Abstract
Objective:
This study was performed to compare health-related quality of life (HRQOL) of gemcitabine plus S-1 (GS), S-1 alone and gemcitabine alone as first-line chemotherapy for locally advanced or metastatic pancreatic cancer in the GEST (Gemcitabine and TS-1 Trial) study and to assess the impacts of adverse events and tumour response on HRQOL.
Methods:
Patients were randomly assigned to receive gemcitabine alone (1000 mg/m2 weekly for 3 of 4 weeks), S-1 alone (80, 100 or 120 mg/day twice daily for 4 of 6 weeks) or GS (gemcitabine at 1000 mg/m2 weekly plus S-1 at 60, 80 or 100 mg/day twice daily for 2 of 3 weeks). HRQOL was assessed using the EuroQoL-5D (EQ-5D) questionnaire at baseline and weeks 6, 12, 24, 48 and 72. EQ-5D scores, quality-adjusted life months (QALMs), quality-adjusted progression-free months (QAPFMs) and time until definitive HRQOL deterioration (TUDD) were compared among the three groups. The impacts of adverse events and tumour response on EQ-5D scores were analysed.
Results:
Including EQ-5D scores after death as 0, the mean profile was significantly better in the GS than gemcitabine group (difference, 0.069; p=0.003), but not the S-1 group (difference, −0.011; p=0.613). The mean profiles until death were similar in the three groups. QALMs, QAPFMs and TUDD were significantly longer in the GS than gemcitabine group (p<0.001, p<0.001 and p=0.004, respectively), but not the S-1 group (p=0.563, p=0.741 and p=0.701, respectively). Fatigue, anorexia and tumour response were significantly associated with changes in EQ-5D scores.
Conclusions:
GS achieved better HRQOL than gemcitabine alone, resulting a good balance between overall survival and HRQOL benefits. S-1 alone provides HRQOL similar to that provided by gemcitabine alone. Preventing fatigue and anorexia and maintaining better response would improve HRQOL.
Keywords: advanced pancreatic cancer, gemcitabine, health-related quality of life, randomized phase III trial, S-1
Key questions.
What is already known about this subject?
As first-line chemotherapy for advanced pancreatic cancer, the oral fluoropyrimidine derivative drug S-1 alone was demonstrated to be non-inferior to gemcitabine alone in overall survival. Gemcitabine plus S-1 was not demonstrated to be superior to gemcitabine alone in overall survival while showing a benefit in prolonging progression-free survival. Health-related quality of life related to these regimens and impacts of adverse events and tumour response on it are unclear.
What does this study add?
S-1 alone provided health-related quality of life similar to that provided by gemcitabine alone. Gemcitabine plus S-1 achieved better health-related quality of life than gemcitabine alone. Fatigue and anorexia were negatively associated with changes in EQ-5D scores while better tumour response was positively associated with it.
How might this impact on clinical practice?
The results suggest that S-1 alone can be used as a convenient oral first-line chemotherapy alternative to gemcitabine alone, and that gemcitabine plus S-1 may be a viable treatment option for some patients. For better health-related quality of life, preventing fatigue and anorexia and maintaining better response would be important.
Introduction
In evaluating therapies for advanced pancreatic cancer, it is important to assess health-related quality of life (HRQOL) together with overall survival (OS) because any benefit associated with a relatively short OS is often counterbalanced by negative impacts on HRQOL. In recent phase III trials for metastatic pancreatic cancer, fluorouracil plus leucovorin plus irinotecan plus oxaliplatin (FOLFIRINOX) and gemcitabine plus albumin-bound paclitaxel (nab-paclitaxel) showed a clear benefit with respect to OS compared with gemcitabine alone.1 2 FOLFIRINOX also showed delayed HRQOL deterioration compared with gemcitabine alone,3 while gemcitabine plus nab-paclitaxel was associated with longer quality-adjusted survival than gemcitabine alone based on non-patient-reported health utilities.4 However, because of their associated toxicities,1 2 these two regimens require a good patient performance status and close monitoring.5 6 A desirable regimen with a good balance between OS and HRQOL for patients who cannot receive the two regimens should be investigated.
