Abstract
This study was carried out to investigate the insecticidal properties of Beauveria bassiana, Metarhizium anisopliae and Heterorhabditis bacteriophora for their virulence against different larval instars of Rhynchophorus ferrugineus (Olivier). Both fungi were either applied alone or in combination, with H. bacteriophora simultaneously or 1 and 2 weeks after fungal application; EPN were also applied alone. Moreover, assessment of host development, diet consumption, frass production and weight gain were observed at sub-lethal dose rates. In combined treatments, additive and synergistic interactions were observed. Synergism was observed more frequently in H. bacteriophora + B. bassiana combinations than in H. bacteriophora + M. anisopliae combinations, and was higher in early instars than old instars. In 2nd and 4th instars, synergy was noted in H. bacteriophora + B. bassiana combinations at 0, 7 and 14 d intervals and in 6th instar synergy was observed only in H. bacteriophora + B. bassiana combinations (at 0 and 7 d intervals). A decrease in pupation, adult emergence and egg hatching was enhanced in the combined treatments. Furthermore, reduced weights and variation in duration of insect developmental stages were observed among entomopathogens and enhanced in H. bacteriophora + B. bassiana combinations. Larvae treated with sub-lethal concentrations exhibited reductions in food consumption, growth and frass production and weight gain.
Introduction
The coleopteran insect pests are ranked among the most voracious pests of economically important crops. Among these notorious insect pests, red palm weevil (RPW) Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) is highly destructive; the insect devastates 29 different palm species, particularly date palms which are economically important crops in the Middle East, Africa and South East Asia1, 2. Synonymously, the pest is known as the Asiatic palm weevil, coconut weevil, red stripe weevil, and hidden enemy, and also called AIDS of palm because of the damage caused and the resulting slow death of palm trees3. The pest has a cryptic nature and mostly damages palm trees younger than 20 years4, 5 for which the crown, trunk and bole are the natural sites of damage. The crowns are the sites of infestation in older plantations. The larvae spend their early stages within the tree trunk, destroying the vascular system and boring into the heart of host, which may lead to tree collapse6. The neonate larvae chew plant fibers and advance towards the interior leaving behind the chewed-up frass which has a typical fermented odor. The completely developed grubs pupate in a cocoon fabricated from chewed fibers, and pupal period lasts for 11–45 days. Adult weevil can interbreed and live within the same host until they are required to colonize to a new palm. If the plant remains untreated the palm can die within 6–8 months7–9.
To combat this insect pest, synthetic insecticides and fumigants have remained the mainstay of date palm growers for decades. However, chemical control is challenging due to the cryptic nature of RPW10. Moreover, the chemical insecticides have exerted negative effects on the environment and human health, and the pest has developed resistance against many of these chemicals5. Alternatively, entomopathogens may have potential for control of R. ferrugineus. Among microbial control agents, entomopathogenic fungi (EPFs) particularly Beauveria bassiana s.l. (Ascomycota: Hypocreales) and Metarhizium anisopliae s.l. (Ascomycota: Hypocreales) are considered promising alternatives to conventional synthetic insecticides. They pose negligible detrimental effect on environment and human health11, and harbor promising insecticidal activities against a number of arthropod pests12, 13.
Several researchers have isolated and successfully deployed these two fungal species against different developmental stages of RPW as biocontrol agents both under laboratory and field conditions14–26. EPFs are attractive relative to a number of other microbial agents due to their novel mode of action by direct contact to the host cuticle instead of ingestion, and their ability to transfer inoculum from treated insects to untreated insects via the new generation of spores27.
Similarly, entomopathogenic nematodes (EPNs) are also promising microbial control agents and are efficient in suppressing a variety of insect pests28–32. They are obligate parasites in the families Steinernematidae and Heterorhabditidae which kill insects with the aid of mutualistic bacterium carried in their intestine (Xenorhabdus spp. and Photorhabdus spp. bacteria are associated with Steinernema spp. and Heterorhabditis spp., respectively)33. Both microbial agents (fungi and nematodes) are considered safe to non-target organisms (vertebrates and invertebrates) and the environment, and they can be successfully integrated with each other, often exhibiting strong additive and synergistic interactions34–37. A number of scientists have evaluated different EPN species against RPW both under laboratory and field conditions28, 29, 38–47.
The objective of this study was to explore the potential for integration of B. bassiana, M. anisopliae and H. bacteriophora in a control program for R. ferrugineus. Mortality, development and growth of R. ferrugineus was studied under laboratory conditions following application of fungus and nematode treatments applied alone, or fungus-nematode combinations. Interactions (synergy, additivity or antagonism) between microbial agents were assessed. Results will assist in decision-making for selecting the most suitable treatments and application times of both agents in future field trials, and eventually lead to successful and environment friendly control of R. ferrugineus populations in date palm systems.
Results
Entomopathogenic fungi and nematodes interaction
In integrated application of H. bacteriophora with B. bassiana or M. anisopliae, additive and synergistic interactions were observed in all the three instars tested when the microbial agents were applied simultaneously or following delayed nematode application (Tables 1, 2 and 3). Antagonism was not observed in any of the combinations tested (Tables 1, 2 and 3). During simultaneous application, 2nd instar larvae exhibited additive effects for B. bassiana and H. bacteriophora for the first two weeks, while synergistic interaction was observed at the third week of application. The degree of synergism increased with the delayed application of H. bacteriophora one or two weeks after B. bassiana treatments. For M. anisopliae additive effects were recorded for simultaneous application, while interactions were shifted towards synergism when delayed nematode application was made after one and two weeks of fungal spore application (Table 1). A similar trend was recorded for 4th and 6th instar larvae but susceptibility was lower among treatments as compared with the 2nd instar larvae, and the instances of synergy also decreased as instar size increased (Tables 1, 2 and 3). Also, synergy was observed in H. bacteriophora and B. bassiana combinations more than in H. bacteriophora and M. anisopliae combinations (Tables 1, 2 and 3).
Table 1.
Mean mortality (% ± SE) of 2nd instar larvae of Rhynchophorus ferrugineus treated with Beauveria bassiana, Metarhizium anisopliae and Heterorhabditis bacteriophora. B. bassiana and M. anisopliae were used each @ 1 × 106 conidia ml−1 and H. bacteriophora was applied @ 100 IJs ml−1 (Bb: Beauveria bassiana, Ma: Metarhizium anisopliae, EPN: Heterorhabditis bacteriophora, IJs: Infective juveniles).
| Treatments | Intervalsa | Weekb | Observed mortality | Expected mortality | Chi Sq. | Type of interaction |
|---|---|---|---|---|---|---|
| Bb | — | 1 | 11.22 | — | — | — |
| — | 2 | 14.28 | — | — | — | |
| — | 3 | 20.40 | — | — | — | |
| Ma | — | 1 | 8.16 | — | — | — |
| — | 2 | 12.24 | — | — | — | |
| — | 3 | 17.34 | — | — | — | |
| EPN | — | 1 | 14.28 | — | — | — |
| — | 2 | 21.42 | — | — | — | |
| — | 3 | 29.59 | — | — | — | |
| Bb + EPN | 0 | 1 | 27.55 | 23.90 | 0.48 | Additive |
| 0 | 2 | 43.87 | 32.65 | 2.87 | Additive | |
| 0 | 3 | 61.22 | 43.96 | 4.86 | Synergistic | |
| Ma + EPN | 0 | 1 | 23.71 | 21.28 | 0.24 | Additive |
| 0 | 2 | 32.98 | 38.21 | 0.82 | Additive | |
| 0 | 3 | 48.45 | 36.94 | 2.73 | Additive | |
| Bb + EPN | 7 | 1 | 32.99 | 26.53 | 1.26 | Additive |
| 7 | 2 | 51.54 | 37.46 | 3.84 | Synergistic | |
| 7 | 3 | 73.19 | 51.86 | 6.21 | Synergistic | |
| Ma + EPN | 7 | 1 | 28.86 | 24.78 | 0.57 | Additive |
| 7 | 2 | 45.36 | 35.05 | 2.33 | Additive | |
| 7 | 3 | 64.94 | 48.27 | 4.28 | Synergistic | |
| Bb + EPN | 14 | 1 | 51.54 | 37.46 | 3.84 | Synergistic |
| 14 | 2 | 69.07 | 51.86 | 4.28 | Synergistic | |
| 14 | 3 | 88.65 | 62.51 | 7.70 | Synergistic | |
| Ma + EPN | 14 | 1 | 44.32 | 35.05 | 1.93 | Additive |
| 14 | 2 | 64.94 | 48.99 | 3.92 | Synergistic | |
| 14 | 3 | 75.25 | 53.95 | 6.02 | Synergistic |
aIntervals between the application of EPFs and EPNs. bWeek after fungal application.
