Abstract
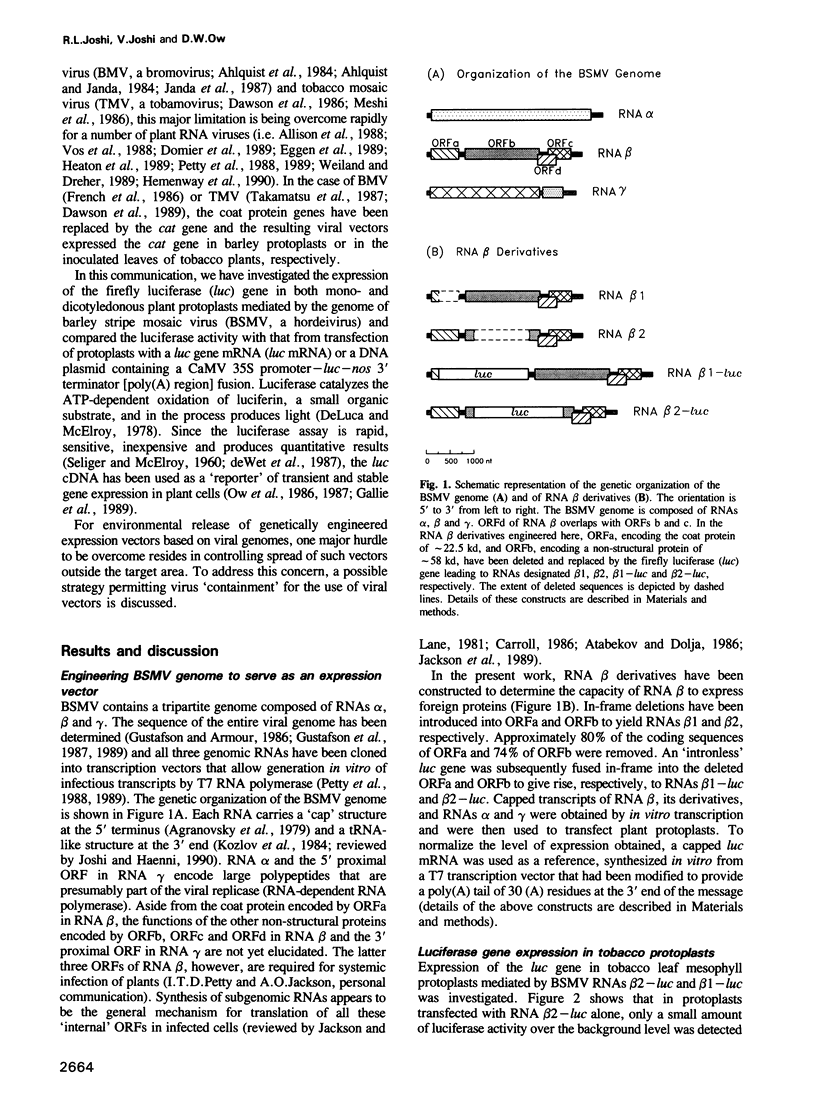
Barley stripe mosaic virus (BSMV) possesses a tripartite genome composed of RNAs alpha, beta and gamma that have been cloned into in vitro transcription vectors from which infectious transcripts can be obtained. The BSMV genome has been engineered here to serve as an expression vector in plant protoplasts. Open reading frame (ORF) b of RNA beta, encoding a non-structural protein, was replaced by the firefly luciferase (luc) reporter gene to yield RNA beta 2-luc. In the presence of both RNAs alpha and gamma, RNA beta 2-luc mediated efficient expression of the luc gene upon transfection into tobacco and maize protoplasts. This expression ranged from 20- to 123-fold higher than the luciferase activity obtained from transfection with a luc gene mRNA. Replication of RNA beta and its derivatives i.e. 'minus' strand synthesis, was confirmed by Northern analysis, indicating that the high level of luc gene expression using RNA beta 2-luc resulted from RNA amplification. ORFa of RNA beta, encoding the coat protein, was also replaced by the luc gene to yield RNA beta 1-luc. Although transfection of RNA beta 1-luc alone produces luciferase efficiently, neither 'minus' strand synthesis nor further increase of luciferase activity was observed in the presence of RNAs alpha and gamma, or RNAs alpha, beta and gamma, suggesting that the deleted sequences within ORFa are cis-acting for replication of RNA beta. Our results demonstrate that a foreign gene can be expressed by replacement of a viral non-structural protein gene that is essential for virus multiplication in plants, leading to a potential strategy for virus 'containment' with use of 'disarmed' plant viral vectors.