In Japan, the oral fluoropyrimidine derivative drug S-1 has been used to treat pancreatic cancer since the early 2000s.7 The randomised phase III GEST (Gemcitabine and TS-1 Trial) study for locally advanced or metastatic pancreatic cancer investigated the superiority of gemcitabine plus S-1 (GS) and the non-inferiority of S-1 alone versus gemcitabine alone on OS. The study confirmed the non-inferiority of S-1, but not the superiority of GS.8 The non-inferiority of S-1 alone and the superiority of GS for progression-free survival (PFS) were shown.8 The detailed results for another secondary endpoint of HRQOL are reported in this article.
The objective of this study was to compare longitudinal HRQOL among the three treatments in the GEST study. The impacts of adverse events and tumour response on HRQOL of patients with advanced pancreatic cancer were also investigated.
Methods
Study design
The full study details were reported previously.8 Briefly, in this open-label randomised phase III study, eligible patients with locally advanced or metastatic pancreatic cancer, no prior chemotherapy or radiotherapy for pancreatic cancer, age of 20 to 79 years and Eastern Cooperative Oncology Group (ECOG) performance status score of 0 to 1 were enrolled. Enrolled patients were randomly assigned in a 1:1:1 ratio to receive gemcitabine alone, S-1 alone or GS using a minimisation method with stratification by disease extent (locally advanced or metastatic disease) and institution. Patients assigned to gemcitabine alone received gemcitabine at a dose of 1000 mg/m2 intravenously on days 1, 8 and 15 of a 28-day cycle. Patients assigned to S-1 alone received S-1 at a dose of 80, 100 or 120 mg/day orally twice daily according to body surface area on days 1 through 28 of a 42-day cycle. Patients assigned to GS received gemcitabine at a dose of 1000 mg/m2 intravenously on days 1 and 8 plus S-1 at a dose of 60, 80 or 100 mg/day orally twice daily according to body surface area on days 1 through 14 of a 21-day cycle.
Assessment of HRQOL, adverse events and tumour response
HRQOL was assessed using the validated EuroQol-5D-3L (EQ-5D) questionnaire, which comprises five items (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) with three status levels.9 Completion of the EQ-5D questionnaire was defined as responses to all five items. Patients’ responses to the five items were converted into a single health utility score using a prescribed algorithm based on societal preferences for health status in general populations.10 EQ-5D scores of 0 and 1 represented death and perfect health, respectively. EQ-5D scores were assessed at baseline and at weeks 6, 12, 24, 48 and 72 after initiation of the study treatment.
All adverse events during the study treatment were assessed in accordance with Common Terminology Criteria for Adverse Events Version 3.0. For each adverse event, the worst grade every 12 weeks was reported. Tumour response to treatment was assessed by the investigators according to Response Evaluation Criteria in Solid Tumours Version 1.0 based on CT or MRI performed every 6 weeks until disease progression.11
Statistical analysis
All statistical analyses were conducted in the full analysis set (FAS). In the GEST study, sample size was calculated for the primary endpoint of OS. The resulting sample size was considered to be enough for HRQOL analyses to detect clinically significant difference between the treatments.
For the first hypothesis, longitudinal EQ-5D scores over 72 weeks were compared using linear mixed-effect models for repeated measures adjusted for baseline EQ-5D scores. Two types of analysis were conducted to handle the problem of truncation by death12: analysis of EQ-5D scores including scores after death as 0 and analysis of only EQ-5D scores until death.
