Abstract
Background
Several fMRI studies in migraine assessed resting state functional connectivity in different networks suggesting that this neurological condition was associated with brain functional alteration. The aim of present study was to explore the association between cognitive functions and cerebral functional connectivity, in default mode network, in migraine patients without and with aura, during interictal episodic attack.
Methods
Twenty-eight migraine patients (14 without and 14 with aura) and 14 matched normal controls, were consecutively recruited. A battery of standardized neuropsychological test was administered to evaluate cognitive functions and all subjects underwent a resting state with high field fMRI examination.
Results
Migraine patients did not show abnormalities in neuropsychological evaluation, while, we found a specific alteration in cortical network, if we compared migraine with and without aura. We observed, in migraine with aura, an increased connectivity in left angular gyrus, left supramarginal gyrus, right precentral gyrus, right postcentral gyrus, right insular cortex.
Conclusion
Our findings showed in migraine patients an alteration in functional connectivity architecture. We think that our results could be useful to better understand migraine pathogenesis.
Keywords: Migraine; Cognitive functions; Functional connectivity, default mode network
Background
Migraine is a common episodic neurological disorder with a complex physiopathology. It is characterized by typical unilateral, often severe, pain throbbing with associated features such as hypersensitivity to multiple stimuli, including visual (photophobia), auditory (phonophobia), and sensory (cutaneous allodynia) stimuli during migraine attacks [1]. Indeed, about one third of patients had experience of aura associated to visual, motor, or somatosensory symptoms during attacks [2, 3].
Migraine is a very common and debilitating disease that causes significant limitations in daily life with effects on emotional-behavioral and relational aspects [4]. Neuropsychological studies suggests that migraine affect also cognitive functions during attacks and interictal periods [5], even though it is unclear the association between cognitive dysfunctions and migraine. Migraineurs could present executive dysfunction which presumably reflects frontal lobe abnormalities [6], or alteration in memory areas. However, while several authors reported significant lower performances in migraine patients, others did not confirm these findings. In other cases authorsdescribed the presence of cognitive deficit only after a long disease duration [7, 8].
Several fMRI studies in migraine assessed resting state functional connectivity in various networks suggesting an association with cortical functional alteration [9]. In particular, some authors reported increased connectivity in specifics cerebral areas, such as right rostral anterior cingulate cortex, prefrontal cortex, orbitofrontal cortex and supplementary motor area [10]. This altered connectivity could indicate intrinsic pathophysiological changes in migraine, even if only a very few studies explored the different functional connectivity in migraine with (MA) and without aura (MO) [11].
The aim of present study was to explore the association between cognitive functions and cerebral functional connectivity (FC) between MO and MA, during interictal episodic attack.
Methods
Twenty-eight migraine patients (14 without aura and 14 with aura) and 14 sex and age matched health controls (HC), were enrolled. Aura included temporary visual or sensory disturbances nausea, and sensitivity to light and sound. The patients were recruited from migraine ambulatory. The diagnosis of definite MA or MO was performed by two neurologist, specialist in headache disorders, blinded to MRI and neuropsychological findings, according to International Headache Society criteria [12] (Headache Classification Committee of the International Headache, 2013).
Control subjects were volunteers recruited from local communities, with no history of neurological diseases. They did not suffer from migraine or headache and were free from medication intake. The study protocol was approved by the Local Ethics Committee according to Declaration of Helsinki. All patients gave written consent to study. All information related to migraine was collected by interviews and examination of medical records. All patients had a clinic diagnosis for at least 10 years. We excluded patients with: 1) other types of headache; 2) vascular disease or trauma; 3) history of major psychiatric disorders; 4) presence of metabolic disorders; 5) other neurological conditions.
Demographic and clinical characteristics were also collected (Table 1). The type of medication, during attack, in patient included: simple analgesics (18/24), simple triptens (4/24), and combination analgesics (6/24).
Table 1.