Table 2.
Mean mortality (% ± SE) of 4th instar larvae of Rhynchophorus ferrugineus treated with Beauveria bassiana, Metarhizium anisopliae and Heterorhabditis bacteriophora. B. bassiana and M. anisopliae were used each @ 1 × 106 conidia ml−1 and H. bacteriophora was applied @ 100 IJs ml−1 (Bb: Beauveria bassiana, Ma: Metarhizium anisopliae, EPN: Heterorhabditis bacteriophora, IJs: Infective Juveniles).
| Treatments | Intervalsa | Weekb | Observed mortality | Expected mortality | Chi Sq. | Type of interaction |
|---|---|---|---|---|---|---|
| Bb | — | 1 | 9.18 | — | — | — |
| — | 2 | 11.22 | — | — | — | |
| — | 3 | 16.32 | — | — | — | |
| Ma | — | 1 | 6.12 | — | — | — |
| — | 2 | 9.18 | — | — | — | |
| — | 3 | 14.28 | — | — | — | |
| EPN | — | 1 | 12.24 | — | — | — |
| — | 2 | 17.34 | — | — | — | |
| — | 3 | 23.46 | — | — | — | |
| Bb + EPN | 0 | 1 | 24.29 | 20.30 | 0.71 | Additive |
| 0 | 2 | 34.69 | 26.62 | 1.87 | Additive | |
| 0 | 3 | 51.02 | 35.96 | 4.44 | Synergistic | |
| Ma + EPN | 0 | 1 | 19.58 | 17.61 | 0.19 | Additive |
| 0 | 2 | 28.86 | 24.93 | 0.53 | Additive | |
| 0 | 3 | 44.32 | 34.40 | 2.22 | Additive | |
| Bb + EPN | 7 | 1 | 27.83 | 22.09 | 1.18 | Additive |
| 7 | 2 | 43.29 | 30.84 | 3.58 | Additive | |
| 7 | 3 | 61.85 | 42.99 | 5.75 | Synergistic | |
| Ma + EPN | 7 | 1 | 23.71 | 20.30 | 0.48 | Additive |
| 7 | 2 | 38.14 | 29.15 | 2.11 | Additive | |
| 7 | 3 | 55.67 | 40.64 | 4.05 | Synergistic | |
| Bb + EPN | 14 | 1 | 42.26 | 30.84 | 3.08 | Additive |
| 14 | 2 | 58.76 | 42.99 | 4.23 | Synergistic | |
| 14 | 3 | 80.41 | 57.39 | 6.58 | Synergistic | |
| Ma + EPN | 14 | 1 | 36.08 | 29.15 | 1.33 | Additive |
| 14 | 2 | 54.63 | 40.64 | 3.58 | Additive | |
| 14 | 3 | 72.16 | 53.95 | 4.50 | Synergistic |
aIntervals between the application of EPFs and EPNs. bWeek after fungal application.
Table 3.
Mean mortality (% ± SE) of 6th instar larvae of Rhynchophorus ferrugineus treated with Beauveria bassiana, Metarhizium anisopliae and Heterorhabditis bacteriophora. B. bassiana and M. anisopliae were used each @ 1 × 106 conidia ml−1 and H. Bacteriophora was applied @ 100 IJs ml−1 (Bb: Beauveria bassiana, Ma: Metarhizium anisopliae, EPN: Heterorhabditis bacteriophora, IJs: Infective Juveniles).
| Treatments | Intervalsa | Weekb | Observed mortality (%) | Expected mortality | Chi Sq. | Type of interaction |
|---|---|---|---|---|---|---|
| Bb | — | 1 | 7.14 | — | — | — |
| — | 2 | 9.18 | — | — | — | |
| — | 3 | 13.26 | — | — | — | |
| Ma | — | 1 | 4.081 | — | — | — |
| — | 2 | 7.14 | — | — | — | |
| — | 3 | 11.22 | — | — | — | |
| EPN | — | 1 | 9.18 | — | — | — |
| — | 2 | 14.28 | — | — | — | |
| — | 3 | 18.36 | — | — | — | |
| Bb + EPN | 0 | 1 | 17.34 | 15.67 | 0.16 | Additive |
| 0 | 2 | 28.57 | 22.15 | 1.43 | Additive | |
| 0 | 3 | 42.85 | 29.19 | 4.35 | Synergistic | |
| Ma + EPN | 0 | 1 | 13.40 | 12.89 | 0.01 | Additive |
| 0 | 2 | 22.68 | 20.40 | 0.22 | Additive | |
| 0 | 3 | 35.05 | 27.53 | 1.61 | Additive | |
| Bb + EPN | 7 | 1 | 21.64 | 17.52 | 0.78 | Additive |
| 7 | 2 | 35.05 | 25.65 | 2.51 | Additive | |
| 7 | 3 | 50.51 | 35.02 | 4.74 | Synergistic | |
| Ma + EPN | 7 | 1 | 17.52 | 15.67 | 0.19 | Additive |
| 7 | 2 | 30.92 | 23.90 | 1.59 | Additive | |
| 7 | 3 | 44.32 | 31.69 | 3.60 | Additive | |
| Bb + EPN | 14 | 1 | 34.02 | 25.65 | 2.05 | Additive |
| 14 | 2 | 48.45 | 35.02 | 3.72 | Additive | |
| 14 | 3 | 71.13 | 51.86 | 5.22 | Additive | |
| Ma + EPN | 14 | 1 | 28.86 | 23.90 | 0.85 | Additive |
| 14 | 2 | 42.26 | 31.69 | 2.64 | Additive | |
| 14 | 3 | 61.85 | 46.03 | 4.04 | Additive |
aIntervals between the application of EPFs and EPNs. bWeek after fungal application.
In a factorial analysis the main effects for pupation adult emergence and egg eclosion were significant while their interaction effects were non-significant except pupation (Table 4). A decrease in pupation, adult emergence and egg hatching was caused by all treatments and the effects were enhanced in the combined treatments (particularly with H. bacteriophora + B. bassiana) (Table 5).
Table 4.
Factorial analysis for pupation, adult emergence and egg eclosion of Rhynchophorus ferrugineus exposed to Beauveria bassiana, Metarhizium anisopliae and Heterorhabditis bacteriophora.
| Source | df | Pupation | Adult emergence | Egg eclosion | |||
|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | ||
| Instar | 2 | 18.78 | <0.01 | 39.94 | <0.01 | 35.0 | <0.01 |
| Treatment | 9 | 114.28 | <0.01 | 84.44 | <0.01 | 90.89 | <0.01 |
| Instar × Treatment | 18 | 10.49 | <0.01 | 0.48 | 0.96 | 0.46 | 0.97 |
| Error | 232 | — | — | — | — | — | — |
| Total | 269 | — | — | — | — | — | — |
Table 5.