For the second hypothesis, quality-adjusted life months (QALMs) and quality-adjusted progression-free months (QAPFMs) were compared. Assuming EQ-5D scores at death as 0, individual QALMs and QAPFMs were calculated as the area under the curve of measured EQ-5D scores from randomisation to events defining OS and PFS with linear interpolation, respectively. QALMs and QAPFMs were analysed by the Kaplan-Meier method and compared using the generalised Wilcoxon test. Sensitivity analyses were conducted for censoring (see online Supplementary file 1).
esmoopen-2016-000151supp001.pdf (141.6KB, pdf)
For the third hypothesis, the time until definitive HRQOL deterioration (TUDD) was compared. Based on the reported minimally important differences,13 definitive HRQOL deterioration was defined as a ≥0.1 point decrease in EQ-5D scores from baseline without a further 0.1-point improvement from baseline or missing scores during the postprogression period after the last completed EQ-5D assessment. Death in the absence of previous definitive HRQOL deterioration was treated as an event. TUDD was analysed by the Kaplan-Meier method and compared using a log-rank test and Cox regression.
Exploratory analyses were conducted to evaluate the impacts of five non-haematologic adverse events (nausea, vomiting, diarrhoea, fatigue and anorexia) and tumour response on HRQOL. Tumour response was categorised into four levels (complete response, partial response, stable disease and progressive disease) or deemed not evaluable. The associations of EQ-5D scores with the grades of these five adverse events and tumour response levels immediately before each EQ-5D assessment were analysed using linear mixed-effect models.
All statistical analyses were conducted with SAS V. 9.1, V. 9.2 and V. 9.4 (SAS Institute). All p value evaluations were two sided. Values of p<0.05 were considered statistically significant without multiplicity adjustment.
Results
Patient characteristics and EQ-5D questionnaire completion
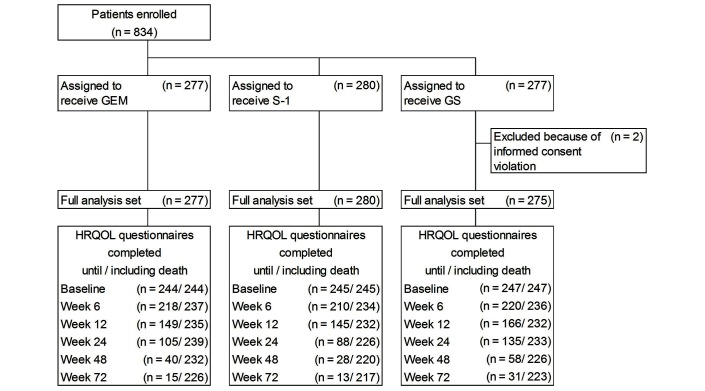
In total, 834 patients were enrolled from July 2007 to October 2009. Among the 832 patients in the FAS (277 in the gemcitabine group, 280 in the S-1 group and 275 in the GS group), 736 (88%) completed the EQ-5D questionnaire at baseline (figure 1). Of those with a completed EQ-5D questionnaire at baseline, 667 (91%) completed at least one EQ-5D questionnaire after randomisation. The demographic and baseline characteristics in the patients with baseline EQ-5D scores were well balanced among the three groups (table 1).
Figure 1.
CONSORT diagram. CONSORT, Consolidated Standards of Reporting Trials. GEM, gemcitabine; GS, gemcitabine plus S-1; HRQOL, health-related quality of life.
Table 1.
