Abstract
In the past 15 years, the outcome for patients with metastatic colorectal cancer has substantially improved owing to the availability of new cytotoxic and biological agents along with many significant advances in molecular selection, the use of personalised therapy and locoregional treatment, a more widespread sharing of specific professional experiences (multidisciplinary teams with oncologists, surgeons, radiotherapists, radiologists, biologists and pathologists), and the adoption of patient-centred healthcare strategies. The Italian Medical Oncology Association (AIOM) has developed evidence-based recommendations to help oncologists and all professionals involved in the management of patients with metastatic colorectal cancer in their daily clinical practice.
Keywords: Metastatic colorectal cancer, guidelines, recommendation, Italian, AIOM
Introduction
Colorectal cancer (CRC) is the third most common cancer in the world. In Italy, 18 756 CRC-related deaths were reported in 2013 and 52 400 new cases have been estimated in 2016.1
Over the past 15 years, the treatment of metastatic colorectal cancer (mCRC) has markedly evolved and significant improvements in the overall treatment plan have been achieved, with median overall survival (mOS) reaching the unprecedented value of over 40 months in molecularly selected patients.
Many advances have led to such notable result being achieved, including the availability of novel efficacious cancer drugs, a more profound knowledge of disease characterisation with molecular biology studies, the application of personalised, patient-centred strategies, the evolution of multidisciplinary teams, and the earlier use of palliative and simultaneous care. In this changing scenario, the Italian Medical Oncology Association (AIOM) has developed evidence-based guidelines to help oncologists, physicians and other healthcare professionals understand the overall disease picture and allow them to easily embrace a more comprehensive and updated treatment strategy.
Methodology
The working group
The AIOM guidelines working group includes professionals from across the country with different professional skills, such as medical oncologists, surgeons, radiation oncologists and molecular biologists, which facilitated the analysis of scientific issues as well as different logistic and regulatory aspects in different regions.
A systematic review of the literature was carried out and every 2 months conference calls between authors were held. During the final consensus meeting, a preliminary report was prepared and sent to reviewers for peer review.
The guidelines were revised by several opinion leaders in CRC and by different scientific societies (table 1).
Table 1.
List of external independent reviewers, including their affiliations and scientific society
| Name | Affiliation | Scientific society |
| Maurizio Cancian | ULSS7, Conegliano Veneto (TV) | SIMG |
| Renato Cannizzaro | Gastroenterology Unit, C.R.O., Aviano (PN) | AIGO |
| Antonino De Paoli | Radiotherapy Unit, C.R.O., Aviano (PN) | AIRO |
| Francesco Di Costanzo | Oncology Unit, Azienda Ospedaliero Universitaria Careggi, Firenze | AIOM |
| Alfredo Falcone | Oncology Unit, Azienda Ospedaliero Universitaria Pisana, Pisa | AIOM |
| Roberto Labianca | Oncology Unit, Azienda Ospedaliero Giovanni XXIII, Bergamo | AIOM |
| Giovanni Lanza | Pathology Unit, Arcispedale S. Anna, Azienda Ospedaliero Universitaria, Ferrara | SIAPEC |
| Salvatore Pucciarelli | Surgery Unit 1, Università, Padova | AIOM |
| Mauro Risio | Pathology Unit, Istituto per la Ricerca e la Cura del Cancro, IRCC, Candiolo (TO) | SIAPEC |
| Francesco Tonelli | Surgery Unit, Università degli Studi di Firenze, Firenze | SICO |
| Vincenzo Valentini | Radiotherapy Unit, Gemelli ART, Fondazione ‘Policlinico A. Gemelli’, Roma | AIOM |
| Alberto Zaniboni | Oncology Unit, Fondazione Poliambulanza, Brescia | AIOM |
AIGO, Italian Association of Gastroenterology; AIOM, Italian Medical Oncology Association; AIRO, Italian Association of Oncologic Radiotherapy; SIAPEC, Italian Society of Pathology and Cytology; SICO, Italian Society of Oncologic Surgery; SIMG, Italian Society of General Medicine.
The final report, including the accepted recommendations of the reviewers, was eventually published online on the AIOM website.
Recommendation’s methodology
Each recommendation has been made based on the guidelines prescribed by the Scottish Intercollegiate Guidelines Network (SIGN).
The quality of evidences according to SIGN reflects both the type of studies that have been considered, as outlined in table 2, and the clinical applicability of results.
Table 2.
Evidence levels according to the Scottish Intercollegiate Guidelines Network
| 1 | Meta-analyses and systematic reviews of randomised clinical trials |
| 1++ | Very low risk of bias |
| 1+ | Low risk of bias |
| 1− | High risk of bias |
| 2 | Systematic reviews of cohort or case and control studies |
| 2++ | Very low risk of bias and high probability of a causal relationship |
| 2+ | Low risk of bias and moderate probability of a causal relationship |
| 2− | High risk of bias and significant risk that the relationship is not causal |
| 3 | Non-analytical studies, such as case reports and case series |
| 4 | Expert opinion |
The quality of evidences according to SIGN is reported using the letters A, B, C or D, as described in table 3.
Table 3.