Pupation, adult emergence and egg eclosion (% ± SE) of 2nd, 4th and 6th instar R. ferrugineus larvae treated with Beauveria bassiana (1 × 106 spore ml−1), Metarhizium anisopliae (1 × 106 spore ml−1) and Heterorhabditis bacteriophora (100 IJs ml−1). Mean sharing the same letters are not significantly different at 5% level (Bb: Beauveria bassiana, Ma: Metarhizium anisopliae, EPN: Heterorhabditis bacteriophora, IJs: Infective Juveniles).
| Treatment | Interval | Second instar | Fourth instar | Sixth instar | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pupation (%) | Adult emergence (%) | Egg eclosion (%) | Pupation (%) | Adult emergence (%) | Egg eclosion (%) | Pupation (%) | Adult emergence (%) | Egg eclosion (%) | ||
| Bb | — | 62.22 ± 2.23bc | 57.77 ± 2.77bc | 53.33 ± 2.33bc | 71.11 ± 3.54b | 66.66 ± 3.08b | 59.55 ± 2.92b | 80.00 ± 2.88bc | 74.44 ± 3.37b | 66.33 ± 3.10b |
| Ma | — | 67.77 ± 2.64b | 61.11 ± 3.21b | 58.88 ± 2.23b | 73.33 ± 3.40b | 68.88 ± 3.51b | 62.22 ± 2.77b | 83.33 ± 2.35ab | 78.88 ± 3.88ab | 72.22 ± 3.22b |
| EPN | — | 56.66 ± 2.33bcd | 50.55 ± 2.69bcd | 45.55 ± 1.67bcd | 62.22 ± 2.77bc | 57.77 ± 2.33bc | 51.11 ± 2.51bc | 69.44 ± 3.42 cd | 65.55 ± 2.75bc | 59.33 ± 2.44bc |
| Bb + EPN | 0 | 45.55 ± 1.93def | 40.33 ± 2.35def | 36.66 ± 1.88def | 48.88 ± 2.09 cd | 43.33 ± 3.08 cd | 38.88 ± 1.60 cd | 54.44 ± 2.76ef | 49.44 ± 2.57de | 43.33 ± 2.35de |
| Ma + EPN | 0 | 51.11 ± 1.51cde | 44.77 ± 2.89cde | 39.44 ± 1.36cde | 54.44 ± 2.12 cd | 49.44 ± 2.16 cd | 44.44 ± 2.23 cd | 60.00 ± 2.88de | 56.66 ± 2.72 cd | 51.11 ± 2.51 cd |
| Bb + EPN | 7 | 39.44 ± 1.69ef | 36.66 ± 1.33def | 31.11 ± 1.51def | 45.55 ± 2.42d | 40.55 ± 1.93d | 35.55 ± 1.93d | 51.11 ± 2.51ef | 45.55 ± 2.93de | 40.55 ± 1.42de |
| Ma + EPN | 7 | 43.55 ± 1.73def | 38.88 ± 1.51def | 34.44 ± 1.37def | 51.11 ± 2.88 cd | 46.11 ± 2.32 cd | 41.11 ± 1.51 cd | 57.77 ± 2.77def | 52.22 ± 2.23cde | 46.66 ± 1.33cde |
| Bb + EPN | 14 | 32.22 ± 1.27 f | 27.77 ± 1.77 f | 22.22 ± 1.46 f | 39.44 ± 2.42d | 36.66 ± 1.33d | 31.11 ± 1.60d | 44.44 ± 2.93 f | 40.22 ± 2.79e | 34.44 ± 1.93e |
| Ma + EPN | 14 | 35.55 ± 1.43 f | 30.55 ± 3.37ef | 26.66 ± 1.68ef | 47.77 ± 2.23 cd | 42.22 ± 2.12 cd | 37.77 ± 1.22 cd | 54.44 ± 2.76ef | 47.77 ± 2.64de | 42.22 ± 1.22de |
| Control | 90.55 ± 2.11a | 86.66 ± 2.88a | 81.11 ± 2.60a | 93.33 ± 2.66a | 90.55 ± 2.11a | 83.33 ± 3.33a | 95.55 ± 1.75a | 92.22 ± 2.22a | 85.55 ± 2.93a | |
| df | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | |
| F | 35.7 | 31.3 | 31.0 | 23.4 | 23.6 | 28.8 | 30.6 | 28.1 | 32.4 | |
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
Development of R. ferrugineus: larvae through adult stages
Growth and development of RPW (larvae to adult stage) was adversely affected by the microbial agents. When larvae were exposed to the sub-lethal doses of H. bacteriophora, B. bassiana and M. anisopliae, significant variations were recorded for larval duration, larval weight, pre-pupal duration, pre-pupal weight, pupal duration, pupal weight, adult longevity and adult weight (larval duration: F 5, 53 = 9.92, P <0.01; larval weight F 5, 53 = 27.3, P <0.01; pre-pupal duration: F 5, 53 = 6.59, P <0.01; pre-pupal weight: F 5, 53 = 6.94, P<0.01; pupal duration F 5, 53 = 5.15, P < 0.01; pupal weight F 5, 53 = 11.10, P <0.01; adult longevity (female F 5, 53 = 3.93, P < 0.01 and male F 5, 53 = 5.58, P < 0.01); adult weight (female F 5, 53 = 4.26, P < 0.01 and male F 5, 53 = 12.7, P < 0.01). For the most part, increases in larval, pre-pupal and pupal duration occurred while decreases in weight among stages were observed for all the treatments tested. On the other hand a decrease in adult life span and weight (male and female) was also recorded. The numerically highest detrimental effect on growth tended to be for combined application of B. bassiana and H. bacteriophora (e.g., see larval and adult weights) followed by M. anisopliae and H. bacteriophora, H. bacteriophora alone, B. bassiana and M. anisopliae (Table 6).
Table 6.
Effect of Beauveria bassiana (1 × 104 spore ml−1), Metarhizium anisopliae (1 × 104 spore ml−1) and Heterorhabditis bacteriophora (50 IJs ml−1) on the development of Rhynchophorus ferrugineus. Mean sharing the same letters within each column are not significantly different at 5% level (Bb: Beauveria bassiana, Ma: Metarhizium anisopliae, EPN: Heterorhabditis bacteriophora, IJs: Infective Juveniles).
| Treatments | Larval duration (days) | Larval weight (g) | Pre-pupal duration (days) | Pre-pupal weight (g) | Pupal duration (days) | Pupal weight (g) | Adult longevity (days) | Adult weight (g) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||||
| Bb | 98.16 ± 3.21b | 4.01 ± 0.12bc | 15.16 ± 0.58bc | 4.02 ± 0.20ab | 22.94 ± 1.20bc | 3.92 ± 0.18abc | 39.05 ± 1.54ab | 42.83 ± 1.78ab | 1.411 ± 0.12ab | 1.14 ± 0.11abc |
| Ma | 96.50 ± 3.88bc | 4.41 ± 0.15ab | 15.94 ± 0.79bc | 4.07 ± 0.19ab | 23.72 ± 1.36abc | 4.11 ± 0.15ab | 41.83 ± 1.40a | 43.61 ± 1.67a | 1.33 ± 0.14ab | 1.28 ± 0.15ab |
| EPN | 101.16 ± 3.63ab | 3.72 ± 0.12 cd | 16.72 ± 0.95abc | 3.85 ± 0.14ab | 24.16 ± 1.41abc | 3.67 ± 0.10bcd | 37.16 ± 1.23ab | 40.38 ± 1.45ab | 1.18 ± 0.1bc | 1.05 ± 0.12abc |
| Bb + EPN | 109.27 ± 4.16a | 3.08 ± 0.12e | 20.16 ± 1.14a | 3.07 ± 0.11c | 27.61 ± 1.24a | 3.21 ± 0.11d | 34.94 ± 1.29b | 36.50 ± 1.78b | 0.84 ± 0.10d | 0.78 ± 0.11c |
| Ma + EPN | 104.38 ± 3.32ab | 3.27 ± 0.11de | 18.50 ± 0.98ab | 3.48 ± 0.12bc | 25.50 ± 1.41ab | 3.43 ± 0.13 cd | 36.16 ± 1.17b | 38.71 ± 1.68ab | 1.01 ± 0.17 cd | 0.92 ± 0.16bc |
| Control | 87.05 ± 1.08c | 4.87 ± 0.14a | 14.38 ± 0.74c | 4.17 ± 0.25a | 21.27 ± 1.36c | 4.24 ± 0.10a | 42.83 ± 1.61a | 45.27 ± 1.43a | 1.56 ± 0.13a | 1.37 ± 0.11a |
| df | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| F | 9.92 | 27.3 | 6.57 | 6.94 | 5.18 | 11.1 | 5.55 | 3.92 | 12.7 | 4.26 |
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
Development of R. ferrugineus: focus on last instar
Based on repeated measures analysis (and Tukey’s separation), diet consumption by 10th instar was significantly influenced by the treatments applied (F 3, 63 = 250.67, P < 0.01); diet consumption was lowest in the combined treatment of H. bacteriophora and B. bassiana compared to their sole applications and all treatments had lower consumption than the control (Fig. 1). Similarly frass production was influenced by treatments applied (F 3, 69 = 386.22, P <0.01), with the lowest frass production for the combined treatment of H. bacteriophora and B. bassiana (0.57 ± 0.04 to 0 ± 0.00 g) and highest in the control (the single-applied treatments were intermediate and not different from each other) (Fig. 2). Untreated larvae (control) and larvae treated with sub-lethal concentrations of B. bassiana and H. bacteriophora gained more weight as compared to their combined application (F 3, 24 = 56.77, P <0.01) (Fig. 3); the single applied treatments were not different from the control whereas the combination treatment was statistically separated from all three others.