Socio-demographic characteristics of patients with aura (n = 14) without aura (n = 14) and controls (n = 14)
| Aura (Mean ± SD) |
No Aura (Mean ± SD) |
HC (Mean ± SD) |
|
|---|---|---|---|
| Age | 41.28 ± 13.44 | 40.75 ± 11.82 | 41.75 ± 12.82 |
| Years of education | 15.8 ± 3.2 | 16.7 ± 4.2 | 16.2 ± 4.1 |
| Disease duration | 10.9 ± 3.7 | 12.3 ± 5.8 | |
| Attack frequency/month (n) | 5.05 ± 2.31 | 6.07 ± 2.81 | |
| Single-Attack duration (hours) | 3.58 ± 2.27 | 4.21 ± 2.99 | |
| Days to next migraine attack after examination | |||
Legend: SD standard deviation
A battery of standardized neuropsychological test to evaluate cognitive functions, was administered by two psychologists, blinded to patients/controls status, diagnosis and MRI findings. Processing speed was assessed using the Trail Making Test, Part A (TMT-A), [13]. Attentional set-shifting was measured using the Trail Making Test, Part B (TMT-B). Memory was assessed using the Rey Auditory Verbal Learning Test (RAVLT) [14]. Language was assessed with semantic and phonemic verbal fluency test [15]. Wisconsin Card Sorting test (WCST) was used for executive function and cognitive flexibility. Finally, Hamilton Rating Scale for depression (HAM-D) and Hamilton Rating Scale for anxiety (HAM-A) were used to asses anxiety and depressive symptoms [16, 17].
All patient underwent to a MRI examination with a scanner operating at 3.0 T (Achieva, Philips Healthcare, Best, The Netherlands), by using a 32-channel SENSE head coil. MRI scans were performed in the interictal stage at least 3 days after migraine attack. For each subject, T1 [TR = 8 ms, TE = 4 ms, slice thickness/gap = 1/0 mm, number of slices = 173, field of view 240 mm], T2-weighted [TR = 3.0 s, TE = 80 ms, slice thickness/gap = 3.0/0.3 mm, number of slices = 30, field of view 230 mm] were acquired. The scan parameters of the resting-state functional magnetic resonance imaging (fMRI) scan were as follows: TR = 3.0 s; TE = 35 msec; flip angle = 90°; and voxel size 1.9 · 1.9 · 4.0 mm, scan duration 10 min. During the resting-state scan, participants were instructed to lie still with their eyes closed and not to fall asleep.
Neuropsychological testing and MRI scanning were performed on same day.
Resting state analysis
fMRI-analysis was performed with FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). The following pre-processing procedure was applied: employing different modules of the FSL-software package. The preprocessing of the resting-state data consisted of motion correction (MCFLIRT) [18], brain extraction [19], spatial smoothing using a Gaussian kernel with a full width at a half maximum of 8 mm. After preprocessing, the functional images were registered to the corresponding high-resolution echo planar images, (co-registered to T1-weighted images,) which were registered to the 2 mm isotropic MNI-152 standard space image [18]. These registration parameters were combined to obtain registration matrix from native (fMRI) space to MNI space and its inverse (from MNI space to native space). Independent component analysis (ICA) was carried out using MELODIC toolbox implementing probabilistic independent component analysis (PICA) [20]. Variance normalization was used and IC maps were thresholded using an alternative hypothesis test based on fitting a Gaussian/gamma mixture model to distribution of voxel intensities within spatial maps and controlling the local false-discovery rate at p < 0.5 [20]. The selection of clusters of interest obtained of MELODIC analysis implied the presence of anatomically relevant areas in each group component map that reproduced the layouts of the main physiological resting state network jointly and consistently across subjects. The artefact components were removed manually from analysis and for all groups we considered IC of the DMN, one of the main networks that are consistently identified when an individual is at wakeful rest and not performing an attention-demanding task. This network includes the precuneus, posterior cingulate cortex (PCC), medial prefrontal cortex, medial temporal lobe and angular gyrus. For inter group analysis was carried out using dual regression (FSL technique) that allows for voxel-wise comparisons of resting-state [21, 22]. This allow, a) to separate fMRI data sets using the group-ICA spatial maps in a linear model fit against, resulting in matrices (time-course matrices) describing the temporal dynamics for each component and subject, and b) estimate subject-specific spatial maps using these time-course matrices. The dual regression analysis was performed with variance normalization because reflects differences in both activity and spatial spread of the network. As a statistical analysis the different component maps are collected across subjects into single 4D files and tested voxel-wise for statistically significant differences between the groups using FSL randomize non parametric permutation testing, with 5000 permutations, using a threshold-free cluster enhanced (TFCE) technique to control for multiple comparisons [23] and corrected for multiple comparisons (across space) within the permutation framework. Age and gender also included in this analysis as nuisance variable. The Harvard-Oxford Cortical structural atlas were used to identify the anatomical characteristics of the resulting PICA maps. Fslstats and fslmaths tools were used to calculate the number of non-zero voxels in the selected difference maps, and their t-score values.
