging-guided chest biopsy; it is most frequently detected after lung biopsy but can also occur even after biopsies of mediastinal, pleural and chest wall lesions. Usually PNX occurs during or immediately after the procedure and it is detected on post-procedural control scans. The incidence of PNX has been reported to be up to 61% with an average risk of 20% [43–46]. Risk factors for PNX can be related to patient or lesions features, but also to the biopsy technique. In particular, the rate of PNX increases with the patient age and severity of underlying lung disease (e.g. emphysema or chronic obstructive disease) [47, 48] as well as in smaller and deeper lesions [49, 50]. Technical risk factors include the type and size of biopsy needle, longer procedure duration, biopsies in the middle or lower lobe, transgression of a fissure and multiple needle repositioning or pleural passes [51]. A PNX developed during the procedure can be immediately aspirated through the introducer needle or a separate needle inserted into the pleural space, preventing further enlargement; however some authors suggest placing a chest tube if aspirated air is greater than 670 ml [40]. PNXs developed after the procedure (Fig. 7) are often small and asymptomatic and can be managed conservatively by monitoring vital signs and performing serial chest films (at 1 and 4 h) [45, 52]. Nevertheless, in a minority of cases (1–14%), PNX can be significant (>30% of lung volume), increase over time or become symptomatic. In these cases small-calibre, 6- to 9-French catheters can be safely and easily placed under CT guidance [41, 53, 54].
Fig. 7.
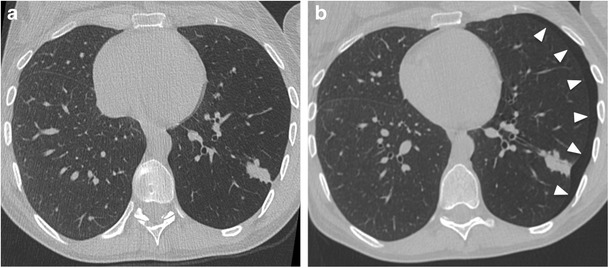
CT-guided chest biopsy of solitary pulmonary nodule in the left lower lobe. (a) Axial CT image before the procedure showing the subpleural nodule. (b) Post-procedural axial CT image showing small PNX (arrows) as a complication of transthoracic biopsy
Pulmonary haemorrhage and haemoptysis
Pulmonary haemorrhage represents the second most common complication after imaging-guided biopsy. PH may occur with or without haemoptysis and can be easily detected on screening post-biopsy CT scan as a perilesional or needle tract ground-glass opacity (Fig. 8). The occurrence rates of PH are estimated to be from 4 to 27% (with an average incidence of 11%), while haemoptysis risk is up to 5% [55, 56]. Usually this complication does not need any treatment and the only recommendations is to place the patient in a lateral position, with the biopsy side down, to avoid aspiration of blood into the unaffected lung [44]. Occasionally a larger, higher-grade PH occurs and oxygen as well as pro-coagulative therapy may be needed. Risk factors for higher-grade PH include older age, female sex, emphysema, pulmonary hypertension, coaxial technique, subsolid lesions, nonsubpleural location and lesion size smaller than 3 cm [57, 58]. Avoiding PH is important to manage patients with abnormal coagulation profiles and to correct the diathesis before the procedure. In such patients, more invasive biopsy techniques that imply the use of the core needle and coaxial technique should be avoided as should prolonged procedures with extended needle paths [45, 59]. Finally, haemothorax is an extremely rare and more severe complication, usually due to puncture of an intercostal or less commonly a large thoracic vessel, or mammary vessels in the case of an anterior parasternal biopsy.
Fig. 8.