Quality of evidences according to the Scottish Intercollegiate Guidelines Network
| A | At least one meta-analysis, systematic review or randomised clinical trial classified as 1++ and directly applicable to the target population |
| Studies classified as 1+ and directly applicable to the target population | |
| B | Studies classified as 2++ and directly applicable to the target population |
| Evidences from studies classified as 1++ or 1+, but not directly applicable to the target population | |
| C | Studies classified as 2+ and directly applicable to the target population |
| Evidences from studies classified as 2++, but not directly applicable to the target population | |
| D | Evidence level 3 or 4 |
| Evidences from studies classified as 2+, but not directly applicable to the target population |
The strength of a recommendation reflects its clinical relevance and is reported as ‘strong for’, ‘strong against’, ‘conditional for’ or ‘conditional against’, as explained in table 4.
Table 4.
Strength of recommendation
| Strength of recommendation | Meaning |
| Strong for | The intervention should be considered as the first treatment option (benefits are higher than risks) |
| Conditional for | The intervention can be considered as a possible treatment option (not sure that benefits are higher than risks) |
| Conditional against | The intervention should not be considered as the first treatment option; it could be considered in selected cases after discussion with the patient (not sure that risks are higher than benefits) |
| Strong against | The intervention should not be considered as a possible treatment option (risks are higher than benefits) |
The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) methodology has been applied only for specific debated interventions. The GRADE approach specifically assesses:
methodological flaws within the component studies
consistency of results across different studies
generalisability of research results to the wider patient base
how effective the treatments have been shown to be.
Treatment comparisons are given one out of four GRADE scores, reflecting the quality of the evidence: high-quality, moderate-quality, low-quality or very low-quality evidence.
mCRC guidelines
The AIOM guidelines consider the management of all CRC settings, including both adjuvant and metastatic settings. Moreover, the guidelines specifically consider rectal cancers. This first report focuses on advanced CRC.
Multidisciplinary team
A multidisciplinary team discussion is crucial for modern diagnostic and therapeutic decision making. Oncologists, surgeons, radiotherapists, molecular biologists and pathologists must give their specific recommendations for an adequate and personalised treatment strategy for each mCRC patient. The treatment quality is directly proportional to the number of treated patients: each multidisciplinary team should discuss and treat at least 50 patients per year (including early stage and advanced disease), while teams dealing with less than 50 cases per year should collaborate with referral hospitals.2–4 Recommendations are provided in table 5.
Table 5.
Multidisciplinary team: SIGN recommendations
| Quality of evidences (SIGN) |
Recommendation | Strength of recommendation |
| D | The diagnostic and therapeutic strategy should be proposed by a multidisciplinary team. Each decision must be recorded and archived.2–4 | Strong for |
| D | The diagnostic and therapeutic decision must be in line with guidelines. Different proposals should be well explained.2–4 | Strong for |
| D | The multidisciplinary team must provide adequate documentary evidence to the patient and the family doctor.2–4 | Strong for |
| D | The treatment quality is directly proportional to the number of treated patients: each multidisciplinary team should treat at least 50 patients per year (including early stage and advanced disease). Teams with less than 50 patients should collaborate with referral hospitals.2 | Strong for |
SIGN, Scottish Intercollegiate Guidelines Network.
Molecular biology
CRCs are characterised by a number of molecular alterations that may combine to determine malignant transformation. Notably, around 80% of CRCs are sporadic, while 15%–20% are familial and 5% are considered genetic or linked to specific genetic syndromes. In the carcinogenic process, three different pathways have been recognised: (1) microsatellite instability, (2) chromosomal instability and (3) DNA methylation. In particular, abnormal hypermethylation has been detected in a significant percentage of CRC patients, and around 20% of CRCs have a methylated phenotype that corresponds to CpG island methylator phenotype (CIMP)-high.
KRAS and NRAS mutations are predictive of resistance to anti-epidermal growth factor receptor (EGFR) drugs. Therefore, mutational analysis of RAS genes is mandatory whenever treatment with an anti-EGFR monoclonal antibody is indicated. In particular, KRAS gene mutations are reported in at least 40% of CRCs. Controversial data are reported regarding the prognostic value of KRAS mutations, and some studies suggest a negative prognostic value for the p.G12V KRAS mutation. The mutational analysis of KRAS and NRAS genes must cover at least codons 12, 13, 59, 61, 117 and 146 of both genes and can be performed either on primary or on metastatic tumour tissue.5 6 The use of circulating tumour DNA for RAS analysis is not routinely recommended, but it could be an option when adequate tissue for molecular testing is not available. AIOM, in collaboration with the Italian Society of Pathology and Cytology (SIAPEC), has implemented a quality control programme for laboratories that perform the RAS mutation test.7 The list of certified laboratories is published in the websites of these two scientific societies.
Although the evidence of BRAF V600E mutation as a predictive factor for resistance to anti-EGFR drugs has not been definitelyascertained, its analysis is recommended owing to its strong negative prognostic value.8
Analysis of mutations in mismatch repair genes is not currently recommended in clinical practice (at the moment it is recommended for genetic counselling), although it could help in selecting patients to be enrolled in specific clinical trials evaluating immunotherapy.9
While a number of studies have suggested that PIK3CA and PTEN mutations may be linked to resistance to EGFR-inhibitors, PI3KCA and PTEN analyses are not currently recommended in clinical practice.
mCRC treatment
About 25% of patients with CRC present with advance disease at the time of diagnosis and a further 35% will develop metastases during the course of the disease. The aims of therapy for mCRC are cure (if possible in very selected cases), prolongation of life, palliation of symptoms, improvement of quality of life, delay disease progression and tumour shrinkage.