Results
Demographic characteristics
Inter group analysis by U Mann Whitney test no highlighted differences between characteristics and clinical scores of patients (Table 1). There were no differences between MA and MO patients in age, (p = 0.84), education (p = 0.35) and disease duration (p = 0.27). Both groups did not show abnormalities in neuropsychological evaluation (Table 2).
Table 2.
Cognitive performances of the migraine patients
| Test | Aura | No Aura | Controls groups | Cut-off |
|---|---|---|---|---|
| Attention | ||||
| Attentive Matrix | 44.60 ± 4.80 | 45.51 ± 6.91 | 43.35 ± 7.87 | 30 |
| Language | ||||
| Fluency Phonemic | 32.08 ± 11.72 | 35.35 ± 10.9 | 30.85 ± 6.63 | 17 |
| Fluency Semantic | 36.25 ± 6.64 | 36.28 ± 5.86 | 37.42 ± 5.74 | 25 |
| Memory | ||||
| RAVLT (Immediate recall) | 40.86 ± 25.01 | 36.28 ± 5.86 | 38.17 ± 4.59 | 28.53 |
| RAVLT (Delayed recall) | 8.2 ± 2.45 | 9.23 ± 3.10 | 6.85 ± 1.65 | 4.69 |
| Executive Functions | ||||
| Trial Making Test-A | 42.62 ± 25.01 | 48.73 ± 55.20 | 55.28 ± 15.52 | 93 |
| Trial Making Test-B | 123.35 ± 56.28 | 155.14 ± 70.16 | 126.64 ± 30.49 | 282 |
Resting state
MA vs MO
MA group showed increased functional connectivity if compared to MO group (blue area, p values are color coded from 0.05 FWE corrected (dark blue) to <0.0001 FWE corrected (light blue). Increased in functional connectivity was found in left angular gyrus, left supramarginal gyrus, right precentral gyrus, right postcentral gyrus, right insular cortex (Fig. 1a, full list of structures are showed in Table 3). No significant voxels for MA < MO were found.
Fig. 1.
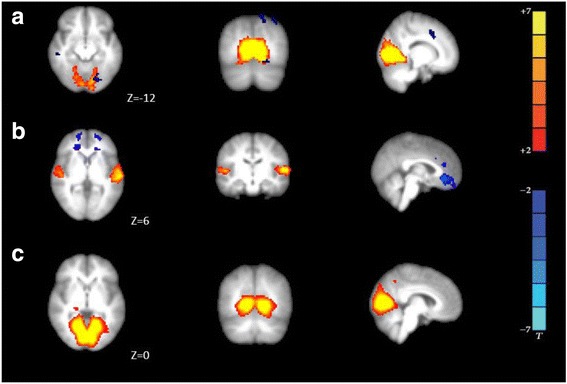
Functional connectivity average DMN of groups: a. MA > MO group; b. MA > HC; c. MO > HC group. MA patients showed increased functional connectivity compared MO (blue areas, p values are color coded from 0.05 FWE corrected (dark blue) to <0.0001 FWE corrected (light blue), full list of structures in Table 2). Axial images are overlaid on transverse slices of MNI-152 standard anatomical image. The left side of the brain corresponds to the right hemisphere and vice versa. Z-coordinates of each slice in the MNI-152 standard space are given
Table 3.