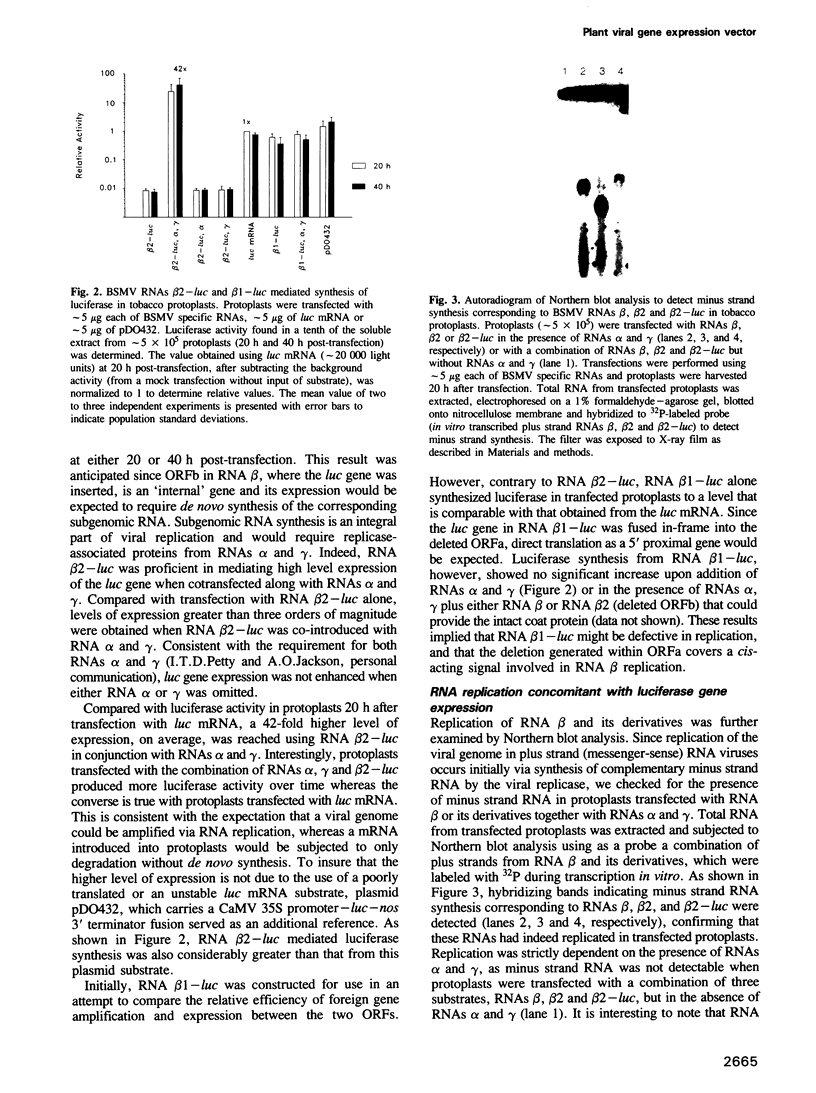
Full text
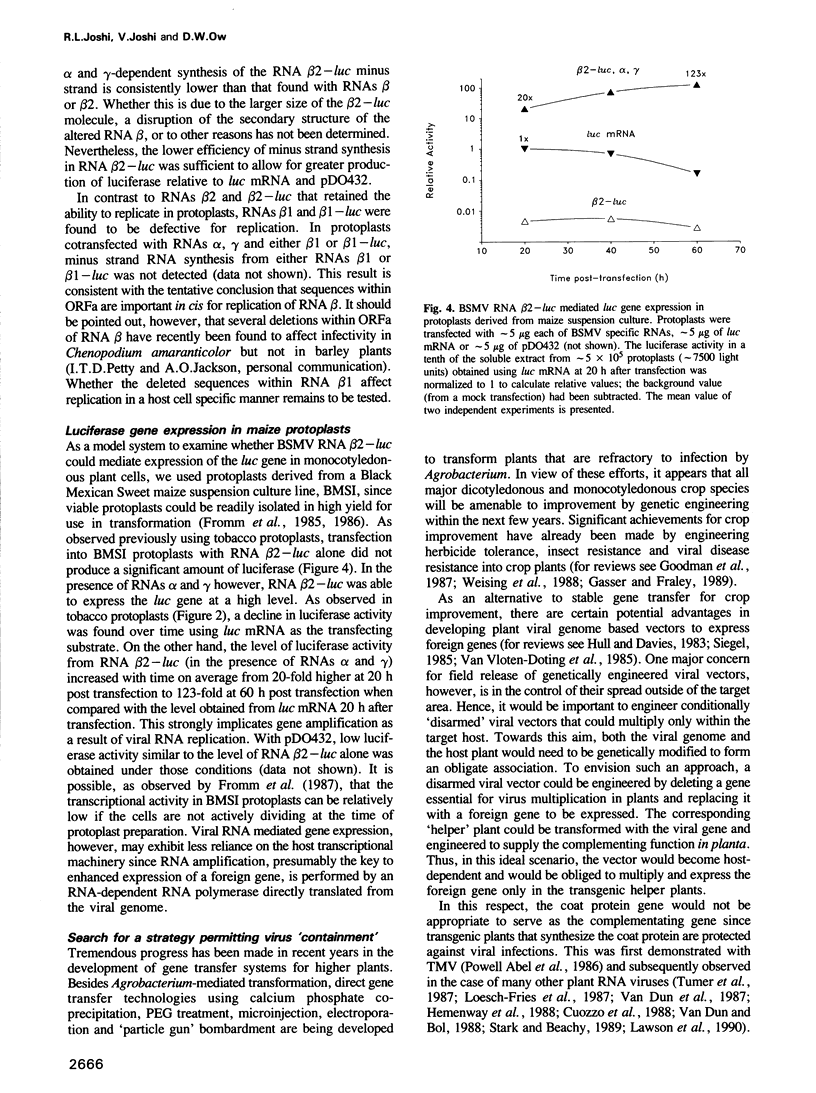
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel P. P., Nelson R. S., De B., Hoffmann N., Rogers S. G., Fraley R. T., Beachy R. N. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science. 1986 May 9;232(4751):738–743. doi: 10.1126/science.3457472. [DOI] [PubMed] [Google Scholar]
- Ahlquist P., French R., Janda M., Loesch-Fries L. S. Multicomponent RNA plant virus infection derived from cloned viral cDNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7066–7070. doi: 10.1073/pnas.81.22.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlquist P., Janda M. cDNA cloning and in vitro transcription of the complete brome mosaic virus genome. Mol Cell Biol. 1984 Dec;4(12):2876–2882. doi: 10.1128/mcb.4.12.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison R. F., Janda M., Ahlquist P. Infectious in vitro transcripts from cowpea chlorotic mottle virus cDNA clones and exchange of individual RNA components with brome mosaic virus. J Virol. 1988 Oct;62(10):3581–3588. doi: 10.1128/jvi.62.10.3581-3588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison R., Thompson C., Ahlquist P. Regeneration of a functional RNA virus genome by recombination between deletion mutants and requirement for cowpea chlorotic mottle virus 3a and coat genes for systemic infection. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1820–1824. doi: 10.1073/pnas.87.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atabekov J. G., Morozov S. Y. Translation of plant virus messenger RNAs. Adv Virus Res. 1979;25:1–91. doi: 10.1016/s0065-3527(08)60568-0. [DOI] [PubMed] [Google Scholar]
- Bujarski J. J., Kaesberg P. Genetic recombination between RNA components of a multipartite plant virus. 1986 May 29-Jun 4Nature. 321(6069):528–531. doi: 10.1038/321528a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dawson W. O., Beck D. L., Knorr D. A., Grantham G. L. cDNA cloning of the complete genome of tobacco mosaic virus and production of infectious transcripts. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1832–1836. doi: 10.1073/pnas.83.6.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson W. O., Lewandowski D. J., Hilf M. E., Bubrick P., Raffo A. J., Shaw J. J., Grantham G. L., Desjardins P. R. A tobacco mosaic virus-hybrid expresses and loses an added gene. Virology. 1989 Sep;172(1):285–292. doi: 10.1016/0042-6822(89)90130-x. [DOI] [PubMed] [Google Scholar]