It is possible to identify four different scenarios with different medical approaches:
patients with limited resectable disease: surgery with perioperative or postoperative ‘adjuvant’ chemotherapy
patients with limited unresectable disease: conversion therapy followed by radical surgery when possible
patients with widespread and aggressive mCRC and disease-related symptoms: palliative therapy with the aim of rapid tumour shrinkage
patients with widespread, unresectable and asymptomatic disease: palliative therapy with the aim of disease control to maintain a good quality of life.
Before planning the treatment strategy, it is essential to consider:
overall conditions and emotional status of patients: fit versus unfit for a combination therapy (triplet vs doublet vs monotherapy), taking into account Eastern Cooperative Oncology Group performance status, age, comorbidities, patient’s attitude as well as his or her disease history (eg, a previous oxaliplatin-based adjuvant treatment);
tumour characteristics and clinical course: indolent versus aggressive tumour, considering disease presentation (synchronous vs metachronous), tumour load and mutational status (RAS and BRAF);
treatment goal: (1) tumour shrinkage to achieve a radical surgery of metastases or palliation of disease-related symptoms and (2) disease control to delay progression and worsening of patient’s general condition
Several drugs are actively used for the treatment of mCRC, such as fluoropyrimidines, irinotecan, oxaliplatin, mytomicin C, EGFR-inhibitors (cetuximab and panitumumab), antiangiogenic drugs (bevacizumab, aflibercept and ramucirumab), regorafenib and TAS-102.
The choice between monochemotherapy versus a polychemotherapy (doublet or triplet) should take into consideration the patient’s fitness and ‘aggressiveness’ of the tumour.
The introduction of biologics, including EGFR- and VEGF-inhibitors, improved chemotherapy efficacy and, consequently, the outcome for patients with mCRC.
As a first-line treatment, bevacizumab can be combined with:
capecitabine, in elderly and/or unfit patients, on the basis of results from the AVEX study which demonstrated a significant improvement of progression-free survival (PFS) in comparison to capecitabine alone10;
FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan), in fit patients younger than 75 years, significantly increased response rate (RR), PFS and OS in comparison to FOLFIRI plus bevacizumab11;
oxaliplatin- or irinotecan-based doublet leading to a significant benefit in patient outcome versus doublet alone.12–15
Several trials suggest that prolonging bevacizumab in combination with fluoropyrimidine as maintenance treatment until disease progression can improve PFS, without a significant improvement in OS. When deciding on maintenance treatment, risks and benefits should be evaluated on an individual basis, taking into account previous toxicities, general condition, comorbidities, disease characteristics and patient’s motivation.16 17
Cetuximab or panitumumab can be combined with a first-line chemotherapy in RAS and BRAF wild-type patients to achieve a significant increase in RR and improve patient outcome.18–24
A head-to-head comparison between bevacizumab and cetuximab has been investigated in two large phase III randomised studies. The FIRE-3 trial, a negative study according to its primary end-point (RR), demonstrated a significant increase in OS in favour of the cetuximab arm25; on the other hand, the CALGB study (OS as primary end-point) did not confirm this difference.26 On the basis of these results, in RAS and BRAF wild-type patients, both bevacizumab and EGFR-inhibitors can be considered valid options, even if recent preliminary data seem to show that the primary tumour site (left vs right) might be a helpful predictive factor in the decision of which biological agent should be combined with a first-line doublet.27
There are several options for second-line antiangiogenic treatment:
bevacizumab beyond progression28 29;
bevacizumab in patients who have not been treated with bevacizumab as first-line treatment30;
aflibercept in combination with FOLFIRI in patients who progressed to a previous oxaliplatin-based therapy31;
ramucirumab in combination with FOLFIRI in patients treated with oxaliplatin and bevacizumab as first-line treatment.32
To date, no specific predictive factors can help us in the choice of the most adequate antiangiogenic drug; therefore, our decision must be based on clinical factors such as the kind of first-line chemotherapy, the magnitude of benefit from first-line bevacizumab and previous toxicities.
Studies evaluating the role of cetuximab and panitumumab in combination with a second-line irinotecan-based chemotherapy demonstrated a significant benefit in terms of RR and PFS, but not in OS.33–35
Considering third and subsequent treatment lines, an increasing number of options is available. Cetuximab (alone or combined with irinotecan) and panitumumab showed prolongation of OS compared with best supportive care (BSC) alone in RAS and BRAF wild-type pretreated patients with mCRC.36 37
Regorafenib, an oral multikinase inhibitor, and TAS-102, an oral combination of the nucleoside analogue trifluridine and a thymidine phosphorylase inhibitor, showed a similar benefit of prolongation of OS in heavily pretreated patients with mCRC compared with BSC alone.38 39
Furthermore, the recent phase II HERACLES study suggested the potential use of HER-inhibitors in HER-2 overexpressed patients with mCRC.40
Algorithms for the management of mCRC are shown in figure 1a–c.
Figure 1.