Increased functional connectivity in MA compared with MO
| Brain Structure | Peak voxel coordinates (MNI) | Peak T-score | ||
|---|---|---|---|---|
| x | y | z | ||
| Right Central Opercular Cortex | 48 | -6 | 6 | 3.89 |
| Right Insular cortex | 42 | −9 | 6 | 4.97 |
| Right first and second Heschl’s Gyrus | 45 | −12 | 6 | 4.12 |
| Left Central Opercular Cortex | −45 | −9 | 6 | 3.17 |
| Left first and second Heschl’s Gyrus | −51 | −15 | 6 | 3.75 |
| Left Superior Temporal gyrus | −69 | −27 | 6 | 3.41 |
| Right Lingual gyrus | 18 | −66 | −12 | 4.61 |
| Right Occipital fusiform gyrus | 18 | −75 | −12 | 5.48 |
| Left occipital pole | −12 | −93 | −12 | 6.60 |
| Left Lingual gyrus | −12 | −84 | −12 | 6.82 |
Harvard-Oxford Cortical structural atlas
For each peak voxel x-, y-, and z-coordinates in the MNI − 152 standard space image are given
MA vs HC
Patients showed increased functional connectivity (blue area, p values are color coded from 0.05 FWE corrected (dark blue) to <0.0001 FWE corrected (light blue)) in bilateral frontal pole, right paracingulate gyrus, in right first and second Heschl’s gyrus, planum temporale, left in first and second Heschl’s gyrus, planum temporale and superior temporal gyrus (Fig. 1b, full list of structures in Table 4). No significant voxels for MA < HC were found.
Table 4.
Increased functional connectivity in MA compared with HC group
| Brain Structure | Peak voxel coordinates (MNI) | Peak T-score | ||
|---|---|---|---|---|
| x | y | z | ||
| Right Heschi’s | 54 | -15 | 6 | 4.20 |
| Right Planum temporale | 54 | −21 | 6 | 3.97 |
| Left Heschi’s gyrus | −54 | −15 | 6 | 3.80 |
| Left Planum temporale | −57 | −21 | 6 | 3.51 |
| Left Superior temporal gyrus | −57 | −33 | 6 | 3.75 |
Harvard-Oxford Cortical structural atlas
For each peak voxel x-, y-, and z-coordinates in the MNI-152 standard space image are given
MO vs HC
Cerebral regions showed increased functional connectivity in the DMN included right lingual gyrus, occipital fusiform gyrus, occipital pole and cingulate gyrus and, in the left side, increase connectivity in lingual gyrus, occipital fusiform gyrus, occipital pole and cingulate gyrus (Fig. 1c, full list of structures in Table 5) in both groups. No significant voxels for MO < HC were found.
Table 5.
Increased functional connectivity in MO compared with HC group
| Brain Structure | Peak voxel coordinates (MNI) | Peak T-score | ||
|---|---|---|---|---|
| x | y | z | ||
| Right Lingual gyrus | 18 | −54 | 0 | 3.54 |
| Right Occipital fusiform gyrus | 21 | −75 | 0 | 3.21 |
| Right Occipital pole | 9 | −93 | 0 | 4.85 |
| Right Cingulate gyrus | 18 | −45 | 0 | 2.8 |
| Left Lingual gyrus | −18 | −54 | 0 | 4.0 |
| Left Occipital fusiform gyrus | −21 | −75 | 0 | 3.1 |
| Left Occipital pole | −9 | −93 | 0 | 4.57 |
| Left Cingulate gyrus | −12 | −45 | 0 | 3.5 |
Harvard-Oxford Cortical structural atlas
For each peak voxel x-, y-, and z-coordinates in the MNI-152 standard space image are given
Discussion
Recently, several studies investigated the activity of resting state network in migraine and showed alterations in brain functional reorganization. Altered functional connectivity was found in cognitive cerebral networks, such as executive control network, default mode network, visual network. It seem to be associated to disease duration, gender, and migraine chronicity [24–26]. The DMN is a cerebral network related to different regions with relatively greater activity during rest-state than during active conditions [27, 28]. It refers to an interconnected group of brain structures that are hypothesized to be part of a functional system. Although the exact functional role of DMN is not completely know, it is thought to be involved in several cognitive processes, such as memory, problem solving and planning [2, 29]. In DMN, there are heteromodal association areas, which have a high number of connections with brain regions involved in integration processes, including pain matrics. In chronic pain DMN is altered [30], and this is possibly due to the increase of baseline activity of other cognitive, salience, or sensorimotor networks. Over time, chronic pain becomes an intrinsic brain activity occurring even in the absence of explicit brain input or output: thus, the alterations in patient’s brain at “rest” could be considered as a different or altered DMN organization [31]. In our study we identified specific alterations, during resting state examination, in cortical DMN if we compared MA, MO and HC. Our findings showed an increase of functional connectivity, in MA, in frontal and parietal lobes, in particular in angular, supramarginal gyrus, somatosensory association cortex, postcentral gyrus and primary somatosensory cortex. Since pain is inherently salient it is rational to speculate that the intrinsic connectivity in this network may be changed in chronic pain patients, like migraine subjects. In addition, in MA patients, we found an altered connectivity in insular cortex. It is know that insula is involved in triggering of pain matrix network and in the subjective pain experience [32]. It is also implicated in cognitive, affective, and regulatory functions, including interoceptive awareness, emotional responses, empathic and attentional processes [33]. The insula seems to be a cortical hub, to process complex sensory and emotional aspects in the migraine condition [34], through connections in frontal, temporal and parietal cortex, basal ganglia, thalamus and limbic structures. It is important to understand if functional connectivity abnormalities in this network could be correlated to minimal impairments in neuropsychological performances, such as processing speed, verbal memory, as reported in migraine in interictal attack period. In fact, although MA showed a cognitive performance lower than MO in executive functions, we did not find a significant impairment in two groups. In other word, in our patients, connectivity altered in DMN dwas not associate to neuropsychological variables and cognitive performances.