- Deom C. M., Oliver M. J., Beachy R. N. The 30-kilodalton gene product of tobacco mosaic virus potentiates virus movement. Science. 1987 Jul 24;237(4813):389–394. doi: 10.1126/science.237.4813.389. [DOI] [PubMed] [Google Scholar]
- Domier L. L., Franklin K. M., Hunt A. G., Rhoads R. E., Shaw J. G. Infectious in vitro transcripts from cloned cDNA of a potyvirus, tobacco vein mottling virus. Proc Natl Acad Sci U S A. 1989 May;86(10):3509–3513. doi: 10.1073/pnas.86.10.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggen R., Verver J., Wellink J., De Jong A., Goldbach R., van Kammen A. Improvements of the infectivity of in vitro transcripts from cloned cowpea mosaic virus cDNA: impact of terminal nucleotide sequences. Virology. 1989 Dec;173(2):447–455. doi: 10.1016/0042-6822(89)90557-6. [DOI] [PubMed] [Google Scholar]
- French R., Janda M., Ahlquist P. Bacterial gene inserted in an engineered RNA virus: efficient expression in monocotyledonous plant cells. Science. 1986 Mar 14;231(4743):1294–1297. doi: 10.1126/science.231.4743.1294. [DOI] [PubMed] [Google Scholar]
- Fromm M. E., Taylor L. P., Walbot V. Stable transformation of maize after gene transfer by electroporation. 1986 Feb 27-Mar 5Nature. 319(6056):791–793. doi: 10.1038/319791a0. [DOI] [PubMed] [Google Scholar]
- Fromm M., Taylor L. P., Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5824–5828. doi: 10.1073/pnas.82.17.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D. R., Lucas W. J., Walbot V. Visualizing mRNA expression in plant protoplasts: factors influencing efficient mRNA uptake and translation. Plant Cell. 1989 Mar;1(3):301–311. doi: 10.1105/tpc.1.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser C. S., Fraley R. T. Genetically engineering plants for crop improvement. Science. 1989 Jun 16;244(4910):1293–1299. doi: 10.1126/science.244.4910.1293. [DOI] [PubMed] [Google Scholar]
- Goodman R. M., Hauptli H., Crossway A., Knauf V. C. Gene transfer in crop improvement. Science. 1987 Apr 3;236(4797):48–54. doi: 10.1126/science.236.4797.48. [DOI] [PubMed] [Google Scholar]
- Grimsley N., Hohn B., Hohn T., Walden R. "Agroinfection," an alternative route for viral infection of plants by using the Ti plasmid. Proc Natl Acad Sci U S A. 1986 May;83(10):3282–3286. doi: 10.1073/pnas.83.10.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson G., Armour S. L., Gamboa G. C., Burgett S. G., Shepherd J. W. Nucleotide sequence of barley stripe mosaic virus RNA alpha: RNA alpha encodes a single polypeptide with homology to corresponding proteins from other viruses. Virology. 1989 Jun;170(2):370–377. doi: 10.1016/0042-6822(89)90427-3. [DOI] [PubMed] [Google Scholar]