CT-guided chest biopsy of a pulmonary nodule. (a) Axial CT image before the procedure showing a pulmonary nodule in the right lower lobe. (b) Post-procedural axial CT image demonstrates perilesional haemorrhage as ground-glass opacity around the nodule (arrows)
Air embolism
The occurrence of systemic air embolism (SAE) in the left atrium, left ventricle or systemic circulation is a rare (incidences between 0.01% and 0.21%), but potentially fatal (by brain or cardiac infarct) event [59] (Fig. 9). There are three mechanisms in particular responsible for SAE during biopsy: placement of the needle tip in a pulmonary vein, formation of a bronchial-venous or alveolar-venous fistula and opening the outer cannula of a coaxial biopsy needle to the atmosphere. Risk factors can be biopsy of cystic or cavitary lesions (i.e. vasculitic granulomas), coughing during the biopsy and positive pressure ventilation [60]. In a study from Freund et al., asymptomatic SAE detectable at CT after lung biopsy was reported to be as high as 3.8% (23/610 patients), whereas the symptomatic cases were 0.49%. In the meta-analysis from Tomiyama et al. [61, 62], the incidence of SAE ranges from 0.001% to 0.003%, being influenced by the depth of needle penetration into the lesion, endotracheal anaesthesia, location of the lesion above the level of the left atrium and prone position of the patients [61].
Fig. 9.
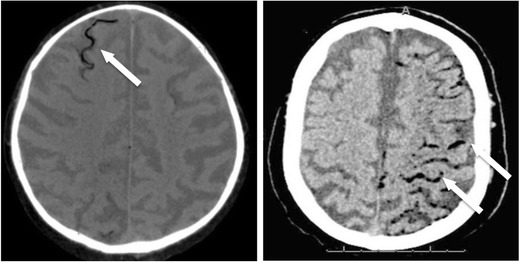
Post-procedural CT scan after chest biopsy showing two different patients with air embolism in cerebral vessels (arrows)
Tumour seeding
Tumour seeding through the needle tract represents a very rare complication with a prevalence reported in the literature between 0.012 and 0.061% [45]. The real clinical relevance is still discussed, but it is obvious that tumour seeding along the needle tract can significantly change patient management and life expectancy and should be strictly avoided [63]. Tumour seeding is reported to be more frequently observed after imaging-guided core needle biopsy of pleural mesothelioma [64].
Complications by imaging guidance technique
In the meta-analysis from Di Bardino et al. US-guided biopsy was generally very well tolerated and safe, with a pooled incidence of PNX of 4.4% (22/503) in the reported papers. This looks favourable compared to CT-guided biopsy, but is also obviously correlated to a statistical bias in lesion position since the targets of US-guided biopsies are usually adjacent to or infiltrating the pleural surface, with a very low risk of PNX.
Compared with the conventional step-and-shoot approach for CT-guided biopsy, CT fluoroscopy is faster and requires fewer needle passes, resulting in a decrease of procedure duration and fewer complications. In particular Heck et al. [48] showed a trend toward a lower PNX rate when using CT fluoroscopy as guidance method. Similar results have been obtained by Kim et al. [65], with a decrease from 27.1% to 11.1% in PNX rates when using CT fluoroscopy.
Conclusions
In conclusion, imaging-guided chest biopsy is an interventional procedure of pivotal importance for several clinical conditions of pneumological, oncological and surgical interest. This procedure may appear very simple and linear, but radiologists approaching it for the first time must consider several clinical and technical variables significantly affecting the final results, in terms of both diagnostic accuracy and patients’ safety.