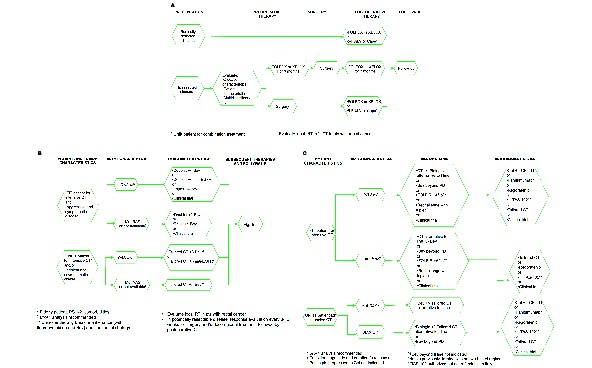
Algorithms for the management of metastatic colorectal cancer: (A) resectable metastatic disease; (B) metastatic disease, first-line; (C) metastatic disease, subsequent lines. 5-FU, 5-fluorouracil; Aflib, aflibercept; Bev, bevacizumab; Cape, capecitabine; Cet, cetuximab; CT, chemotherapy; EGFR, epidermal growth factor receptor; FOLFIRI, 5-fluorouracil+lederfolin+irinotecan; FOLFIRI, folinic acid, 5-FU and irinotecan; FOLFOX, folinic acid, 5-FU and oxaliplatin; LV, lederfolin; mut, mutant; PD, progressive disease; PS, performace status; pts, patients; RT, radiotherapy; wt, wild type; XELOX, capecitabine+oxaliplatin.
SIGN and GRADE recommendations are provided in tables 6 and 7, respectively.
Table 6.
Metastatic colorectal cancer treatment: SIGN recommendations
| Quality of evidences(SIGN) | Recommendation | Strength of recommendation |
| C | RAS status must be evaluated for the decision of treatment strategy for metastatic disease.18 | Strong for |
| D* | BRAF status should be evaluated for the decision of treatment strategy for metastatic disease. | Conditional for |
| A | The combination of 5-fluorouracil (continuous infusion is preferable) and oxaliplatin and/or irinotecan must be used in patients deemed fit for a combination treatment (the combination with anti-VEGF or anti-EGFR monoclonal antibodies is preferable). For unfit patients the option is fluoropyrimidine±bevacizumab.10–15 19–22 44–50 | Strong for |
| A | Capecitabine can substitute for monotherapy with 5-fluorouracil+folinic acid. When a monotherapy is indicated, capecitabine is the first option, preferably with bevacizumab.10 50 | Strong for |
| A | Capecitabine can be used in combination with oxaliplatin.51–53 Capecitabine plus irinotecan, due to increased toxicity, should be used only if there are contraindications to infusional 5-fluorouracil.54 55 | Strong for |
| A | If no contraindications, bevacizumab can be used in combination with first-line chemotherapy.10–15 49 50 | Strong for |
| A | If no contraindications, bevacizumab can be used in combination with second-line chemotherapy in patients not treated with bevacizumab as first-line treatment.30 | Strong for |
| B | Bevacizumab beyond progression in combination with chemotherapy can be a treatment option.28 29 | Conditional for |
| A | A second-line treatment must be always considered in fit patients. A third- and fourth-line treatment can be considered in several cases.56 57 |
Strong for |
| A | Cetuximab can be used in RAS wild-type patients in combination with irinotecan-based regimens (irrespective of treatment line) or as monotherapy in advanced lines.19 36 | Strong for |
| B | Cetuximab can be associated with first-line oxaliplatin-based treatment. In this case, continuous infusion of 5-fluorouracil without bolus is preferable.21 23 24 | Strong for |
| A | Panitumumab (anti-EGFR) can be used as monotherapy in advanced lines, in RAS wild-type patients not previously treated with cetuximab or after a severe infusion reaction to cetuximab.37 | Strong for |
| A | In RAS wild-type patients, panitumumab can be used in combination with first-line FOLFOX or FOLFIRI,20 22 and with second-line FOLFIRI.33 | Strong for |
| A | The combination of aflibercept with second-line FOLFIRI in patients previously treated with an oxaliplatin-based treatment (with or without a biological drug) can be an option.31 | Conditional for |
| B | A sequential and less toxic strategy can be considered in case of indolent disease.44 45 | Conditional for |
| B | FOLFOXIRI plus bevacizumab should be considered as first-line treatment in BRAF mutated and fit patients.58 | Strong for |
| B | To reduce treatment-related toxicity a ‘stop-and-go’ strategy or a less intensive treatment can be considered.59–61 | Conditional for |
| B | In patients pretreated or not considered candidates for all the available drugs, regorafenib can be an option.38 TAS-102 could be a further option in this setting.‡ 39 | Conditional for |
*Panel opinion.
‡At the moment authorised but not refundable in Italy.
EGFR, epidermal growth factor receptor; FOLFIRI, folinic acid, 5-fluorouracil and irinotecan; FOLFOX, folinic acid, 5-fluorouracil and oxaliplatin; SIGN, Scottish Intercollegiate Guidelines Network; VEGF, vascular endothelial growth factor.
Table 7.
mCRC treatment: GRADE recommendations
| Quality of evidences (GRADE) | Recommendation | Strength of clinical recommendation |
| Very low | Starting a treatment for metastatic disease at the time of diagnosis, also without disease-related symptoms, is recommended. A wait-and-see period might be considered in well-selected cases (elderly, comorbidities, minimal tumour load) after an adequate evaluation of risks/benefits.62 63 | Strong for |
| Moderate | A maintenance treatment with bevacizumab±fluoropyrimidine can be considered in patients with mCRC after a first-line treatment with bevacizumab, after an adequate evaluation of risks/benefits and patient’s motivation.16 17 | Conditional for |
GRADE, Grading of Recommendations, Assessment, Development and Evaluations; mCRC, metastatic colorectal cancer.