- Gustafson G., Armour S. L. The complete nucleotide sequence of RNA beta from the type strain of barley stripe mosaic virus. Nucleic Acids Res. 1986 May 12;14(9):3895–3909. doi: 10.1093/nar/14.9.3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson G., Hunter B., Hanau R., Armour S. L., Jackson A. O. Nucleotide sequence and genetic organization of barley stripe mosaic virus RNA gamma. Virology. 1987 Jun;158(2):394–406. doi: 10.1016/0042-6822(87)90211-x. [DOI] [PubMed] [Google Scholar]
- Hanley-Bowdoin L., Elmer J. S., Rogers S. G. Transient expression of heterologous RNAs using tomato golden mosaic virus. Nucleic Acids Res. 1988 Nov 25;16(22):10511–10528. doi: 10.1093/nar/16.22.10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton L. A., Carrington J. C., Morris T. J. Turnip crinkle virus infection from RNA synthesized in vitro. Virology. 1989 May;170(1):214–218. doi: 10.1016/0042-6822(89)90368-1. [DOI] [PubMed] [Google Scholar]
- Hemenway C., Fang R. X., Kaniewski W. K., Chua N. H., Tumer N. E. Analysis of the mechanism of protection in transgenic plants expressing the potato virus X coat protein or its antisense RNA. EMBO J. 1988 May;7(5):1273–1280. doi: 10.1002/j.1460-2075.1988.tb02941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemenway C., Weiss J., O'Connell K., Tumer N. E. Characterization of infectious transcripts from a potato virus X cDNA clone. Virology. 1990 Apr;175(2):365–371. doi: 10.1016/0042-6822(90)90421-m. [DOI] [PubMed] [Google Scholar]
- Hull R., Davies J. W. Genetic engineering with plant viruses, and their potential as vectors. Adv Virus Res. 1983;28:1–33. doi: 10.1016/s0065-3527(08)60720-4. [DOI] [PubMed] [Google Scholar]
- Kozlov YuV, Rupasov V. V., Adyshev D. M., Belgelarskaya S. N., Agranovsky A. A., Mankin A. S., Morozov SYu, Dolja V. V., Atabekov J. G. Nucleotide sequence of the 3'-terminal tRNA-like structure in barley stripe mosaic virus genome. Nucleic Acids Res. 1984 May 11;12(9):4001–4009. doi: 10.1093/nar/12.9.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C., Kaniewski W., Haley L., Rozman R., Newell C., Sanders P., Tumer N. E. Engineering resistance to mixed virus infection in a commercial potato cultivar: resistance to potato virus X and potato virus Y in transgenic Russet Burbank. Biotechnology (N Y) 1990 Feb;8(2):127–134. doi: 10.1038/nbt0290-127. [DOI] [PubMed] [Google Scholar]