Figure 1.
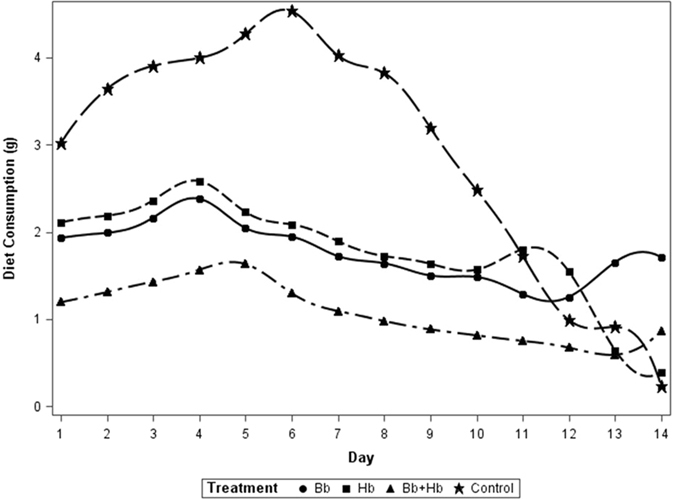
Diet consumption (g) in last instar larvae of Rhynchophorus ferrugineus when treated with B. bassiana (1 × 104 spore ml−1) and H. bacteriophora (50 IJs ml−1); (Bb: Beauveria bassiana, Hb: Heterorhabditis bacteriophora, IJs: Infective Juveniles).
Figure 2.
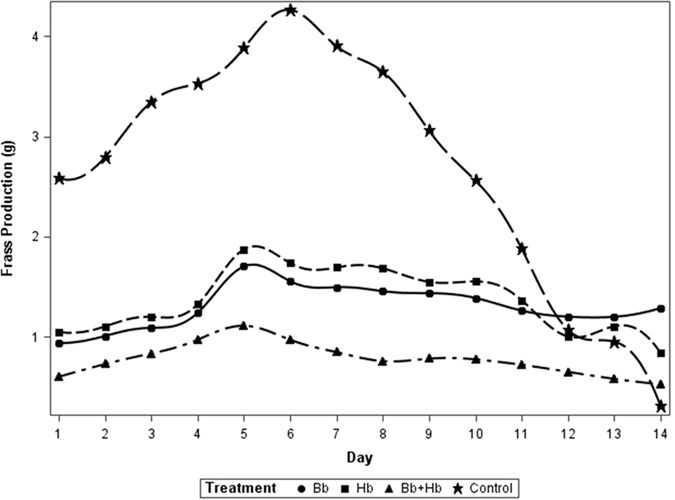
Frass production (g) in last instar larvae of Rhynchophorus ferrugineus when treated with B. bassiana (1 × 104 spore ml−1) and H. bacteriophora (50 IJs ml−1); (Bb: Beauveria bassiana, Hb: Heterorhabditis bacteriophora, IJs: Infective Juveniles).
Figure 3.
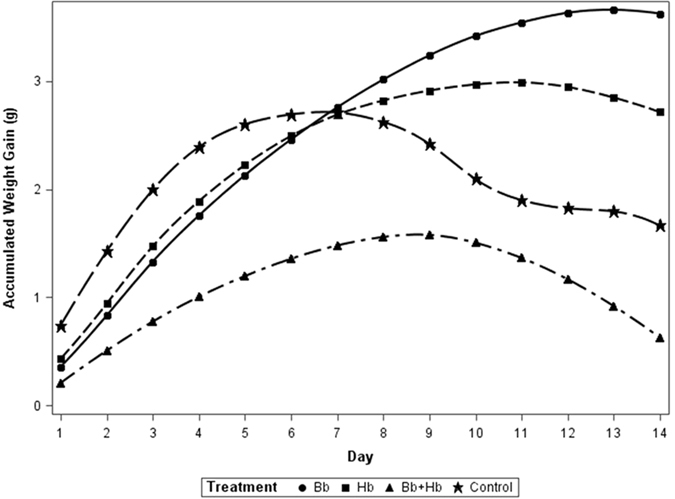
Cumulative weight gain (g) in last instar larvae of Rhynchophorus ferrugineus when treated with B. bassiana (1 × 104 spore ml−1) and H. bacteriophora (50 IJs ml−1); (Bb: Beauveria bassiana, Hb: Heterorhabditis bacteriophora, IJs: Infective Juveniles).
Discussion
This is the first study to investigate the combined effects of fungal isolates and H. bacteriophora against larvae of RPW. The results revealed that the microbial agents applied alone displayed pathogenicity. However, when fungi and nematodes were combined (either applied simultaneously or delayed application of H. bacteriophora), the level of virulence was enhanced in an additive or synergistic fashion. Synergistic levels of virulence were observed more frequently in H. bacteriophora + B. bassiana combinations than in H. bacteriophora and M. anisopliae combinations. Additionally, synergy appeared to be more prevalent in the younger instars tested than the later instars, and our findings are similar to the findings of Ansari et al.48, 49 indicating positive interactions with the combined application of H. megidis or S. glaseri with M. anisopliae CLO 53 against 3rd instar H. philanthus under laboratory and greenhouse conditions, and between H. bacteriophora and M. anisopliae CLO 53 under field conditions. Similarly synergistic effects were observed in combined treatments of H. bacteriophora and M. anisopliae isolate against barley chafer grub, C. curtipennis after 5 weeks of M. anisopliae application50. They have suggested exposing grubs 3 or 4 weeks before addition of nematodes to get stronger synergistic interactions. In our study enhanced efficacy and stronger interactions were recorded at 1 or 2 weeks delayed application of H. bacteriophora.
In prior research, synergistic interactions were also observed between entomopathogenic nematodes and fungi. For example51 laboratory trials were conducted against Otiorhynchus sulcatus (the black vine weevil) using a combination of M. anisopliae and EPN. Synergy occurred with either H. bacteriophora or S. kraussei and mortality with S. feltiae was recorded as additive. They also performed greenhouse trials with the combination of these agents applied at various concentrations. The combination of M. anisopliae and H. bacteriophora resulted in synergistic interactions in all treatments. The combinations of M. anisopliae and S. feltiae only resulted in synergy when a high concentration of fungus was used with a low concentration of nematodes; all other interactions were additive51. When overwintering black vine weevils in greenhouses were exposed to M. anisopliae and S. kraussei the interaction recorded was synergistic in the first trial, but when the trial was repeated results were additive52. This shows that the combination of these pathogens may not be consistent. It was hypothesised that the inconsistent results may be due to the sensitivity of the fungus to temperature52.