Baseline characteristics of patients with baseline EQ-5D scores in the FAS
| Characteristic | GEM (n=244) |
S-1 (n=245) |
GS (n=247) |
|||
| n | % | n | % | n | % | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 153 | 62.7 | 148 | 60.4 | 145 | 58.7 |
| Female | 91 | 37.3 | 97 | 39.6 | 102 | 41.3 |
| Age, years | ||||||
| <65 | 119 | 48.8 | 119 | 48.6 | 125 | 50.6 |
| ≥65 | 125 | 51.2 | 126 | 51.4 | 122 | 49.4 |
| ECOG PS | ||||||
| 0 | 156 | 63.9 | 148 | 60.4 | 155 | 62.8 |
| 1 | 88 | 36.1 | 97 | 39.6 | 92 | 37.2 |
| Extent of disease | ||||||
| Locally advanced | 53 | 21.7 | 58 | 23.7 | 57 | 23.1 |
| Metastatic | 191 | 78.3 | 187 | 76.3 | 190 | 76.9 |
| Type of tumour | ||||||
| Adenocarcinoma | 240 | 98.4 | 241 | 98.4 | 244 | 98.8 |
| Adenosquamous carcinoma |
4 | 1.6 | 4 | 1.6 | 3 | 1.2 |
| Pancreatic excision | ||||||
| No | 222 | 91.0 | 231 | 94.3 | 221 | 89.5 |
| Yes | 22 | 9.0 | 14 | 5.7 | 26 | 10.5 |
| Tumour location* | ||||||
| Head | 108 | 44.3 | 91 | 37.1 | 102 | 41.3 |
| Body | 76 | 31.1 | 109 | 44.5 | 88 | 35.6 |
| Tail | 61 | 25.0 | 50 | 20.4 | 61 | 24.7 |
*Including patients with tumours involving multiple sites.
ECOG PS, Eastern Cooperative Oncology Group performance status; EQ-5D, EuroQol-5D-3L; FAS, full analysis set; GEM, gemcitabine; GS, gemcitabine plus S-1.
Longitudinal profiles of EQ-5D scores
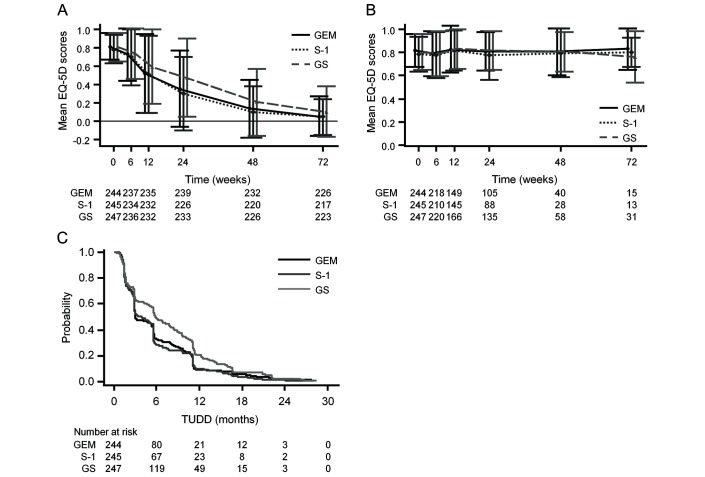
The EQ-5D scores at baseline were comparable among the three groups (figure 2A). When EQ-5D scores after death as 0 were included, a better longitudinal profile of mean scores was observed in the GS than gemcitabine group, while the profiles in the S-1 and gemcitabine groups were similar (figure 2A). Compared with the gemcitabine group, EQ-5D scores until 72 weeks were significantly better in the GS group (difference 0.069; 95% CI 0.024 to 0.115; p=0.003), but not in the S-1 group (difference −0.011; 95% CI −0.054 to 0.032; p=0.613).
Figure 2.
Health-related quality of life (HRQOL) results. (A) Longitudinal mean EQ-5D scores including EQ-5D scores after death as 0. (B) Longitudinal mean EQ-5D scores including only EQ-5D scores until death. (C) Kaplan-Meier estimates of time until definitive HRQOL deterioration. Error bars in (A) and (B) represent ±1 SD. EQ-5D, EuroQol-5D-3L; GEM, gemcitabine; GS, gemcitabine plus S-1.
After restricting the analysis data to only EQ-5D scores until death, similar longitudinal profiles of mean scores were observed in the three groups (figure 2B). Compared with the gemcitabine group, there were no significant differences in the GS group (difference, 0.018; 95% CI, −0.020 to 0.057; p=0.349) or S-1 group (difference, 0.009; 95% CI, −0.035 to 0.052; p=0.693).
Subgroup analyses including scores after death as 0 showed that the adjusted mean EQ-5D scores in the S-1 and gemcitabine groups were similar irrespective of disease extent and ECOG performance status, while those in the subgroups of locally advanced disease and ECOG performance status of 1 were remarkably better in the GS than gemcitabine group (see online supplementary file).