Moreover, we found in MA a greater cortical hyperexcitability than MO: resting-state abnormal activity could play a key role in the pathogenesis knowledge of migraine attacks with aura [35]. In particular, alterations of the DMN functional connectivity in migraine may lead to changes in pain modulating network, which could be considered as a neuroimaging biomarkers for disease pathophysiology.
Conclusions
The importance of various frequencies of BOLD fluctuations is not yet known, even if recently few studies started to explore this feature, especially in pain conditions. Brain dysfunction affecting intrinsic connectivity in migraine, possibly reflecting the impact of long lasting and constant pain on brain function.
Although our study was limited to a small sample size, our results confirmed that brain functional connectivity in migraine patients showed an alteration of DMN connectivity, suggesting that pain has a widespread impact on brain function, since modify the complex brain networks and beyond pain perception. Although migraine is one of the most investigated neurologic disorders, specific neuroimaging biomarker for its pathophysiology has not been found.Altered intrinsic functional connectivity architecture was identified in migraine patients and our finding could provide a new perspective to understand the pathogenesis of MA and MO migraine, in order to find a more appropriate therapeutic management.
Authors’ contributions
VLB contributed to study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript and critical revision; LB has made substantial contributions to the statistical analysis of data. FC and LRP have performed the clinical data collection. RL, RG, GDL have made substantial contributions to interpretation of data. PB and SM have been involved in analysis and interpretation data and in the drafting of manuscript and critical revision. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Participants provided written informed consent. The study protocol was approved by the Local Ethics Committee according to Declaration of Helsinki.
Competing interests
The authors have no competing interests to report.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schwedt TJ. Multisensory integration in migraine. Curr Opin Neurol. 2013;26(3):248. doi: 10.1097/WCO.0b013e328360edb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tessitore A, Russo A, Giordano A, Conte F, Corbo D, De Stefano M, Cirillo S, Cirillo M, Esposito F, Tedeschi G. Disrupted default mode network connectivity in migraine without aura. J Headache Pain. 2013;14:89. doi: 10.1186/1129-2377-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen BK, Olesen J. Migraine with aura and migraine without aura: an epidemiological study. Cephalalgia. 1992;12(4):221–228. doi: 10.1046/j.1468-2982.1992.1204221.x. [DOI] [PubMed] [Google Scholar]
- 4.Corallo F, De Cola MC, Lo Buono V, Grugno R, Pintabona G, Presti L, et al. Assessment of anxiety, depressive disorders and pain intensity in migraine and tension headache patients. Acta Med Austriaca. 2015;31:615. [Google Scholar]
- 5.Santangelo G, Russo A, Trojano L, Falco F, Marcuccio L, Siciliano M, Conte F, Garramone TA, Tedeschi G. Cognitive dysfunctions and psychological symptoms in migraine without aura: a cross-sectional study. J Headache Pain. 2016;17(1):76. doi: 10.1186/s10194-016-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Pira F, Reggio E, Quattrocchi G, Sanfilippo C, Maci T, Cavallaro T, Zappia M. Executive dysfunctions in migraine with and without aura: what is the role of white matter lesions? Headache. 2014;54(1):125–130. doi: 10.1111/head.12158. [DOI] [PubMed] [Google Scholar]
- 7.Pearson AJ, Chronicle EP, Maylor EA, Bruce LA. Cognitive function is not impaired in people with a long history of migraine: a blinded study. Cephalalgia. 2006;26:74–80. doi: 10.1111/j.1468-2982.2005.01001.x. [DOI] [PubMed] [Google Scholar]