- Loesch-Fries L. S., Halk E. L., Nelson S. E., Krahn K. J. Human leukocyte interferon does not inhibit alfalfa mosaic virus in protoplasts or tobacco tissue. Virology. 1985 Jun;143(2):626–629. doi: 10.1016/0042-6822(85)90402-7. [DOI] [PubMed] [Google Scholar]
- Loesch-Fries L. S., Merlo D., Zinnen T., Burhop L., Hill K., Krahn K., Jarvis N., Nelson S., Halk E. Expression of alfalfa mosaic virus RNA 4 in transgenic plants confers virus resistance. EMBO J. 1987 Jul;6(7):1845–1851. doi: 10.1002/j.1460-2075.1987.tb02442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T., Ishikawa M., Motoyoshi F., Semba K., Okada Y. In vitro transcription of infectious RNAs from full-length cDNAs of tobacco mosaic virus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5043–5047. doi: 10.1073/pnas.83.14.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen D. A., Shapiro D. J. Preparation of capped RNA transcripts using T7 RNA polymerase. Nucleic Acids Res. 1986 Jul 25;14(14):5936–5936. doi: 10.1093/nar/14.14.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow D. W., DE Wet J. R., Helinski D. R., Howell S. H., Wood K. V., Deluca M. Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science. 1986 Nov 14;234(4778):856–859. doi: 10.1126/science.234.4778.856. [DOI] [PubMed] [Google Scholar]
- Ow D. W., Jacobs J. D., Howell S. H. Functional regions of the cauliflower mosaic virus 35S RNA promoter determined by use of the firefly luciferase gene as a reporter of promoter activity. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4870–4874. doi: 10.1073/pnas.84.14.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty I. T. A plasmid vector for cloning directly at the transcription initiation site of a bacteriophage T7 promoter. Nucleic Acids Res. 1988 Sep 12;16(17):8738–8738. doi: 10.1093/nar/16.17.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty I. T., Hunter B. G., Jackson A. O. A novel strategy for one-step cloning of full-length cDNA and its application to the genome of barley stripe mosaic virus. Gene. 1988 Dec 30;74(2):423–432. doi: 10.1016/0378-1119(88)90175-8. [DOI] [PubMed] [Google Scholar]
- Petty I. T., Hunter B. G., Wei N., Jackson A. O. Infectious barley stripe mosaic virus RNA transcribed in vitro from full-length genomic cDNA clones. Virology. 1989 Aug;171(2):342–349. doi: 10.1016/0042-6822(89)90601-6. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELIGER H. H., McELROY W. D. Spectral emission and quantum yield of firefly bioluminescence. Arch Biochem Biophys. 1960 May;88:136–141. doi: 10.1016/0003-9861(60)90208-3. [DOI] [PubMed] [Google Scholar]