References
- 1.Viggiano RW, Swensen SJ, Rosenow EC., 3rd Evaluation and management of solitary and multiple pulmonary nodules. Clin Chest Med. 1992;13(1):83–95. [PubMed] [Google Scholar]
- 2.Nomori H, Watanabe K, Ohtsuka T, Naruke T, Suemasu K, Uno K. Evaluation of F-18 fluorodeoxyglucose (FDG) PET scanning for pulmonary nodules less than 3 cm in diameter, with special reference to the CT images. Lung Cancer. 2004;45(1):19–27. doi: 10.1016/j.lungcan.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Hammerschlag G, Cao J, Gumm K, Irving L, Steinfort D. Prevalence of incidental pulmonary nodules on computed tomography of the thorax in trauma patients. Intern Med J. 2015;45(6):630–633. doi: 10.1111/imj.12755. [DOI] [PubMed] [Google Scholar]
- 4.Momen M. Wahidi, Joseph A. Govert, Ranjit K. Goudar, et al. Evidence for the Treatment of Patients With Pulmonary Nodules: When Is It Lung Cancer? ACCP Evidence-Based Clinical Practice Guidelines (2nd Edition) [DOI] [PubMed]
- 5.Manhire A, Charig M, Clelland C, Gleeson F, Miller R, Moss H, Pointon K, Richardson C. Sawicka E; BTS. Guidelines for radiologically guided lung biopsy. Thorax. 2003;58(11):920–936. doi: 10.1136/thorax.58.11.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laroche C, Fairbairn I, Moss H, Pepke-Zaba J, Sharples L, Flower C, Coulden R. Role of computed tomographic scanning of the thorax prior to bronchoscopy in the investigation of suspected lung cancer. Thorax. 2000;55(5):359–363. doi: 10.1136/thorax.55.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins CD, Breatnach E, Nath PH. Percutaneous needle biopsy of lung nodules following failed bronchoscopic biopsy. Eur J Radiol. 1992;15(1):49–53. doi: 10.1016/0720-048X(92)90203-L. [DOI] [PubMed] [Google Scholar]
- 8.Goldsmith SJ, Kostakoglu L. Role of nuclear medicine in the evaluation of the solitary pulmonary nodule. Semin Ultrasound CT MR. 2000;21(2):129–138. doi: 10.1016/S0887-2171(00)90019-2. [DOI] [PubMed] [Google Scholar]
- 9.Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 2001;21;285(7):914–24 [DOI] [PubMed]
- 10.Erasmus JJ, McAdams HP, Connolly JE. Solitary pulmonary nodules: part II. Evaluation of the indeterminate nodule. Radiographics. 2000;20(1):59–66. doi: 10.1148/radiographics.20.1.g00ja0259. [DOI] [PubMed] [Google Scholar]
- 11.Erasmus JJ, McAdams HP, Patz EF, Jr, Coleman RE, Ahuja V, Goodman PC. Evaluation of primary pulmonary carcinoid tumors using FDG PET. AJR Am J Roentgenol. 1998;170(5):1369–1373. doi: 10.2214/ajr.170.5.9574618. [DOI] [PubMed] [Google Scholar]
- 12.Higashi K, Ueda Y, Seki H, Yuasa K, Oguchi M, Noguchi T, Taniguchi M, Tonami H, Okimura T, Yamamoto I. Fluorine-18-FDG PET imaging is negative in bronchioloalveolar lung carcinoma. J Nucl Med. 1998;39(6):1016–1020. [PubMed] [Google Scholar]
- 13.MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP, Patz EF., Jr Swensen SJ; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395–400. doi: 10.1148/radiol.2372041887. [DOI] [PubMed] [Google Scholar]
- 14.Gruden JF, Klein SJ, Webb WR. Percutaneous transthoracic needle biopsy in AIDS: analysis in 32 patients. Radiology. 1993;189:567–571. doi: 10.1148/radiology.189.2.8105506. [DOI] [PubMed] [Google Scholar]
- 15.Sinner WN. Fine needle biopsy of tuberculomas. Chest. 1984;85:836. doi: 10.1378/chest.85.6.836a. [DOI] [PubMed] [Google Scholar]
- 16.Hoffer FA, Gow K, Flynn PM, et al. Accuracy of percutaneous lung biopsy for invasive pulmonary aspergillosis. Pediat Radiol. 2001;31:144–152. doi: 10.1007/s002470000402. [DOI] [PubMed] [Google Scholar]
- 17.Nosari A, Anghilieri M, Carrafiello G, Guffanti C, et al. Utility of percutaneous lung biopsy for diagnosing filamentous fungal infections in hematologic malignancies. Haematologica. 2003;88(12):1405–1409. [PubMed] [Google Scholar]