QALMs and QAPFMs
The total number of events for QALMs was 630 (86%) in the three groups. The median QALMs were 5.0 (95% CI 4.3 to 5.6) in the gemcitabine group, 4.8 (95% CI 4.0 to 6.1) in the S-1 group and 6.1 (95% CI 5.5 to 6.8) in the GS group. The proportion of patients with more than 12 QALMs (ie, one quality-adjusted life year) was 17.7% (95% CI 13.2% to 22.8%) in the gemcitabine group, 17.0% (95% CI 12.4% to 22.2%) in the S-1 group and 23.4% (95% CI 18.2% to 29.0%) in the GS group. The QALMs in the GS group was significantly longer than those in the gemcitabine group (p<0.001), while the distributions of QALMs between the S-1 and gemcitabine groups did not differ significantly (p=0.563).
The total number of events for QAPFMs was 701 (95%) in the three groups. The median QAPFMs were 2.5 (95% CI 1.9 to 3.0) in the gemcitabine group, 2.4 (95% CI 2.1 to 2.9) in the S-1 group and 3.7 (95% CI 3.3 to 4.3) in the GS group. The QAPFMs in the GS group were significantly longer than those in the gemcitabine group (p<0.001), while the distributions of QAPFMs in the S-1 and gemcitabine groups were similar (p=0.741).
Time until definitive HRQOL deterioration
The total number of events for TUDD was 711 (97%), of which 76% were based on definitive HRQOL deterioration. The median TUDD was 3.0 (95% CI 2.9 to 5.4) months in the gemcitabine group, 3.8 (95% CI 2.9 to 5.6) months in the S-1 group and 5.8 (95% CI 5.6 to 7.7) months in the GS group (figure 2C). Compared with the gemcitabine group, TUDD in the GS group was significantly prolonged (HR, 0.76; 95% CI 0.64 to 0.92; p=0.004), while that in the S-1 group was not (HR, 1.04; 95% CI 0.87 to 1.24; p=0.701).
Exploratory analysis: impacts of adverse events and tumour response
Analyses of each individual adverse event revealed that all adverse events except diarrhoea were significantly associated with impaired EQ-5D scores (table 2). However, in the simultaneous analysis of all five adverse events, only fatigue and anorexia were significantly associated with decreased EQ-5D scores (table 2). Better tumour responses had also significant favourable effects on EQ-5D scores (table 2).
Table 2.
Impacts of adverse events and tumour response on EQ-5D scores
| Separate analyses | Simultaneous analysis | |||||
| Estimate | 95% CI | p | Estimate | 95% CI | p | |
|---|---|---|---|---|---|---|
| Adverse event (per one-grade increase) | ||||||
| Nausea | −0.050 | −0.065 to −0.035 | <0.001 | −0.008 | −0.027 to 0.011 | 0.421 |
| Vomiting | −0.053 | −0.070 to −0.036 | <0.001 | −0.018 | −0.038 to 0.001 | 0.061 |
| Diarrhoea | −0.013 | −0.030 to 0.005 | 0.167 | 0.009 | −0.008 to 0.026 | 0.283 |
| Fatigue | −0.057 | −0.070 to −0.043 | <0.001 | −0.034 | −0.049 to −0.018 | <0.001 |
| Anorexia | −0.053 | −0.065 to −0.042 | <0.001 | −0.032 | −0.047 to −0.017 | <0.001 |
| Response (per one-level improvement) | ||||||
| 0.059 | 0.045 to 0.073 | <0.001 | - | - | - | |
EQ-5D, EuroQol-5D-3L.
Discussion
In this study, the mean profile of HRQOL, QALMs, QAPFMs and TUDD were significantly better in the GS than gemcitabine group, while all aspects of HRQOL were similar between the S-1 and gemcitabine groups. Exploratory analyses suggested that fatigue and anorexia impaired HRQOL, while better tumour responses improved HRQOL.