- 8.Kalaydjian A, Zandi PP, Swartz KL, Eaton WW, Lyketsos C. How migraines impact cognitive function findings from the Baltimore ECA. Neurology. 2007;68(17):1417–1424. doi: 10.1212/01.wnl.0000268250.10171.b3. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Su J, Wang M, Zhao Y, Yao Q, Zhang Q, Wu YL. Increased default mode network connectivity and increased regional homogeneity in migraineurs without aura. J Headache Pain. 2016;17(1):98. doi: 10.1186/s10194-016-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu D, Yuan K, Zhao L, Zhao L, Dong M, Liu P, Wang G, Liu J, Sun J, Zhou G, von Deneen KM, Liang F, Qin W, Tian J. Regional homogeneity abnormalities in patients with interictal migraine without aura: a resting-state study. JNMR Biomed. 2012;25(5):806–812. doi: 10.1002/nbm.1796. [DOI] [PubMed] [Google Scholar]
- 11.Faragó P, Tuka B, Tóth E, Szabó N, Király A, Csete G, et al. Interictal brain activity differs in migraine with and without aura: resting state fMRI study. J Headache Pain. 2017;18(1):8. doi: 10.1186/s10194-016-0716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Headache Classification Committee of the International Headache S The international classification of headache disorders, 3rd edition (beta version) Cephalalgia. 2013;33(9):629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 13.Tombaugh TN. Trail making test a and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M. Rey auditory verbal learning test: a handbook. Los Angeles: Western Psychological Services; 1996. p. 1996. [Google Scholar]
- 15.Troyer AK, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency: evidence from younger and older healthy adults. Neuropsychology. 1997;11(1):138. doi: 10.1037/0894-4105.11.1.138. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- 19.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE TransMedImaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 21.Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl AcadSci USA. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abou-Elseoud A, Starck T, Remes J, Nikkinen J, Tervonen O, Kiviniemi V. The effect of model order selection in group PICA. Hum BrainMapp. 2010;31:1207–1216. doi: 10.1002/hbm.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nichols TE, Holmes AP. Non parametric permutation tests for functional neuroimaging: a primer with examples. HumBrainMapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guidetti V, Faedda N, Siniatchkin M. Migraine in childhood: biobehavioural or psychosomatic disorder? J Headache Pain. 2016;17(1):82. doi: 10.1186/s10194-016-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Zhao L, Li G, Xiong S, Nan J, Li J, Yuan K, von Deneen KM, Liang F, Qin W, Tian J. Hierarchical alteration of brain structural and functional networks in female migraine sufferers. PLoS One. 2012;7(12):e51250. doi: 10.1371/journal.pone.0051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol. 2011;70:838–845. doi: 10.1002/ana.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network. Ann N Y Acad Sci. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 28.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coppola G, Di Renzo A, Tinelli E, Lepre C, Di Lorenzo C, Di Lorenzo G, et al. Thalamo-cortical network activity between migraine attacks: insights from MRI-based microstructural and functional resting-state network correlation analysis. J Headache Pain. 2016;17(1):100. doi: 10.1186/s10194-016-0693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28(6):1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foss JM, Apkarian AV, Chialvo DR. Dynamics of pain: fractal dimension of temporal variability of spontaneous pain differentiates between pain states. J Neurophysiol. 2006;95(2):730–736. doi: 10.1152/jn.00768.2005. [DOI] [PubMed] [Google Scholar]
- 32.Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, Coghill RC. Roles of the insular cortex in the modulation of pain: insights from brain lesions. J Neurosci. 2009;29(9):2684–2694. doi: 10.1523/JNEUROSCI.5173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borsook D, Veggeberg R, Erpelding N, Borra R, Linnman C, Burstein R, Becerra L. The Insula a “hub of activity” in migraine. Neuroscientist. 2015;22(6):632–652. doi: 10.1177/1073858415601369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H, Ge H, Xiang J, Miao A, Tang L, Wu T, et al. Resting state brain activity in patients with migraine: a magnetoencephalography study. J Headache Pain. 2015;16(1):1–10. doi: 10.1186/s10194-015-0525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