We speculate that the longer grubs are exposed to the fungus, the more debilitated they become and subsequently are more susceptible to the EPN. It is also conceivable that the debilitated insects respire more, attracting the EPNs, which follow a CO2 gradient to their hosts51. It is also suggested that the stressed insects are more vulnerable to pathogen infection, which thus enhances insect mortality or facilitates a higher speed of kill leading to synergistic effects in combined treatments53. For example, Paenibacillus popilliae (Dutky) applied against scarab larvae acted as a stressor to nematode infection which caused elevated larval mortality34, 35. Similarly entomopathogenic fungi have been shown to alter the behavior of the host insect, reducing locomotion, feeding and increasing irritability54. Grubs suffering from a fungal infection may not be able to feed or utilize food normally. This could adversely affect morphological, behavioral and physiological mechanism that grubs have evolved in order to defend against natural enemies in the soil55, thereby making the grub more susceptible to nematode penetration. Synergistic interactions between fungi and nematodes have been reported by many researchers34–37, 56–58. Contrarily59 found antagonism between EPNs and Isaria fumosorosea (Wize) when virulence to pecan weevil, Curculio caryae (Horn). Also, in contrast to this study59, also observed antagonism between certain combinations of B. bassiana and EPN, Steinernema carpocapsae (Weiser) or Heterorhabditis indica Poinar, Karunakar & David, and antagonism in combinations of S. carpocapsae and M. anisopliae. These discrepancies emphasize that synergistic interactions depend heavily on the host target as well as the pathogens that are being combined, and timing of applications (simultaneous versus sequential).
Growth and development are required for successful completion of the insect life-cycle and reproduction. Any delay may render the insect susceptible to biotic and abiotic factors (such as natural enemies or environmental regimes) that ultimately limit their growth and development. In this regard larval stages are vulnerable towards such phenomena60. In our study, treatments caused reduced food consumption, reduced longevity of the adult stage, and reduced weights in life-stages, which thus affected the insect’s fecundity and survival into the next generation.
The present study showed that B. bassiana and M. anisopliae isolates in integration with H. bacteriophora under laboratory conditions caused high mortality against larvae of the red palm weevil. The pathogens exerted detrimental effects on survival, growth and development of different developmental stages of R. ferrugineus. Hence, integrated application of H. bacteriophora in sequential manners with B. bassiana and M. anisopliae might be effectively used for the successful control of red palm weevil. This approach (of combined applications) promises to provide superior efficacy than previous attempts to control the insect pest with singly applied treatments but further research is needed (e.g. under field conditions) for confirmation of the method’s success in date palm orchards.
Materials and Methods
RPW collection and rearing
A survey was conducted for collection of R. ferrugineus in date palm growing areas of west Punjab, Pakistan. Different developmental stages (larvae, pupae and adults) were collected from fallen and infested date palm trees with the permission of farmers (owners). All the stages collected were kept separately in plastic jars until brought to Microbial Control Laboratory, Department of Entomology, University of Agriculture, Faisalabad (UAF), Pakistan. Larvae were fed with sets of sugarcane (Saccharum officinarum L.; Poales: Poaceae) and pupation occurred within the stalks, while shredded sugarcane pieces were offered to adults for feeding and as a substrate for oviposition. After pupation, pupal cocoons were kept in separate plastic jars for adult emergence. After adult emergence beetles were shifted to the adult’s jar for feeding, mating and oviposition. The colony was developed in plastic boxes (30 × 60 × 60 cm) having a lid covered with mesh wire gauze (60 mesh size, 10 cm diameter) in the middle for aeration. The adult’s diet was changed every three days, and used sugarcane pieces were kept in separate jars (8 × 8 × 12 cm) for egg hatching. After egg hatching neonate larvae were allowed to feed for some time in the same set, after 3 days larvae were transferred to new sugarcane sets for feeding and pupation. Larvae were shifted to the new sugarcane sets after every week until pupation. The rearing conditions were maintained at 25 ± 2 °C, 65 ± 5% RH and a 12:12 (D: L) hour photoperiod.
Entomopathogenic nematodes
Infective juveniles (IJs) of H. bacteriophora culture were obtained from Microbial Control Laboratory and used for the bioassays against 2nd, 4th and 6th instars of R. ferrugineus. H. bacteriophora was cultured in the final instar Galleria mellonella L. (Lepidoptera: Pyralidae) following the procedures of Kaya and Stock61.
Entomopathogenic fungi
Two isolates of entomopathogenic fungi B. bassiana (WG-11) and M. anisopliae (WG-02) used in the study were obtained from the culture collection of the Microbial Control Laboratory; the strains were originally isolated from soils of vegetables and crop fields, respectively. Culturing of the fungi was accomplished by inoculating Petri plates containing Potato Dextrose Agar (PDA) media (BD, France)62. Spore concentration of 1 × 106 spore ml−1 was determined with a Neubauer haemocytometer.
Treatment with entomopathogenic fungi
The larvae (2nd, 4th and 6th instars) were directly immersed in 100 ml conidial suspension for 60 s individually and both treatments and the control were applied in an aqueous solution of 0.01% Tween-80 (Merck, KGaA, Darmstadt, Germany)63; control larvae were dipped into a suspension of 0.01% Tween-80 (without fungal spores). The treated and control larvae were then individually moved to 150 ml cylindrical plastic cups, each measuring 6 cm in height and 6 cm diameter. The top of the cups were covered with a fine mesh in order to prevent the insects from escaping. A piece of 2 × 2 cm2 artificial diet (Agar, brewer’s yeast, wheat germ, corn flour, ascorbic acid, benzoic acid, amino acid-vitamin mix, chloramphenicol and nipagin)64 was kept in the center of each cup. The cups were kept at 27 ± 2 °C, 65 ± 5% RH and a 12:12 (D:L) hour photoperiod in an incubator (Sanyo, Japan). Three replicates of 10 larvae were treated to the fungal suspensions or control. Each cup was opened daily and checked for mortality, and the old diet was replaced with fresh artificial diet until insect mortality or pupation was observed. After the insect reached the last instar stage, dry coir (coconut) was provided to the surviving larvae for pupation. The entire bioassay was repeated thrice.
Treatment with H. bacteriophora
Nematode suspensions were prepared with concentrations of 100 IJs ml−1 in glass jars and 1 ml of suspension was poured into the cylindrical plastic cups lined with Whatman filter paper (described above). After pouring 30 minutes allowing the nematodes to distribute evenly on the filter paper, a small piece of artificial diet 2 × 2 cm2 was placed in the middle of the cups as a food source. Ten larvae for each treatment were used individually in each cup and each treatment was replicated three times, while control treatment received 1 ml of distilled water (without nematodes). The cups were maintained at above mentioned environmental conditions. The cups were opened daily to note mortality until insect mortality or pupation was observed. After the insect reached the last instar stage, dry coir was provided to the surviving larvae for pupation. The entire bioassay was repeated thrice.
Treatment with entomopathogenic fungi and nematodes
The fungi (B. bassiana or M. anisopliae) were tested in combination with H. bacteriophora; combinations of the two fungi together (B. bassiana plus M. anisopliae) were not tested. In combined treatments fungi and nematodes were applied simultaneously or at different time intervals as follows:
B. bassiana or M. anisopliae plus H. bacteriophora were applied simultaneously: larvae were immersed in fungal suspensions and transferred to the cylindrical plastic cups lined with moistened filter paper treated with H. bacteriophora IJs, and maintained at the conditions described above.
Insects were first inoculated with B. bassiana or M. anisopliae, maintained at 27 ± 2 °C and 65 ± 5% RH for one week, then transferred to cylindrical plastic cups lined with moistened filter paper treated with H. bacteriophora IJs, and maintained at above mentioned conditions.
Insects were first inoculated with B. bassiana and M. anisopliae, maintained at 27 ± 2 °C and 65 ± 5% RH for two weeks, transferred to cylindrical plastic cups lined with moistened filter paper treated with H. bacteriophora IJs, and maintained at above mentioned conditions.
Control insects were immersed in aqueous solution with 0.01% Tween-80 and maintained in cylindrical plastic cups lined with moistened filter paper using conditions stated above.