The analyses that included scores after death as 0 showed that the mean HRQOL profile in the GS group was significantly better than that in the gemcitabine group. This result partly reflects the prolonged trend in OS in the GS group (median, 10.1 months) compared with the gemcitabine group (median, 8.8 months).8 The QALMs in the GS group were also significantly longer than those in the gemcitabine group. QALMs is a measure that explicitly takes both OS and HRQOL into account. Based on these results, GS accomplished a good balance between OS and HRQOL. Depending on patients’ characteristics and monitoring circumstances, GS may be a viable treatment option for some patients.
Another reason for the better HRQOL associated with GS could be related to the better tumour response. Associations between tumour response and HRQOL have been reported for other types of cancer, such as colorectal cancer and lung cancer, based on phase III randomised study data.14 15 The exploratory analyses in this study also indicated that better tumour responses would improve the HRQOL of patients with advanced pancreatic cancer. The objective response rate in the GS group was 29.3% in the GEST study, while those in the gemcitabine and S-1 groups were 13.3% and 21.0%, respectively.8 The HRQOL benefit from the higher response to S-1 alone may have been cancelled out by negative factors such as fatigue and anorexia, which significantly affected HRQOL in the exploratory analyses. In terms of other regimens for advanced pancreatic cancer, FOLFIRINOX also had a higher objective response rate and delayed HRQOL impairment than did gemcitabine alone.1 3 In contrast, gemcitabine plus erlotinib and gemcitabine plus capecitabine, which did not achieve better HRQOL compared with gemcitabine alone,16 17 had objective response rates similar to those with gemcitabine alone.16 18
The HRQOL results were similar between the S-1 and gemcitabine groups in all relevant analyses in this study. These results indicate that S-1 alone is equivalent to gemcitabine alone with respect to HRQOL. The additional HRQOL results in this study support the conclusion suggested by Ueno et al 8 that S-1 alone can be used as a convenient oral first-line chemotherapy alternative to gemcitabine alone for advanced pancreatic cancer.
Some limitations should be taken into account in interpreting the results of this study. First, missing HRQOL data cannot be avoided in randomised studies assessing HRQOL. Because the baseline characteristics were not changed by excluding approximately 10% of patients who did not respond to the baseline questionnaire,8 the results of this study are generalisable to the original FAS population. Some of the missing scores after randomisation were likely to arise through deterioration of patients’ health, especially after disease progression. This could produce upward bias in the mean EQ-5D profiles; however, if equal amounts of missing scores occurred in each group, the bias between groups would be offset.
The second limitation is that patients with both locally advanced and metastatic pancreatic cancer were enrolled in the GEST study. The GEST study was planned before the recommendation that patients with locally advanced disease should be studied separately from patients with metastatic disease.19 The subgroup analyses of EQ-5D scores indicated that the results for S-1 could be applicable to the two entities, while GS could be remarkably beneficial for locally advanced disease.
In conclusion, longitudinal HRQOL in patients with locally advanced or metastatic pancreatic cancer given GS as first-line chemotherapy was significantly better than that in patients given gemcitabine alone. GS is considered one of the regimens that can accomplish a good balance between OS and HRQOL benefits. The longitudinal HRQOL of S-1 alone was similar to that of gemcitabine alone. Better tumour responses with appropriate supportive care for cancer-related fatigue and anorexia could contribute to maintenance of good HRQOL in patients with advanced pancreatic cancer.
Acknowledgments
The authors appreciate all of the patients and their families who participated in the study, as well as the investigators and medical staff who were dedicated to the study. The authors also thank Takeko Oishi for assistance with writing an earlier version of the manuscript.
Footnotes
Contributors: YO, TO, HU, TI, NB, SE, TH, JF, KM, SO, TY, KY, AF, AS and MT were contributed to conception and design. TO, HU, TI, NB, SE, TH, JF, KM, SO, TY, KY, AF, ALC and MT were contributed to collection and assembly of data. YH, YO, TO, HU, TI, NB, SE, TH, JF, KM, SO, TY, KY, AF, KK, AS and MT were contributed to data analysis and interpretation. All authors were contributed to manuscript writing. All authors have approved the final version of the manuscript ahead of submission.