Evaluation of elderly patients
Comprehensive geriatric assessment (CGA) is recommended to define the presence or absence of frailty. Frailty could be detrimental if patients are to receive chemotherapy, both in adjuvant and metastatic settings. To improve this evaluation, several simpler tests have been recently introduced; the G8 test is one such recommended test.
In patients over 75 years, due to weak evidence, CGA and evaluation of expected remaining life are recommended. Recommendations are provided in table 8.
Table 8.
Evaluation of elderly patients: SIGN recommendations
| Quality of evidences(SIGN) | Recommendation | Strength of recommendation |
| D | Functional evaluation, before treatment, is recommended. Prescreening with a fast test (G8 test) helps the individuation of patients for evaluation according to CGA.64 | Strong for |
CGA, comprehensive geriatric assessment; SIGN, Scottish Intercollegiate Guidelines Network.
Surgery for advanced disease
Many factors may affect the timing and type of surgery in patients with unresected primary tumour and synchronous metastatic disease: performance status, extension of metastatic disease and symptoms from primary tumour. A multidisciplinary evaluation must define the best strategy. Radical surgery of liver metastases can be curative, but it is dependent on the surgeon’s expertise. Radical resection of lung metastases may also be considered in selected cases. Recommendations are provided in table 9.
Table 9.
Surgery: SIGN recommendations
| Quality of evidences(SIGN) | Recommendation | Strength of recommendation |
| D* | The timing and type of surgery in patients with unresected primary tumour and synchronous metastatic disease depends on performance status, extension of metastatic disease and symptoms from primary tumour. A multidisciplinary evaluation is recommended in the decision of the best strategy. | Strong for |
| D* | In patients with symptomatic rectal cancer and synchronous metastasis, polychemotherapy plus radiotherapy can be considered. | Conditional for |
| D | Radical (R0: negative margins) liver resection can be curative in selected cases.65 | Strong for |
| D* | The number of liver metastasis is not related to a worse prognosis if the surgeon is an expert and the surgery is radical. | Conditional for |
| D* | Liver resection in borderline resectable disease must be considered after tumour shrinkage is achieved with chemotherapy. | Strong for |
| D | Medical treatment must be stopped when disease becomes resectable. The prosecution of chemotherapy could increase liver toxicity and surgery risks.66 A radiological complete response does not mean a pathological complete response; it could create difficulty for the surgeon in the individuation of metastasis.67 | Strong for |
| D | Preoperative bevacizumab must be interrupted 5–6 weeks before surgery. | Strong for |
| B | Patients with resectable disease can receive a perioperative treatment.41 42 | Conditional for |
| D | Radical (R0: negative margins) lung resection can be curative in selected cases.68 | Strong for |
*Panel opinion.
SIGN, Scottish Intercollegiate Guidelines Network.
Chemotherapy after radical liver resection
The vast majority of evidence on this point are retrospective. Nevertheless, patients can receive postoperative treatment with fluoropyrimidine with or without oxaliplatin after surgical resection of secondary lesions or a perioperative treatment with FOLFOX or XELOX (capecitabine+oxaliplatin).41 42
Locoregional treatments
Liver-directed therapies
Liver-directed therapies could represent an effective treatment option for unresectable liver metastases. Oligometastatic liver metastases could be treated by ablative techniques (radiofrequency ablation, microwaves, cryoablation) or by external irradiation with stereotactic procedures (stereotactic body irradiation, 3D conformal radiation therapy, intensity modulated radiotherapy).
Although all these procedures are active and well tolerated, the lack of randomised trials on these procedures limits the understanding of their role in the treatment algorithm.
For ‘extended’ liver metastases, ablative techniques and external irradiation are contraindicated. In these situations, the use of radioembolisation with 90Y resin microspheres, intrahepatic chemotherapy and transcathether arterial chemoembolisation with DEBIRI (irinotecan-loaded drug-eluting beads) might be considered.
A single phase III randomised trial evaluated the role of 90Y resin microspheres associated with FOLFOX-based chemotherapy in patients with dominant liver metastatic disease. Although the trial failed its primary endpoint (overall PFS), an advantage was observed in terms of liver PFS for patients receiving 90Y resin microspheres.43 Recommendations are provided in table 10.
Table 10.
Liver-directed therapies: SIGN recommendations
| Quality of evidences (SIGN) | Recommendation | Strength of recommendation |
| B | Patients with liver-limited disease who are not candidates for radical surgery can benefit from a combination strategy with systemic therapy and RFA.69 | Conditional for |
| D* | Ablative techniques (RFA, MW, cryoablation) or external irradiation (SBRT, 3D CRT, IMRT) could be useful in selected oligometastatic liver disease unsuitable for surgery. | Conditional for |
| D* | Intrahepatic radioembolisation in combination with a systemic treatment can achieve liver disease control. | Conditional for |
| D* | Intrahepatic chemotherapy and TACE (ideally with DEBIRI) could represent a therapeutic option only for patients unsuitable for standard systemic treatment in I, II and III line. |
Conditional against |
*Panel opinion.
CRT, confocal radiation therapy; DEBIRI, irinotecan-loaded drug-eluting beads; IMRT, intensity modulated radiotherapy; MW, micowaves; RFA, radiofrequency ablation; SBRT, stereotactic body radiation therapy; SIGN, Scottish Intercollegiate Guidelines Network; TACE, transcathether arterial chemoembolisation.
