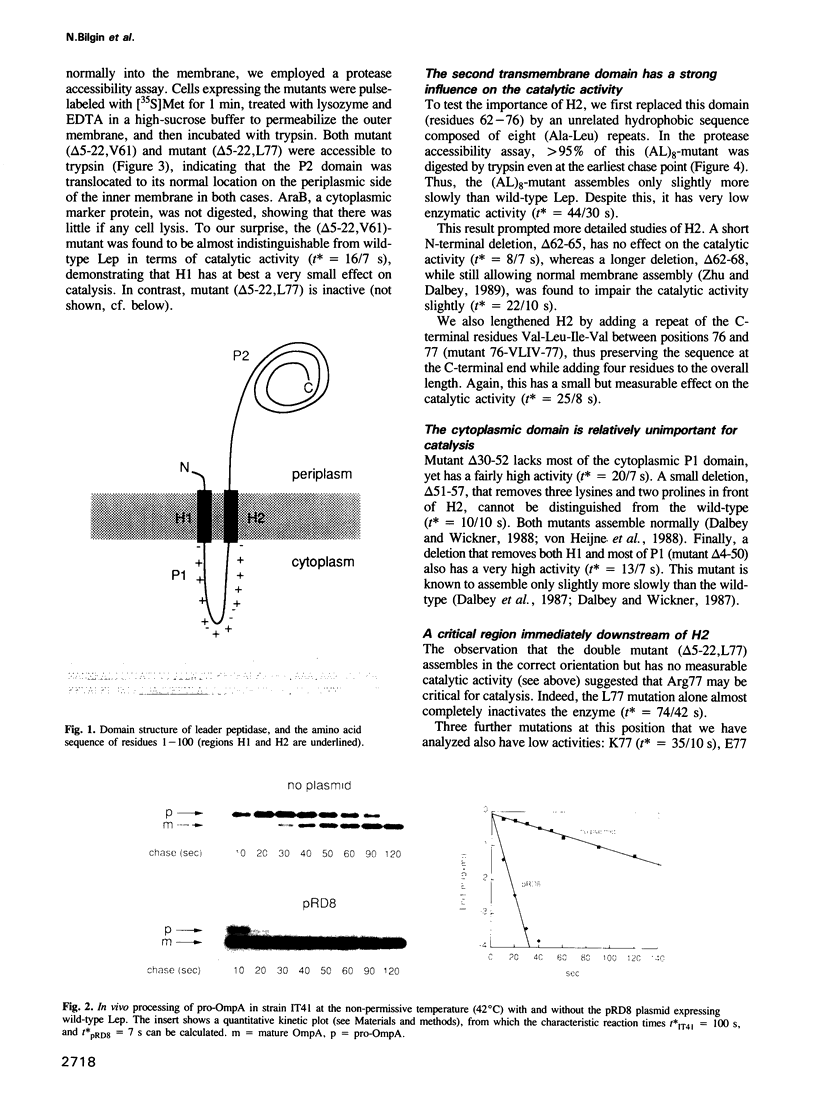
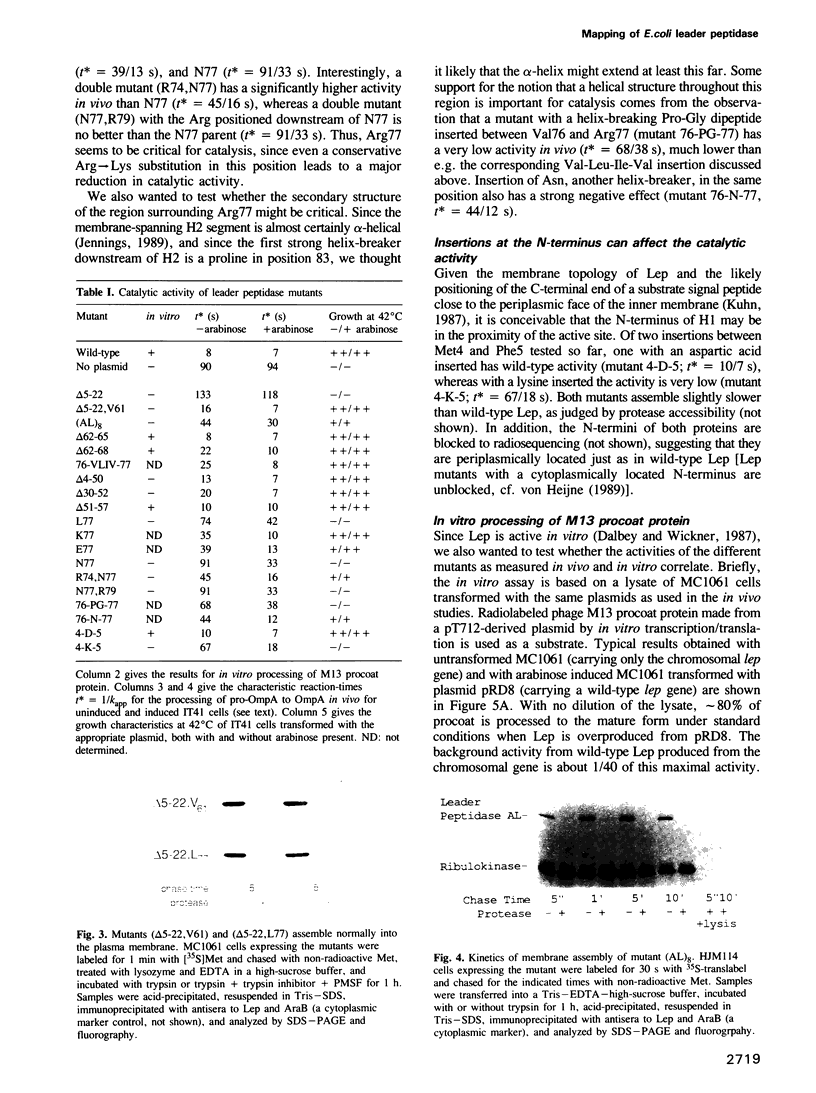
Abstract
Leader peptidase (Lep) is a central component of the secretory machinery of Escherichia coli, where it serves to remove signal peptides from secretory proteins. It spans the inner membrane twice with a large C-terminal domain protruding into the periplasmic space. To investigate the importance of the different structural domains for the catalytic activity, we have studied the effects of a large panel of Lep mutants on the processing of signal peptides, both in vivo and in vitro. Our data suggest that the first transmembrane and cytoplasmic regions are not directly involved in catalysis, but that the second transmembrane region and the region immediately following it may be in contact with the signal peptide and/or located spatially close to the active site of Lep.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boeke J. D., Russel M., Model P. Processing of filamentous phage pre-coat protein. Effect of sequence variations near the signal peptidase cleavage site. J Mol Biol. 1980 Dec 5;144(2):103–116. doi: 10.1016/0022-2836(80)90027-3. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Dalbey R. E., Kuhn A., Wickner W. The internal signal sequence of Escherichia coli leader peptidase is necessary, but not sufficient, for its rapid membrane assembly. J Biol Chem. 1987 Sep 25;262(27):13241–13245. [PubMed] [Google Scholar]
- Dalbey R. E., Wickner W. Characterization of the internal signal-anchor domain of Escherichia coli leader peptidase. J Biol Chem. 1988 Jan 5;263(1):404–408. [PubMed] [Google Scholar]
- Dalbey R. E., Wickner W. Leader peptidase catalyzes the release of exported proteins from the outer surface of the Escherichia coli plasma membrane. J Biol Chem. 1985 Dec 15;260(29):15925–15931. [PubMed] [Google Scholar]
- Dalbey R. E., Wickner W. Leader peptidase of Escherichia coli: critical role of a small domain in membrane assembly. Science. 1987 Feb 13;235(4790):783–787. doi: 10.1126/science.3544218. [DOI] [PubMed] [Google Scholar]