Funding: This work was supported by Taiho Pharmaceutical Co., Ltd. (no grant number is applicable) and TTY Biopharm Co., Ltd. (no grant number is applicable).
Competing interests: YH is contractually employed by J-CRSU. YO has a leadership position at Statcom and J-CRSU; has stock in Statcom; received honoraria from Sanofi, Shionogi, Taiho, Kowa, Chugai and Eisai; and received research funding from Eisai. TO received honoraria from Chugai, Pfizer Japan, Novartis, Taiho, Merck Serono, Eli Lilly Japan, Sumitomo Dainippon, Eisai, Bayer, Yakult, Nobelpharma, Nippon Kayaku, Baxter and Astellas; received rewards for an advisory role from Eli Lilly Japan, Sumitomo Dainippon, Taiho, Ono, Nippon Boehringer Ingelheim, Nano Carrier and Zeria; and received research funding from Chugai, Eli Lilly Japan, Eisai, Novartis, Shizuoka Industry, Takeda Bio Development Center, Yakult, Onco Therapy Science, Otsuka, Taiho, Sceti Medical Labo, Nippon Boehringer Ingelheim, Kowa, Kyowa Hakko Kirin, Merck Serono, Ono, Bayer, Pfizer Japan, AstraZeneca, Sumitomo Dainippon, Nobelpharma, Zeria and Glaxo Smith Kline. HU received honoraria from Taiho and Chugai and received research funding from Taiho, NanoCarrier and Baxalta Japan. TI received rewards for an advisory role from Taiho, Yakult, Baxalta and JCRO; served on the speakers’ bureau of Taiho, Yakult, Daiichi Sankyo, Eisai, Mochida and Fujifilm; and received research funding from Taiho, AstraZeneca, Glaxo Smith Kline, Nihon Zouki, Zeria and Merck Sereno. NB received honoraria from Taiho and Eli Lilly. JF received honoraria from Taiho, Yakult, Eli Lilly Japan, Chugai, Eisai, Ono, Daiichi Sankyo, Merck Serono, Bayer, Novartis, Sumitomo Dainippon, Mitsubishi Tanabe, MSD, AstraZeneca, Sawai, Mochida, Takeda, Pfizer and Astellas; received rewards for an advisory role from Taiho, Yakult, Fujifilm, Chugai, Kyowa Hakko Kirin, Otsuka, Zeria and J-Pharma; served on the speakers’ bureau of Taiho and Yakult; and received research funding from J-Pharma, Taiho, Sumitomo Dainippon, Janssen, Daiichi Sankyo, MSD, Yakult, Takeda, Chugai, Ono, Astellas, Zeria, Novartis, Nanocarrier, Shionogi, Onco Therapy Science, Eli Lilly Japan, Bayer, Bristol-Myers Squibb, Merck Serono, Kyowa Hakko Kirin, Eisai and Mochida. SO received honoraria from Eli Lilly, Taiho, Yakult, Otsuka and Sandoz; received rewards for an advisory role from Boehringer Ingelheim; and received research funding from AstraZeneca. ALC received rewards for an advisory role from Bayer, Eisai, Novartis and Merck KGaA. AS received honoraria from Taiho and received rewards for an advisory role from Taiho. MT received honoraria from Taiho and Eli Lilly and received research funding from Eli Lilly. All remaining authors have declared no conflict of interest.