Non-liver-directed therapies
Isolated peritoneal carcinomatosis is a clinical condition characterised by an extremely poor prognosis. In this population, cytoreductive surgery and intraperitoneal chemohyperthermia performed in centres of expertise could improve PFS and survival, especially in cases with limited peritoneal spread and without macroscopic residual disease after surgery.
Radiation therapy is effective in bone metastases to improve pain control and to reduce the risk of bone fracture or spinal cord compression in cases of spine involvement.
Patients with local recurrence or unresectable T4b lesions with no distant metastases could undergo concomitant radiochemotherapy with the aim of achieving a radical resection of the tumour.
In selected patients with pulmonary oligometastatic disease unsuitable for surgery, stereotactic radiotherapy treatment may be indicated. Recommendations are provided in table 11.
Table 11.
Non-liver-directed therapies: SIGN recommendations
| Quality of evidences (SIGN) | Recommendation | Strength of recommendation |
| B | Cytoreductive surgery and intraperitoneal chemohyperthermia performed in centres of expertise can be attempted in patients with isolated peritoneal carcinomatosis.70 | Conditional for |
| D* | Radiation therapy is efficacious for palliative treatment of bone metastases. | Strong for |
| D* | Radiation therapy +/- chemotherapy can be used for palliation or with a cytoreductive intent in patients with resectable recurrent disease localised in the pelvis, lymph nodes and lung. | Conditional for |
*Panel opinion.
SIGN, Scottish Intercollegiate Guidelines Network.
Conclusions
To date, several treatment options are available for patients with mCRC, and the complex choice of the optimal strategy must take into consideration patient and tumour characteristics. Owing to the progress in medical therapy, surgery and loregional approaches, the outcome for patients with mCRC has notably improved.
In this changing scenario, the AIOM guidelines aim to simplify the complexity in the choice of the optimal treatment strategy by providing evidence-based recommendations to help Italian oncologists in their daily clinical practice.
The methodology followed while writing and updating the AIOM guidelines, the multidisciplinary nature of the working group and the final systematic review by independent CRC experts and different medical societies have contributed to the strong scientific value of the current Italian guidelines.
Footnotes
Contributors: Drafting the manuscript: LS, GA, GDB; revising the manuscript and approval of the final version of the manuscript: all authors; responsible for the overall content as guarantors: LS, GA, GDB.
Funding: This work received no specific grant from any funding agency in the public, commercial or not-forprofit sectors.
Competing interests: None declared.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1. Köhne CH, Hofheinz R, Mineur L, et al. I numeri del cancro in italia—2016. http://www.registri-tumori.it/cms/it/node/4572.
- 2. Steele RJ, Rey JF, Lambert R; International Agency for Research on Cancer. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—professional requirements and training. Endoscopy 2012;44(Suppl 3):SE106–15. 10.1055/s-0032-1309796 [DOI] [PubMed] [Google Scholar]
- 3. Borras JM, Albreht T, Audisio R, et al. ; European Partnership Action Against Cancer consensus group. Policy statement on multidisciplinary cancer care. Eur J Cancer 2014;50:475–80. 10.1016/j.ejca.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 4. Kesson EM, Allardice GM, George WD, et al. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ 2012;344:e2718. 10.1136/bmj.e2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Artale S, Sartore-Bianchi A, Veronese SM, et al. Mutations of KRAS and BRAF in primary and matched metastatic sites of colorectal cancer. J Clin Oncol 2008;26:4217–9. 10.1200/JCO.2008.18.7286 [DOI] [PubMed] [Google Scholar]
- 6. Knijn N, Mekenkamp LJ, Klomp M, et al. KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer 2011;104:1020–6. 10.1038/bjc.2011.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. AIOM. Contatta la segreteria. http://www.aiom.it/area+pubblica/area+medica/prodotti+scientifici/tavoli+di+lavoro/Tavolo+di+Lavoro+AIOM+-+SIAPEC/1,604,1.
- 8. Souglakos J, Philips J, Wang R, et al. Prognostic and predictive value of common mutations for treatment response and survival in patients with metastatic colorectal cancer. Br J Cancer 2009;101:465–72. 10.1038/sj.bjc.6605164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Le DT, Uram JN, Wang H, et al. PD-1blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cunningham D, Lang I, Marcuello E, et al. ; AVEX study investigators. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol 2013;14:1077–85. 10.1016/S1470-2045(13)70154-2 [DOI] [PubMed] [Google Scholar]
- 11. Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014;371:1609–18. 10.1056/NEJMoa1403108 [DOI] [PubMed] [Google Scholar]
- 12. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42. 10.1056/NEJMoa032691 [DOI] [PubMed] [Google Scholar]
- 13. Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013–9. 10.1200/JCO.2007.14.9930 [DOI] [PubMed] [Google Scholar]
- 14. Kozloff M, Yood MU, Berlin J, et al. ; Investigators of the BRiTE study. Clinical outcomes associated with bevacizumab-containing treatment of metastatic colorectal cancer: the BRiTE observational cohort study. Oncologist 2009;14:862–70. 10.1634/theoncologist.2009-0071 [DOI] [PubMed] [Google Scholar]