- Dalbey R. E., Wickner W. The role of the polar, carboxyl-terminal domain of Escherichia coli leader peptidase in its translocation across the plasma membrane. J Biol Chem. 1986 Oct 15;261(29):13844–13849. [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Dierstein R., Wickner W. Requirements for substrate recognition by bacterial leader peptidase. EMBO J. 1986 Feb;5(2):427–431. doi: 10.1002/j.1460-2075.1986.tb04228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T., Court D. L., Ito K., Nakamura Y. Conditionally lethal amber mutations in the leader peptidase gene of Escherichia coli. J Bacteriol. 1989 Jan;171(1):585–587. doi: 10.1128/jb.171.1.585-587.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings M. L. Topography of membrane proteins. Annu Rev Biochem. 1989;58:999–1027. doi: 10.1146/annurev.bi.58.070189.005031. [DOI] [PubMed] [Google Scholar]
- Johnston S., Lee J. H., Ray D. S. High-level expression of M13 gene II protein from an inducible polycistronic messenger RNA. Gene. 1985;34(2-3):137–145. doi: 10.1016/0378-1119(85)90121-0. [DOI] [PubMed] [Google Scholar]
- Kuhn A. Bacteriophage M13 procoat protein inserts into the plasma membrane as a loop structure. Science. 1987 Dec 4;238(4832):1413–1415. doi: 10.1126/science.3317833. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws J. K., Dalbey R. E. Positive charges in the cytoplasmic domain of Escherichia coli leader peptidase prevent an apolar domain from functioning as a signal. EMBO J. 1989 Jul;8(7):2095–2099. doi: 10.1002/j.1460-2075.1989.tb03618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. E., Miura S. A small hydrophobic domain anchors leader peptidase to the cytoplasmic membrane of Escherichia coli. J Biol Chem. 1987 Jun 25;262(18):8806–8813. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. Mechanisms of membrane assembly: general lessons from the study of M13 coat protein and Escherichia coli leader peptidase. Biochemistry. 1988 Feb 23;27(4):1081–1086. doi: 10.1021/bi00404a001. [DOI] [PubMed] [Google Scholar]
- Wolfe P. B., Silver P., Wickner W. The isolation of homogeneous leader peptidase from a strain of Escherichia coli which overproduces the enzyme. J Biol Chem. 1982 Jul 10;257(13):7898–7902. [PubMed] [Google Scholar]