- 18.Carrafiello G, Laganà D, Nosari AM, Guffanti C, et al. Utility of computed tomography (CT) and of fine needle aspiration biopsy (FNAB) in early diagnosis of fungal pulmonary infections. Study of infections from filamentous fungi in haematologically immunodeficient patients. Radiol Med. 2006;111(1):33–41. doi: 10.1007/s11547-006-0004-9. [DOI] [PubMed] [Google Scholar]
- 19.Raptakis T, Boura P, Tsimpoukis S, Gkiozos I, Syrigos KN. Endoscopic and endobronchial ultrasound-guided needle aspiration in the mediastinal staging of non-small cell lung cancer. Anticancer Res. 2013;33(6):2369–2376. [PubMed] [Google Scholar]
- 20.Lange TJ, Kunzendorf F, Pfeifer M, Arzt M, Schulz C. Endobronchial ultrasound-guided transbronchial needle aspiration in routine care - plenty of benign results and follow-up tests. Int J Clin Pract. 2012;66(5):438–445. doi: 10.1111/j.1742-1241.2012.02907.x. [DOI] [PubMed] [Google Scholar]
- 21.Carter BW, Marom EM, Detterbeck FC. Approaching the patient with an anterior mediastinal mass: a guide for clinicians. J Thorac Oncol. 2014;9(9 Suppl 2):S102–S109. doi: 10.1097/JTO.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 22.Carter BW, Okumura M, Detterbeck FC, Marom EM. Approaching the patient with an anterior mediastinal mass: a guide for radiologists. J Thorac Oncol. 2014;9(9 Suppl 2):S110–S118. doi: 10.1097/JTO.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 23.Chouaid C, Dujon C, Monnet I, Madroszyk A, Le Caer H, et al. Feasibility and clinical impact of re-biopsy in advanced non small-cell lung cancer: a prospective multicenter study in a real-world setting (GFPC study 12-01) Lung Cancer. 2014;86(2):170–173. doi: 10.1016/j.lungcan.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Patel IJ, Davidson JC, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(6):727–736. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Laurent F, Montaudon M, Latrabe V, Bégueret H. Percutaneous biopsy in lung cancer. Eur J Radiol. 2003;45(1):60–68. doi: 10.1016/S0720-048X(02)00286-3. [DOI] [PubMed] [Google Scholar]
- 26.Klein JS, Zarka MA. Transthoracic needle biopsy: an overview. J Thorac Imaging. 1997;12(4):232–249. doi: 10.1097/00005382-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Sheth S, Harper UH, Stanley DB, et al. US guidance for thoracic biopsy: a valuable alternative to CT. Radiology. 1999;210(3):721–726. doi: 10.1148/radiology.210.3.r99mr23721. [DOI] [PubMed] [Google Scholar]
- 28.Winokur RS, Pua BB, Sullivan BW, Madoff DC. Percutaneous lung biopsy: technique, efficacy, and complications. Semin Intervent Radiol. 2013;30(2):121–127. doi: 10.1055/s-0033-1342952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anzidei M, Argirò R, Porfiri A, Boni F, et al. Preliminary clinical experience with a dedicated interventional robotic system for CT-guided biopsies of lung lesions: a comparison with the conventional manual technique. Eur Radiol. 2015;25(5):1310–1316. doi: 10.1007/s00330-014-3508-z. [DOI] [PubMed] [Google Scholar]
- 30.Romanes GJ. Cunningham’s textbook of anatomy. London: Oxford University Press; 1981. [Google Scholar]
- 31.Glassberg RM, Sussman SK, Glickstein MF. CT anatomy of the internal mammary vessels: importance in planning percutaneous transthoracic procedures. AJR. 1990;155:397–400. doi: 10.2214/ajr.155.2.2115273. [DOI] [PubMed] [Google Scholar]
- 32.Yankelevitz DF, Vazquez M, Henschke CI. Special techniques in transthoracic needle biopsy of pulmonary nodules. Radiol Clin N Am. 2000;38:267–279. doi: 10.1016/S0033-8389(05)70162-7. [DOI] [PubMed] [Google Scholar]
- 33.Lang EK, Ghavami R, Schreiner VC, Archibald S, Ramirez J. Autologous blood clot seal to prevent pneumothorax at CT-guided lung biopsy. Radiology. 2000;216(1):93–96. doi: 10.1148/radiology.216.1.r00jl3293. [DOI] [PubMed] [Google Scholar]