- 15. Van Cutsem E, Rivera F, Berry S, et al. ; First BEAT investigators. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol 2009;20:1842–7. 10.1093/annonc/mdp233 [DOI] [PubMed] [Google Scholar]
- 16. Koeberle D, Betticher DC, von Moos R, et al. Bevacizumab continuation versus no continuation after first-line chemotherapy plus bevacizumab in patients with metastatic colorectal cancer: a randomized phase III non-inferiority trial (SAKK 41/06). Ann Oncol 2015;26:709–14. 10.1093/annonc/mdv011 [DOI] [PubMed] [Google Scholar]
- 17. Simkens LH, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet 2015;385:1843–52. 10.1016/S0140-6736(14)62004-3 [DOI] [PubMed] [Google Scholar]
- 18. Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023–34. 10.1056/NEJMoa1305275 [DOI] [PubMed] [Google Scholar]
- 19. Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408–17. 10.1056/NEJMoa0805019 [DOI] [PubMed] [Google Scholar]
- 20. Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697–705. 10.1200/JCO.2009.27.4860 [DOI] [PubMed] [Google Scholar]
- 21. Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009;27:663–71. 10.1200/JCO.2008.20.8397 [DOI] [PubMed] [Google Scholar]
- 22. Köhne CH, Hofheinz R, Mineur L, et al. First-line panitumumab plus irinotecan/5-fluorouracil/leucovorin treatment in patients with metastatic colorectal cancer. J Cancer Res Clin Oncol 2012;138:65–72. 10.1007/s00432-011-1061-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maughan TS, Adams RA, Smith CG, et al. ; MRC COIN Trial Investigators. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103–14. 10.1016/S0140-6736(11)60613-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tveit KM, Guren T, Glimelius B, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol 2012;30:1755–62. 10.1200/JCO.2011.38.0915 [DOI] [PubMed] [Google Scholar]
- 25. Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1065–75. 10.1016/S1470-2045(14)70330-4 [DOI] [PubMed] [Google Scholar]
- 26. Venook AP, Niedzwiecki D, Lenz H-J, et al. Cancer and Leukemia Group B (Alliance), SWOG, and ECOG. CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol 2014;32:5s (suppl; abstr LBA3; ASCO 2014) 10.1200/jco.2014.32.18_suppl.lba3 [DOI] [Google Scholar]
- 27. Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal Cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol 2017;3:194–201. 10.1001/jamaoncol.2016.3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bennouna J, Sastre J, Arnold D, et al. ; ML18147 Study Investigators. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 2013;14:29–37. 10.1016/S1470-2045(12)70477-1 [DOI] [PubMed] [Google Scholar]
- 29. Masi G, Salvatore L, Boni L, et al. ; BEBYP Study Investigators. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: final results of the randomized BEBYP trial. Ann Oncol 2015;26:724–30. 10.1093/annonc/mdv012 [DOI] [PubMed] [Google Scholar]
- 30. Giantonio BJ, Catalano PJ, Meropol NJ, et al. ; Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539–44. 10.1200/JCO.2006.09.6305 [DOI] [PubMed] [Google Scholar]
- 31. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499–506. 10.1200/JCO.2012.42.8201 [DOI] [PubMed] [Google Scholar]
- 32. Tabernero J, Yoshino T, Cohn AL, et al. ; RAISE Study Investigators. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol 2015;16:499–508. 10.1016/S1470-2045(15)70127-0 [DOI] [PubMed] [Google Scholar]
- 33. Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706–13. 10.1200/JCO.2009.27.6055 [DOI] [PubMed] [Google Scholar]
- 34. Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:2311–19. 10.1200/JCO.2007.13.1193 [DOI] [PubMed] [Google Scholar]
- 35. Seymour MT, Brown SR, Middleton G, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol 2013;14:749–59. 10.1016/S1470-2045(13)70163-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jonker DJ, O'Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007;357:2040–8. 10.1056/NEJMoa071834 [DOI] [PubMed] [Google Scholar]
- 37. Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007;25:1658–64. 10.1200/JCO.2006.08.1620 [DOI] [PubMed] [Google Scholar]
- 38. Grothey A, Van Cutsem E, Sobrero A, et al. ; CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12. 10.1016/S0140-6736(12)61900-X [DOI] [PubMed] [Google Scholar]
- 39. Mayer RJ, Van Cutsem E, Falcone A, et al. ; RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–19. 10.1056/NEJMoa1414325 [DOI] [PubMed] [Google Scholar]
- 40. Sartore-Bianchi A, Trusolino L, Martino C, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17:738–46. 10.1016/S1470-2045(16)00150-9 [DOI] [PubMed] [Google Scholar]
- 41. Nordlinger B, Sorbye H, Glimelius B, et al. ; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und-tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007–16. 10.1016/S0140-6736(08)60455-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nordlinger B, Sorbye H, Glimelius B, et al. ; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 2013;14:1208–15. 10.1016/S1470-2045(13)70447-9 [DOI] [PubMed] [Google Scholar]
- 43. van Hazel GA, Heinemann V, Sharma NK, et al. SIRFLOX: randomized phase III trial comparing First-Line mFOLFOX6 (Plus or minus bevacizumab) Versus mFOLFOX6 (Plus or minus bevacizumab) Plus selective internal radiation therapy in patients with metastatic colorectal Cancer. J Clin Oncol 2016;34:1723–31. 10.1200/JCO.2015.66.1181 [DOI] [PubMed] [Google Scholar]