- Wolfe P. B., Zwizinski C., Wickner W. Purification and characterization of leader peptidase from Escherichia coli. Methods Enzymol. 1983;97:40–46. doi: 10.1016/0076-6879(83)97116-1. [DOI] [PubMed] [Google Scholar]
- Yamane K., Ichihara S., Mizushima S. In vitro translocation of protein across Escherichia coli membrane vesicles requires both the proton motive force and ATP. J Biol Chem. 1987 Feb 15;262(5):2358–2362. [PubMed] [Google Scholar]
- Zalkin H., Yanofsky C., Squires C. L. Regulated in vitro synthesis of Escherichia coli tryptophan operon messenger ribonucleic acid and enzymes. J Biol Chem. 1974 Jan 25;249(2):465–475. [PubMed] [Google Scholar]
- Zhu H. Y., Dalbey R. E. Both a short hydrophobic domain and a carboxyl-terminal hydrophilic region are important for signal function in the Escherichia coli leader peptidase. J Biol Chem. 1989 Jul 15;264(20):11833–11838. [PubMed] [Google Scholar]
- Zwizinski C., Date T., Wickner W. Leader peptidase is found in both the inner and outer membranes of Escherichia coli. J Biol Chem. 1981 Apr 10;256(7):3593–3597. [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Control of topology and mode of assembly of a polytopic membrane protein by positively charged residues. Nature. 1989 Oct 5;341(6241):456–458. doi: 10.1038/341456a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Net N-C charge imbalance may be important for signal sequence function in bacteria. J Mol Biol. 1986 Nov 20;192(2):287–290. doi: 10.1016/0022-2836(86)90365-7. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Towards a comparative anatomy of N-terminal topogenic protein sequences. J Mol Biol. 1986 May 5;189(1):239–242. doi: 10.1016/0022-2836(86)90394-3. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Wickner W., Dalbey R. E. The cytoplasmic domain of Escherichia coli leader peptidase is a "translocation poison" sequence. Proc Natl Acad Sci U S A. 1988 May;85(10):3363–3366. doi: 10.1073/pnas.85.10.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]