- 34.Wagner JM, Hinshaw JL. LubnerMG, et al. CT-guided lung biopsies: pleural blood patching reduces the rate of chest tube placement for postbiopsy pneumothorax. AJR Am J Roentgenol. 2011;197(4):783–788. doi: 10.2214/AJR.10.6324. [DOI] [PubMed] [Google Scholar]
- 35.DiBardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis. 2015;7(Suppl 4):S304–S316. doi: 10.3978/j.issn.2072-1439.2015.12.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capalbo E, Peli M, Lovisatti M, Cosentino M, et al. Trans-thoracic biopsy of lung lesions: FNAB or CNB? Our experience and review of the literature. Radiol Med. 2014;119(8):572–594. doi: 10.1007/s11547-013-0360-1. [DOI] [PubMed] [Google Scholar]
- 37.Schneider F, Smith MA, Lane MC, et al. Adequacy of core needle biopsy specimens and fine-needle aspirates for molecular testing of lung adenocarcinomas. Am J Clin Pathol. 2015;143(2):193–200. doi: 10.1309/AJCPMY8UI7WSFSYY. [DOI] [PubMed] [Google Scholar]
- 38.Layfield LJ, Coogan A, Johnston WW, et al. Transthoracic fine needle aspiration biopsy. Sensitivity in relation to guidance technique and lesion size and location. Acta Cytol. 1996;40:687–690. doi: 10.1159/000333940. [DOI] [PubMed] [Google Scholar]
- 39.Yamagami T, Kato T, Iida S, et al. Percutaneous needle biopsy for small lung nodules beneath the rib under CT scan fluoroscopic guidance with gantry tilt. Chest. 2004;126:744–747. doi: 10.1378/chest.126.3.744. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Jiang F, Tan X, Tian P. CT-guided percutaneous transthoracic needle biopsy for paramediastinal and nonparamediastinal lung lesions: diagnostic yield and complications in 1484 patients. Medicine. 2016;95(31):e4460. doi: 10.1097/MD.0000000000004460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu CC, Maher MM, Shepard JA. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol. 2011;196(6):W678–W682. doi: 10.2214/AJR.10.4659. [DOI] [PubMed] [Google Scholar]
- 42.Anzidei M, Sacconi B, Fraioli F, Saba L, et al. Development of a prediction model and risk score for procedure-related complications in patients undergoing percutaneous computed tomography-guided lung biopsy. Eur J Cardiothorac Surg. 2015;48(1):e1–e6. doi: 10.1093/ejcts/ezv172. [DOI] [PubMed] [Google Scholar]
- 43.Birchard KR. Transthoracic needle biopsy. Semin Intervent Radiol. 2011;28:87–97. doi: 10.1055/s-0031-1273943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Traill ZC, Gleeson FV. Delayed pneumothorax after CT-guided percutaneous fine needle aspiration lung biopsy. Thorax. 1997;52:581–582. doi: 10.1136/thx.52.6.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu CC, Maher MM, Shepard JA. Complications of CT- guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol. 2011;196:W678–W682. doi: 10.2214/AJR.10.4659. [DOI] [PubMed] [Google Scholar]
- 46.Geraghty PR, Kee ST, McFarlane G, et al. CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. Radiology. 2003;229:475–481. doi: 10.1148/radiol.2291020499. [DOI] [PubMed] [Google Scholar]
- 47.CollingsCL WJL, BansonNL LRC. Pneumothorax and dependent versus nondependent patient position after needle biopsy of the lung. Radiology. 1999;210(1):59–64. doi: 10.1148/radiology.210.1.r99ja1759. [DOI] [PubMed] [Google Scholar]
- 48.Heck SL, Blom P, Berstad A. Accuracy and complications in computed tomography fluoroscopy-guided needle biopsies of lung masses. Eur Radiol. 2006;16(6):1387–1392. doi: 10.1007/s00330-006-0152-2. [DOI] [PubMed] [Google Scholar]
- 49.Cox JE, Chiles C, McManus CM, Aquino SL, Choplin RH. Transthoracic needle aspiration biopsy: variables that affect risk of pneumothorax. Radiology. 1999;212(1):165–168. doi: 10.1148/radiology.212.1.r99jl33165. [DOI] [PubMed] [Google Scholar]
- 50.Kazerooni EA, Lim FT, Mikhail A, Martinez FJ. Risk of pneumothorax in CT-guided transthoracic needle aspiration biopsy of the lung. Radiology. 1996;198(2):371–375. doi: 10.1148/radiology.198.2.8596834. [DOI] [PubMed] [Google Scholar]