Larval mortality was recorded after one, two and three weeks post application. For all treatments, artificial diet was offered to the larvae as food source. Larvae that failed to respond on slight prodding by a blunt needle were considered dead. After the insect reached the last instar stage, dry coir was provided to the surviving larvae for pupation. Percent pupation, adult emergence and egg eclosion were also recorded.
Effects of entomopathogens on R. ferrugineus development
The effects of sub-lethal entomopathogen concentrations on development of RPW was assessed. Fourth instars were exposed to the sub-lethal dose of fungal entomopathogens (1 × 104 conidia ml−1) and H. bacteriophora (50 IJs ml−1). The sub-lethal dosages were determined through preliminary experimentation (unpublished data). The larvae were fed on artificial diet and transferred to the treatment cups. Dry coir was provided to the each larva before pupation for cocoon formation. Adult insects, upon emergence, were offered shredded sugarcane pieces. Developmental parameters of each stage were recorded including larval duration, larval weight, pre-pupal duration, pre-pupal weight, pupal duration, pupal weight, adult longevity (male and female) and adult weight (male and female).
Effects of entomopathogens on larval development
An additional assessment was made on the impact of sub-lethal concentrations on the development of last instar RPW; this experiment only involved B. bassiana and H. bacteriophora (because these pathogens were found to be the most compatible in terms of synergistic interactions, see Results section). Last instar RPW were exposed to sub-lethal doses of B. bassiana (1 × 104 conidia ml−1) and H. bacteriophora (50 IJs ml−1). Before exposure in all the treatments larvae were weighed first and transferred to the rearing cups with artificial diet. Larvae continued to feed until they pupated; the insects were maintained under experimental conditions at 25 ± 2 °C, 65 ± 5% RH and a (12: 12) L: D hour photoperiod. Every day until the larvae pupated in the control (14 d), larvae were changed to a new clean cup and a new piece of artificial diet was offered. Frass produced during this period was separated from vials using a fine camel-hair brush, and weighed. Diet left unused in each vial was recovered, oven dried at 80 °C, and weighed. Prior to the assay, diet in fifteen vials was dried to obtain an estimate of the dry weight. Diet consumption of each larva was thus determined by subtracting the mass after feeding from before feeding estimate. Cumulative weight gain of larvae was also determined. Three replicates of ten insects were used for each treatment and same count of larvae fed on normal diet served as untreated check. The entire experiment was repeated thrice.
Statistical analysis
The fungus-nematode interactions (synergistic, additive or antagonistic) were based on a comparison of observed versus expected values of insect mortality59. Expected mortality was calculated using formula P E = P 0 + (1 − P 0) (P 1) + (1 − P 0) (1 − P 1) (P 2), where P E is the expected mortality of the combination, P 0 is the control mortality, P 1 is the mortality from one pathogen treatment applied alone, and P 2 is the mortality from the other pathogen applied alone. A X 2 test was applied to the observed and expected results: X 2 = (L 0 − L E)2/L E + (D 0 − D E)2/D E, where L 0 is the number of living larvae observed, L E the number of living larvae expected, D 0 the number of dead larvae observed, and D E the number of dead larvae expected. Interactions were additive if X 2 < 3.84, antagonistic if X 2 > 3.84 and P C < P E, and synergistic if X 2 > 3.84 and P C > P E, where P C is the observed mortality from the combination and P E is the expected mortality from the combination. Data for pupation, adult emergence, egg eclosion and developmental parameters were subjected to one way analysis of variance (ANOVA) in Minitab65; means were separated using Tukey’s Kramer test (HSD)66 at a 5% significance level. To inspect the impact of microbial agents on the diet consumption, cumulative weight gain and frass production, data were analyzed by repeated measures (Proc Glimmix); based on residual plots data that were log transformed (back-transformed means are presented in the associated figures).
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Acknowledgements
This study was supported through grant (2AV1-263) by Higher Education Commission (HEC), Islamabad, Pakistan. We thank Kim Mai and Debbie Boykin (USDA) for assistance with statistical analysis.
Author Contributions
W.W. conceived and designed research, M.Y. and W.W. conducted experiments. M.Y., D.S.I. and W.W. analyzed data. M.Y. and W.W. wrote, W.W. and D.S.I. edited and finalized the manuscript. All authors read and approved the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Waqas Wakil, Email: waqaswakeel@hotmail.com.
David Shapiro-Ilan, Email: david.shapiro@ars.usda.gov.
References
- 1.Wakil, W., Faleiro, J. R. & Miller, T. A. Sustainable Pest Management in Date Palm: Current Status and Emerging Challenges, Sustainability in Plant and Crop Protection. Springer International Publishing Switzerland. p. 445 (2015).
- 2.Dembilio, Ó. & Jaques, J. A. Biology and management of red palm weevil. In: Wakil, W., Faleiro, J. R. & Miller, T. A. (Eds), Sustainable Pest Management in Date Palm: Current Status and Emerging Challenges, Sustainability in Plant and Crop Protection. Springer International Publishing Switzerland. pp. 13–36 (2015).
- 3.Khamiss, O. & Abdel Badeea, A. Initiation, characterization and karyotyping of a new cell line from red palm weevil Rhynchophorus ferrugineus adapted at 27 °C. AFPP - Palm Pest Mediterranean Conference, 16–18 January 2013, Nice, France. (2013).
- 4.Nirula KK. Investigations on the pests of coconut palm. Part 4. Rhynchophorus ferrugineus. Ind. Coc. J. 1956;9:229–247. [Google Scholar]
- 5.Abraham VA, et al. An integrated management approach for red palm weevil Rhynchophorus ferrugineus Oliv a key pest of date palm in the Middle East. Proc. Inter. Conf. on Integrated Pest Management (Muscat, Sultanate of Oman). Sultan Qaboos Uni. J. Sci. Res. 1998;3:77–83. [Google Scholar]
- 6.Ju RT, Wang F, Wan FH, Li B. Effect of host plants on development and reproduction of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) J. Pest Sci. 2011;84:33–39. doi: 10.1007/s10340-010-0323-4. [DOI] [Google Scholar]
- 7.Kurian C, Mathen K. Red palm weevil hidden enemy of coconut palm. Ind. Farmi. 1971;21:29–31. [Google Scholar]
- 8.Avand-Faghih A. The biology of red palm weevil, Rhynchophorus ferrugineus Oliv. (Coleoptera, Curculionidae) in Saravan region (Sistan & Balouchistan Province, Iran) Appl. Entomol. Phytopathol. 1996;63:16–18. [Google Scholar]
- 9.Rajamanickam K, Kennedy JS, Christopher A. Certain components of integrated management for red palm weevil, Rhynchohphorus ferrugineus F. (Curculionidae: Coleoptera) on coconut. Mededelingen Faculteit Landbouwkundige en Toegepaste Biologische Wetenschappen. 1995;60:803–805. [Google Scholar]
- 10.Hussain A, Haq MRU, Al-Jabr AM, Al-Ayied HY. Managing invasive populations of red palm weevil: A worldwide perspective. J. Food Agric. Environ. 2013;11:456–463. [Google Scholar]
- 11.Khan S, et al. Entomopathogenic fungi as microbial biocontrol agent. Mol. Plant Breed. 2012;3:63–79. [Google Scholar]
- 12.Charnley, A. & Collins, S. A. Entomopathogenic fungi and their role in pest control. In: Howard, D. H. & Miller J. D. (Eds), The Mycota IV: Environmental and Microbial Relationships, Springer-Verlag, Berlin, Heidelberg, Germany. pp. 159–187 (2007).
- 13.de Faria MR, Wraight SP. Mycoinsecticides and Mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control. 2007;43:237–256. doi: 10.1016/j.biocontrol.2007.08.001. [DOI] [Google Scholar]
- 14.Deadman, M. L., Azam, K. M., Ravzi, S. A. & Kaakeh, W. Preliminary investigation into the biological control of the red palm weevil using Beauveria bassiana. Proceedings of the Second International Conference on Date Palm, March 25–27, Al-Ain, UAE. 225–232 (2001).