Ethics approval: Ethics committees or institutional review boards of all participating centres.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1. Conroy T, Desseigne F, Ychou M, et al. ; Groupe Tumeurs Digestives of Unicancer, PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 2. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic Cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–703. 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol 2013;31:23–9. 10.1200/JCO.2012.44.4869 [DOI] [PubMed] [Google Scholar]
- 4. Reni M, Wan Y, Solem C, et al. Quality-adjusted survival with combination nab-paclitaxel + gemcitabine vs gemcitabine alone in metastatic pancreatic cancer: a Q-TWiST analysis. J Med Econ 2014;17:338–46. 10.3111/13696998.2014.903122 [DOI] [PubMed] [Google Scholar]
- 5. Saif MW, Chabot J. Chemotherapy: metastatic pancreatic cancer-is FOLFIRINOX the new standard? Nat Rev Clin Oncol 2011;8:452–3. 10.1038/nrclinonc.2011.107 [DOI] [PubMed] [Google Scholar]
- 6. O'Reilly EM. Evolving panorama of treatment for metastatic pancreas adenocarcinoma. J Clin Oncol 2013;31:1621–3. 10.1200/JCO.2013.48.7660 [DOI] [PubMed] [Google Scholar]
- 7. Sudo K, Nakamura K, Yamaguchi T. S-1 in the treatment of pancreatic Cancer. World J Gastroenterol 2014;20:15110–8. 10.3748/wjg.v20.i41.15110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ueno H, Ioka T, Ikeda M, et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 2013;31:1640–8. 10.1200/JCO.2012.43.3680 [DOI] [PubMed] [Google Scholar]
- 9. EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 10. Tsuchiya A, Ikeda S, Ikegami N, et al. Estimating an EQ-5D population value set: the case of Japan. Health Econ 2002;11:341–53. 10.1002/hec.673 [DOI] [PubMed] [Google Scholar]
- 11. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. european organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- 12. Kurland BF, Egleston BL. For health related quality of life and other longitudinal data, analysis should distinguish between truncation by death and data missing because of nonresponse. J Clin Oncol 2016;34: 4449. 10.1200/JCO.2016.69.1220 [DOI] [PubMed] [Google Scholar]
- 13. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007;5:70. 10.1186/1477-7525-5-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bezjak A, Tu D, Seymour L, et al. ; National Cancer Institute of Canada Clinical Trials Group Study BR.21. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group study BR.21. J Clin Oncol 2006;24:3831–7. 10.1200/JCO.2006.05.8073 [DOI] [PubMed] [Google Scholar]
- 15. Au HJ, Karapetis CS, O'Callaghan CJ, et al. Health-related quality of life in patients with advanced colorectal cancer treated with cetuximab: overall and KRAS-specific results of the NCIC CTG and AGITG co.17 trial. J Clin Oncol 2009;27:1822–8. 10.1200/JCO.2008.19.6048 [DOI] [PubMed] [Google Scholar]
- 16. Moore MJ, Goldstein D, Hamm J, et al. ; National Cancer Institute of Canada Clinical Trials Group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960–6. 10.1200/JCO.2006.07.9525 [DOI] [PubMed] [Google Scholar]
- 17. Bernhard J, Dietrich D, Scheithauer W, et al. ; Central European Cooperative Oncology Group. Clinical benefit and quality of life in patients with advanced pancreatic cancer receiving gemcitabine plus capecitabine versus gemcitabine alone: a randomized multicenter phase III clinical trial-SAKK 44/00-CECOG/PAN.1.3.001. J Clin Oncol 2008;26:3695–701. 10.1200/JCO.2007.15.6240 [DOI] [PubMed] [Google Scholar]
- 18. Herrmann R, Bodoky G, Ruhstaller T, et al. ; Swiss Group for Clinical Cancer Research, Central European Cooperative Oncology Group. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the swiss group for clinical Cancer research and the central European cooperative oncology group. J Clin Oncol 2007;25:2212–7. 10.1200/JCO.2006.09.0886 [DOI] [PubMed] [Google Scholar]
- 19. Philip PA, Mooney M, Jaffe D, et al. Consensus report of the national cancer institute clinical trials planning meeting on pancreas cancer treatment. J Clin Oncol 2009;27:5660–9. 10.1200/JCO.2009.21.9022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2016-000151supp001.pdf (141.6KB, pdf)