- 51.Ko JP, Shepard JO, Drucker EA, et al. Factors influencing pneumothorax rate at lung biopsy: are dwell time and angle of pleural puncture contributing factors? Radiology. 2001;218(2):491–496. doi: 10.1148/radiology.218.2.r01fe33491. [DOI] [PubMed] [Google Scholar]
- 52.Khankan AA, Al-Muaikeel M. Image-guided percutaneous transthoracic biopsy in lung cancer emphasis on CT-guided technique. J Infect Public Health. 2012;5(Suppl 1):S22–S30. doi: 10.1016/j.jiph.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 53.YamagamiT NT, Iida S, Kato T, Nishimura T. Management of pneumothorax after percutaneous CT-guided lung biopsy. Chest. 2002;121(4):1159–1164. doi: 10.1378/chest.121.4.1159. [DOI] [PubMed] [Google Scholar]
- 54.Henry M, Arnold T, Harvey J. BTS guidelines for the management of spontaneous pneumothorax. In: BTS guidelines for the management of pleural disease. Thorax. 2003;58(Suppl II):ii39–ii52. doi: 10.1136/thx.58.suppl_2.ii39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinner WN. Complications of percutaneous transthoracic needle aspiration biopsy. Acta Radiol Diagn (Stockh) 1976;17(6):813–828. doi: 10.1177/028418517601700609. [DOI] [PubMed] [Google Scholar]
- 56.Berquist TH, Bailey PB, Cortese DA, et al. Transthoracic needle biopsy: accuracy and complications in relation to location and type of lesion. Mayo Clin Proc. 1980;55:475–481. [PubMed] [Google Scholar]
- 57.Tai R, Dunne R, Trotman-Dickenson B, Jacobson F, et al. Frequency and severity of pulmonary hemorrhage in patients undergoing percutaneous CT-guided transthoracic lung biopsy: single-institution experience of 1175 cases. Radiology. 2016;279(1):287–296. doi: 10.1148/radiol.2015150381. [DOI] [PubMed] [Google Scholar]
- 58.Milner LB, Ryan K, Gullo J. Fatal intratho- racic hemorrhage after percutaneous aspiration lung biopsy. AJR Am J Roentgenol. 1979;132(2):280–281. doi: 10.2214/ajr.132.2.280. [DOI] [PubMed] [Google Scholar]
- 59.Khankan A, Sirhan S, Aris F. Common complications of nonvascular percutaneous thoracic interventions: diagnosis and management. Semin Intervent Radiol. 2015;32(2):174–181. doi: 10.1055/s-0035-1549843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freund MC, Petersen J, Goder KC, Bunse T, Wiedermann F, Glodny B. Systemic air embolism during percutaneous core needle biopsy of the lung: frequency and risk factors. BMC Pulm Med. 2012;12:2. doi: 10.1186/1471-2466-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomiyama N, Yasuhara Y, Nakajima Y, Adachi S, et al. CT-guided needle biopsy of lung lesions: a survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol. 2006;59(1):60–64. doi: 10.1016/j.ejrad.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 62.Hare SS, Gupta A, Goncalves AT, Souza CA, Matzinger F, Seely JM. Systemic arterial air embolism after percutaneous lung biopsy. Clin Radiol. 2011;66(7):589–596. doi: 10.1016/j.crad.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Voravud N, Shin DM, Dekmezian RH, Dimery I, Lee JS, Hong WK. Implantation metastasis of carcinoma after percutaneous fine-needle aspiration biopsy. Chest. 1992;102(1):313–315. doi: 10.1378/chest.102.1.313. [DOI] [PubMed] [Google Scholar]
- 64.Agarwal PP, Seely JM, Matzinger FR, MacRae RM, Peterson RA, Maziak DE, Dennie CJ. Pleural mesothelioma: sensitivity and incidence of needle track seeding after image-guided biopsy versus surgical biopsy. Radiology. 2006;241(2):589–594. doi: 10.1148/radiol.2412051020. [DOI] [PubMed] [Google Scholar]
- 65.Kim GR, Hur J, Lee SM et al (2011) CT fluoroscopy-guided lung biopsy versus conventional CT-guided lung biopsy: a prospective controlled study to assess radiation doses and diagnostic performance. Eur Radiol [DOI] [PubMed]