- 15.Gindin G, Levski S, Glazer I, Soroker V. Evaluation of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana against the red palm weevil Rhynchophorus ferrugineus. Phytoparasitica. 2006;34:370–379. doi: 10.1007/BF02981024. [DOI] [Google Scholar]
- 16.El-Sufty R, et al. Biological control of red palm weevil, Rhynchophorus ferrugineus (Col.: Curculionidae) by the entomopathogenic fungus Beauveria bassiana in United Arab Emirates. Proceedings of the 3rd International Conference on Date Palm. Acta Hortic. 2007;736:399–404. doi: 10.17660/ActaHortic.2007.736.36. [DOI] [Google Scholar]
- 17.El-Sufty R, et al. Pathogenicity of the fungus Beauveria bassiana (Bals.) Vuill to the red palm weevil, Rhynchophorus ferrugineus (Oliv.) (Col.: Curculionidae) under laboratory and field conditions. Egypt. J. Biol. Pest Cont. 2009;19:81–85. [Google Scholar]
- 18.El-Sufty R, et al. A trap for auto-dissemination of the entomopathogenic fungus Beauveria bassiana by the red palm weevil adults in date palms plantations. Egypt. J. Biol. Pest Cont. 2011;21:271–276. [Google Scholar]
- 19.Sewify GH, Belal MH, Al-Awash SA. Use of the entomopathogenic fungus, Beauveria bassiana for the biological control of the red palm weevil, Rhynchophorus ferrugineus Olivier. Egypt J. Biol. Pest Cont. 2009;19:157–163. [Google Scholar]
- 20.Torta L, et al. Microrganismi fungini associati a Rhynchophorus ferrugineus (Olivier) in Sicilia e valutazione dell’efficacia entomopatogena di 484. Ann. Microbiol. 2009;65:477–485. [Google Scholar]
- 21.Vitale AV, Leone L, Burruano TS, Polizzi G. Prove preliminari di lotta biologica con Beauveria bassiana e Metarhizium anisopliae nei confronti del punteruolo rosso. In Regione Siciliana - Assessorato Agricoltura e Foreste. La ricerca scientifica sul Punteruolo rosso e gli altri fitofagi delle palme in Sicilia, Palermo, Italy. 2009;1:169–172. [Google Scholar]
- 22.Güerri-Agulló B, et al. Infection of the red palm weevil (Rhynchophorus ferrugineus) by the entomopathogenic fungus Beauveria bassiana: a SEM study. Microsco. Res. Tech. 2010;73:714–725. doi: 10.1002/jemt.20812. [DOI] [PubMed] [Google Scholar]
- 23.Merghem A. Susceptibility of the red palm weevil, Rhynchophorus ferrugineus (Olivier) to the green muscardine fungus, Metarhizium anisopliae (Metsch.) in the laboratory and in palm tree orchards. Egypt. J. Biol. Pest Cont. 2011;21:179–183. [Google Scholar]
- 24.Francardi V, et al. Entomopathogenicity of Beauveria bassiana (Bals.) Vuill. and Metarhizium anisopliae (Metsch.) Sorokin isolated from different sources in the control of Rhynchophorus ferrugineus (Olivier) (Coleoptera Curculionidae) Redia. 2012;95:49–55. [Google Scholar]
- 25.Ricaño J, et al. Evaluation of the pathogenicity of multiple isolates of Beauveria bassiana (Hypocreales: Clavicipitaceae) on Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) for the assessment of a solid formulation under simulated field conditions. Florida Entomol. 2013;96:1311–1324. doi: 10.1653/024.096.0410. [DOI] [Google Scholar]
- 26.Cito A, et al. Roversi. Characterization and comparison of Metarhizium strains isolated from Rhynchophorus ferrugineus. FEMS Microbiol. Lett. 2014;355:108–115. doi: 10.1111/1574-6968.12470. [DOI] [PubMed] [Google Scholar]
- 27.Quesada-Moraga E, Santos-Quiros R, Valverde-Garcia P, Santiago-Alvarez C. Virulence, horizontal transmission, and sublethal reproductive effects of Metarhizium anisopliae (Anamorphic fungi) on the German cockroach (Blattodea: Blattellidae) J. Invert. Pathol. 2004;87:51–58. doi: 10.1016/j.jip.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Llácer E, Martínez de Altube MM, Jacas JA. Evaluation of the efficacy of Steinernema carpocapsae in a chitosan formulation against the red palm weevil, Rhynchophorus ferrugineus, in Phoenix canariensis. BioCont. 2009;54:559–565. doi: 10.1007/s10526-008-9208-3. [DOI] [PubMed] [Google Scholar]
- 29.Dembilio Ó, Llácer E, Martínez de Altube MM, Jacas JA. Field efficacy of imidacloprid and Steinernema carpocapsae in a chitosan formulation against the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Curculionidae) in Phoenix canariensis. Pest Manag. Sci. 2010;66:365–370. doi: 10.1002/ps.1882. [DOI] [PubMed] [Google Scholar]
- 30.Lacey LA, et al. Insect pathogens as biological control agents: back to the future. J. Invert. Pathol. 2015;132:1–41. doi: 10.1016/j.jip.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro-Ilan, H, R. & Qiu, X. Production of entomopathogenic nematodes. In: Morales-Ramos, J. A., Rojas, M. G. & Shapiro-Ilan, D. I. (Eds). Mass Production of Beneficial Organisms: Invertebrates and Entomopathogens. Academic Press, San Diego, California, USA. pp. 321–356 (2014).
- 32.Shapiro-Ilan, D. I., Hazir, S. & Glazer, I. Basic and Applied Research: Entomopathogenic Nematodes. In: Lacey, L. A. (Ed), Microbial Agents for Control of Insect Pests: From Theory to Practice. Academic Press, San Diego, California, USA. pp. 91–105 (2017).
- 33.Poinar, G. O. Jr. Taxonomy and biology of Steinernematidae and Heterorhabditidae. In: Gaugler, R. & Kaya H. K. (Eds), Entomopathogenic Nematodes in Biological Control. CRC Press, Boca Raton, Florida, USA. pp. 23-61 (1990).