- 44. Seymour MT, Maughan TS, Ledermann JA, et al. ; FOCUS Trial Investigators; National Cancer Research Institute Colorectal Clinical Studies Group. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet 2007;370:143–52. 10.1016/S0140-6736(07)61087-3 [DOI] [PubMed] [Google Scholar]
- 45. Koopman M, Antonini NF, Douma J, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet 2007;370:135–42. 10.1016/S0140-6736(07)61086-1 [DOI] [PubMed] [Google Scholar]
- 46. de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000;18:2938–47. 10.1200/JCO.2000.18.16.2938 [DOI] [PubMed] [Google Scholar]
- 47. Folprecht G, Grothey A, Alberts S, et al. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol 2005;16:1311–19. 10.1093/annonc/mdi246 [DOI] [PubMed] [Google Scholar]
- 48. Masi G, Loupakis F, Pollina L, et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg 2009;249:420–5. 10.1097/SLA.0b013e31819a0486 [DOI] [PubMed] [Google Scholar]
- 49. Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol 2005;23:3502–8. 10.1200/JCO.2005.10.017 [DOI] [PubMed] [Google Scholar]
- 50. Kabbinavar FF, Hambleton J, Mass RD, et al. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol 2005;23:3706–12. 10.1200/JCO.2005.00.232 [DOI] [PubMed] [Google Scholar]
- 51. Cassidy J, Tabernero J, Twelves C, et al. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol 2004;22:2084–91. 10.1200/JCO.2004.11.069 [DOI] [PubMed] [Google Scholar]
- 52. Díaz-Rubio E, Tabernero J, Gómez-España A, et al. ; Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. Phase III study of capecitabine plus oxaliplatin compared with continuous-infusion fluorouracil plus oxaliplatin as first-line therapy in metastatic colorectal cancer: final report of the Spanish Cooperative Group for the Treatment of Digestive Tumors Trial. J Clin Oncol 2007;25:4224–30. 10.1200/JCO.2006.09.8467 [DOI] [PubMed] [Google Scholar]
- 53. Cassidy J, Clarke S, Díaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 2008;26:2006–12. 10.1200/JCO.2007.14.9898 [DOI] [PubMed] [Google Scholar]
- 54. Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 2007;25:4779–86. 10.1200/JCO.2007.11.3357 [DOI] [PubMed] [Google Scholar]
- 55. Fuchs CS, Marshall J, Barrueco J, Randomized BJ. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C Study. J Clin Oncol 2008;26:689–90. 10.1200/JCO.2007.15.5390 [DOI] [PubMed] [Google Scholar]
- 56. Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229–37. 10.1200/JCO.2004.05.113 [DOI] [PubMed] [Google Scholar]
- 57. Grothey A, Sargent D. Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. J Clin Oncol 2005;23:9441–2. 10.1200/JCO.2005.04.4792 [DOI] [PubMed] [Google Scholar]
- 58. Loupakis F, Cremolini C, Salvatore L, et al. FOLFOXIRI plus bevacizumab as first-line treatment in BRAF mutant metastatic colorectal cancer. Eur J Cancer 2014;50:57–63. 10.1016/j.ejca.2013.08.024 [DOI] [PubMed] [Google Scholar]
- 59. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer—a GERCOR study. J Clin Oncol 2006;24:394–400. 10.1200/JCO.2005.03.0106 [DOI] [PubMed] [Google Scholar]
- 60. Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol 2009;27:5727–33. 10.1200/JCO.2009.23.4344 [DOI] [PubMed] [Google Scholar]
- 61. Chibaudel B, Tournigand C, Bonnetain F, et al. Platinum-sensitivity in metastatic colorectal cancer: towards a definition. Eur J Cancer 2013;49:3813–20. 10.1016/j.ejca.2013.07.150 [DOI] [PubMed] [Google Scholar]
- 62. Nordic Gastrointestinal Tumor Adjuvant Therapy Group. Expectancy or primary chemotherapy in patients with advanced asymptomatic colorectal Cancer: a randomized trial. J Clin Oncol 1992;10:904–11. 10.1200/JCO.1992.10.6.904 [DOI] [PubMed] [Google Scholar]
- 63. Price TJ, Townsend AR, Beeke C, et al. "Watchful waiting" for metastatic colorectal cancer antediluvian or an option to be considered again? Asia Pac JCO 2012;8:10–13. 10.1111/j.1743-7563.2011.01458.x [DOI] [PubMed] [Google Scholar]
- 64. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol 2015;26:288–300. 10.1093/annonc/mdu210 [DOI] [PubMed] [Google Scholar]
- 65. Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25:4575–80. 10.1200/JCO.2007.11.0833 [DOI] [PubMed] [Google Scholar]
- 66. Karoui M, Penna C, Amin-Hashem M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg 2006;243:1–7. 10.1097/01.sla.0000193603.26265.c3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Benoist S, Brouquet A, Penna C, et al. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol 2006;24:3939–45. 10.1200/JCO.2006.05.8727 [DOI] [PubMed] [Google Scholar]
- 68. Hishida T, Okumura T, Boku N, et al. Surgical outcome for pulmonary metastasis of colorectal cancer in the modern chemotherapy era: results of a retrospective Japanese multicenter study. J Clin Oncol 2014;32:5s (suppl; abstr 3528; ASCO 2014). [Google Scholar]
- 69. Ruers T, Punt C, Van Coevorden F, et al. ; EORTC Gastro-Intestinal Tract Cancer Group, Arbeitsgruppe Lebermetastasen und—tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO) and the National Cancer Research Institute Colorectal Clinical Study Group (NCRI CCSG). Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol 2012;23:2619–26. 10.1093/annonc/mds053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737–43. 10.1200/JCO.2003.04.187 [DOI] [PubMed] [Google Scholar]