- Stanley J. The molecular biology of geminiviruses. Adv Virus Res. 1985;30:139–177. doi: 10.1016/s0065-3527(08)60450-9. [DOI] [PubMed] [Google Scholar]
- Takamatsu N., Ishikawa M., Meshi T., Okada Y. Expression of bacterial chloramphenicol acetyltransferase gene in tobacco plants mediated by TMV-RNA. EMBO J. 1987 Feb;6(2):307–311. doi: 10.1002/j.1460-2075.1987.tb04755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumer N. E., O'connell K. M., Nelson R. S., Sanders P. R., Beachy R. N., Fraley R. T., Shah D. M. Expression of alfalfa mosaic virus coat protein gene confers cross-protection in transgenic tobacco and tomato plants. EMBO J. 1987 May;6(5):1181–1188. doi: 10.1002/j.1460-2075.1987.tb02352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos P., Jaegle M., Wellink J., Verver J., Eggen R., Van Kammen A., Goldbach R. Infectious RNA transcripts derived from full-length DNA copies of the genomic RNAs of cowpea mosaic virus. Virology. 1988 Jul;165(1):33–41. doi: 10.1016/0042-6822(88)90655-1. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Meinkoth J. L., Kimmel A. R. Northern and Southern blots. Methods Enzymol. 1987;152:572–581. doi: 10.1016/0076-6879(87)52064-x. [DOI] [PubMed] [Google Scholar]
- Ward A., Etessami P., Stanley J. Expression of a bacterial gene in plants mediated by infectious geminivirus DNA. EMBO J. 1988 Jun;7(6):1583–1587. doi: 10.1002/j.1460-2075.1988.tb02983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland J. J., Dreher T. W. Infectious TYMV RNA from cloned cDNA: effects in vitro and in vivo of point substitutions in the initiation codons of two extensively overlapping ORFs. Nucleic Acids Res. 1989 Jun 26;17(12):4675–4687. doi: 10.1093/nar/17.12.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weising K., Schell J., Kahl G. Foreign genes in plants: transfer, structure, expression, and applications. Annu Rev Genet. 1988;22:421–477. doi: 10.1146/annurev.ge.22.120188.002225. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dun C. M., Bol J. F. Transgenic tobacco plants accumulating tobacco rattle virus coat protein resist infection with tobacco rattle virus and pea early browning virus. Virology. 1988 Dec;167(2):649–652. doi: 10.1016/s0042-6822(88)90131-6. [DOI] [PubMed] [Google Scholar]