- 34.Thurston GS, Kaya HK, Burlando TM, Harrison RE. Milky disease bacteria as a stressor to increase susceptibility of scarabaeid larvae to an entomopathogenic nematode. J. Invert. Pathol. 1993;61:167–172. doi: 10.1006/jipa.1993.1030. [DOI] [Google Scholar]
- 35.Thurston GS, Kaya HK, Gaugler R. Characterization of enhanced susceptibility of milky disease infected scarabaeid grubs to entomopathogenic nematodes. Biol. Control. 1994;4:67–73. doi: 10.1006/bcon.1994.1012. [DOI] [Google Scholar]
- 36.Koppenhöfer AM, Kaya HK. Additive and Synergistic interactions between entomopathogenic nematodes and Bacillus thuringiensis for scarab grub control. Biol. control. 1997;8:131–137. doi: 10.1006/bcon.1996.0498. [DOI] [Google Scholar]
- 37.Koppenhöfer AM, et al. Increased field and greenhouse efficacy against scarab grubs with a combination of an entomopathogenic nematode and Bacillus thuringiensis. Biol. Control. 1999;14:37–44. doi: 10.1006/bcon.1998.0663. [DOI] [Google Scholar]
- 38.Abbas MST, Hanonik SP. Pathogenicity of entomopathogenic nematodes to red palm weevil, Rynchophorus ferrugineus. Int. J. Nematol. 1999;9:84–86. [Google Scholar]
- 39.Salama HS, Abd-Elgawad MM. Isolation of heterorhabditid nematodes from palm tree planted areas and their implications in the red palm weevil control. Anzeiger für Schädlingskunde. 2001;74:43–45. doi: 10.1111/j.1493-0280.2001.01010.x. [DOI] [Google Scholar]
- 40.Monzer MA. Response of Heterorhabditis indica infective juveniles to host diffusates in a modified laboratory bioassay. Egypt. J. Biol. Pest Cont. 2004;14:309–313. [Google Scholar]
- 41.Elawad SA, et al. Efficacy of entomopathogenic nematodes against red palm weevil in UAE. Acta Hortic. 2007;736:415–420. doi: 10.17660/ActaHortic.2007.736.38. [DOI] [Google Scholar]
- 42.Chapin E, André N. Nouveau moyen de lutte biologique contre le papillon palmivore. Phytoma La Défense des Végétaux. 2010;635:27–30. [Google Scholar]
- 43.Pérez L, et al. Palmier, efficacité curative du nématode Steinernema carpocapsae contre le papillon palmivore Paysandisia archon: résultats d’essais conduits dans des jardins et espaces verts. Phytoma La Défense des Végétaux. 2010;637:14–17. [Google Scholar]
- 44.Triggiani O, Tarasco E. Evaluation of the autochthonous and commercial isolates of Steinernematidae and Heterorhabditidae on Rhynchophorus ferrugineus. Bull. Insectol. 2011;64:175–180. [Google Scholar]
- 45.Manachini B, Schillaci D, Arizza V. Biological responses of Rhynchophorus ferrugineus (Coleoptera: Curculionidae) to Steinernema carpocapsae (Nematoda: Steinernematidae) J. Econ. Entomol. 2013;106:1582–1589. doi: 10.1603/EC13031. [DOI] [PubMed] [Google Scholar]
- 46.Atwa AA, Hegazi EM. Comparative susceptibilities of different life stages of the red palm weevil (Coleoptera: Curculionidae) treated by entomopathogenic nematodes. J. Econ. Entomol. 2014;107:1339–1347. doi: 10.1603/EC13438. [DOI] [PubMed] [Google Scholar]
- 47.Santhi VS, et al. Attraction of entomopathogenic nematodes Steinernema carpocapsae and Heterorhabditis bacteriophora to the red palm weevil (Rhynchophorus ferrugineus) Biol. Control. 2015;83:75–81. doi: 10.1016/j.biocontrol.2015.01.003. [DOI] [Google Scholar]
- 48.Ansari MA, Vestergaard S, Tirry L, Moens M. Selection of a highly virulent fungal isolate, Metarhizium anisopliae CLO 53, for controlling Hoplia philanthus. J. Invert. Pathol. 2004;85:89–96. doi: 10.1016/j.jip.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Ansari MA, Shah FA, Tirry L, Moens M. Field trials against Hoplia philanthus (Coleoptera: Scarabaeidae) with a combination of an entomopathogenic nematode and the fungus Metarhizium anisopliae CLO 53. Biol. Control. 2006;39:453–459. doi: 10.1016/j.biocontrol.2006.07.004. [DOI] [Google Scholar]
- 50.Anbesse SA, Adge BJ, Gebru WM. Laboratory screening for virulent entomopathogenic nematodes (Heterorhabditis bacteriophora and Steinernema yirgalemense) and fungi (Metarhizium anisopliae and Beauveria bassiana) and assessment of possible synergistic effects of combined use against grubs of the barley chafer Coptognathus curtipennis. Nematol. 2008;10:701–709. doi: 10.1163/156854108785787217. [DOI] [Google Scholar]
- 51.Ansari MA, Adhikari N, Ali F, Moens M. Susceptibility of Hoplia philanthus (Coleptera: Scarabaeidae) larvae and pupae to entomopathogenic nematodes (Rhabditida: Steinernematidae, Heterorhabditidae) Biol. Control. 2008;47:315–321. doi: 10.1016/j.biocontrol.2008.08.021. [DOI] [Google Scholar]
- 52.Ansari MA, Shah FA, Butt TM. The entomopathogenic nematode Steinernema kraussei and Metarhizium anisopliae work synergistically in controlling overwintering larvae of the black vine weevil, Otiorhynchus sulcatus, in strawberry growbags. Biocon. Sci. Technol. 2009;20:99–105. doi: 10.1080/09583150903420031. [DOI] [Google Scholar]
- 53.Steinhaus EA. Stress as a factor in insect disease. Proceedings of the Xth Inter. Cong. Entomology. 1958;4:725–730. [Google Scholar]
- 54.Samuels RI, Charnley AK, Reynolds SE. 1988. The role of destruxins in the pathogenicity of 3 strains of Metarhizium anisopliae for the tobacco hornworm Manduca sexta. Mycopathol. 1988;104:51–58. doi: 10.1007/BF00437924. [DOI] [Google Scholar]
- 55.Gaugler R, Wang Y, Campbell JF. Aggressive and evasive behaviors in Popillia japonica (Coleoptera: Scarabaeidae) larvae: Defenses against entomopathogenic nematode attack. J. Invert. Pathol. 1994;64:193–199. doi: 10.1016/S0022-2011(94)90150-3. [DOI] [Google Scholar]
- 56.Kermarrec A, Mauleon H. Synergy between chlordecone and Neoaplectana carpocapsae Weiser (Nematoda: Steinernematidae) in the control of Cosmopolites sordidus (Coleoptera: Curculionidae) Rev. Nematol. 1989;12:324–325. [Google Scholar]
- 57.Barbercheck ME, Kaya HK. Interactions between Beauveria bassiana and the entomogenous nematodes Steinernema feltiae and Heterorhabditis heliothidis. J. Invert. Pathol. 1990;55:225–234. doi: 10.1016/0022-2011(90)90058-E. [DOI] [Google Scholar]
- 58.Wu S, Youngman RR, Kok LT, Laub CA, Pfeiffer DG. Interaction between entomopathogenic nematodes and entomopathogenic fungi applied to third instar southern masked chafer white grubs, Cyclocephala lurida (Coleoptera: Scarabaeidae), under laboratory and greenhouse conditions. Biol. Control. 2014;76:65–73. doi: 10.1016/j.biocontrol.2014.05.002. [DOI] [Google Scholar]
- 59.Shapiro-Ilan DI, Jackson M, Reilly CC, Hotchkiss MW. Effects of combining an entomopathogenic fungi or bacterium with entomopathogenic nematodes on mortality of Curculio caryae (Coleoptera: Curculionidae) Biol. Control. 2004;30:119–126. doi: 10.1016/j.biocontrol.2003.09.014. [DOI] [Google Scholar]
- 60.Marzban R, He Q, Liu X, Zhang Q. Effects of Bacillus thuringiensis toxin Cry1Ac and cytoplasmic polyhedrosis virus of Helicoverpa armigera (Hübner) (HaCPV) on cotton bollworm (Lepidoptera: Noctuidae) J. Invert. Pathol. 2009;101:71–76. doi: 10.1016/j.jip.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 61.Kaya, H. K. & Stock, S. P. Techniques in insect nematology. In: Lacey, L. A. (Ed), Manual of Techniques in Insect Pathology. Academic Press, London, UK. p. 281 (1997).
- 62.Goettel, S. & Inglis, G. D. Fungi: Hyphomycetes. In: Lacey L. A. (Ed), Manual of Techniques in Insect Pathology. Academic Press, London, UK. pp. 213–249 (1997).
- 63.Dembilio Ó, Quesada-Moraga E, Santiago-Alvarez C, Jacas JA. Potential of an indigenous strain of the entomopathogenic fungus Beauveria bassiana as a biological control agent against the red palm weevil, Rhynchophorus ferrugineus. J. Invert. Pathol. 2010;104:214–221. doi: 10.1016/j.jip.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Martín MM, Cabello T. Manejo de la cría del picudo rojo de la palmera, Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera, Dryophthoridae), en dieta artificial y efectos en su biometría y biología. Boletín de Sanidad Vegetal de Plagas. 2006;32:631–641. [Google Scholar]
- 65.Minitab, MINITAB Release 14 for Windows. Minitab Inc., State College, Pennsylvania, USA (2003).
- 66.Sokal, R. R. & Rohlf, F. J. Biometry. Freeman, New York, USA (1995).


