Abstract
Objective
Australia has a publicly funded universal healthcare system which heavily subsidises the cost of most registered anticancer drugs. The use of anticancer drugs that are unfunded, that is, not subsidised by the government, entails substantial out-of-pocket costs for patients. We sought to determine how frequently Australian medical oncologists discuss and prescribe unfunded anticancer drugs, and their attitudes and beliefs about their use.
Methods
Members of the Medical Oncology Group of Australia (MOGA) completed an online survey about their clinical practices over a recent 3-month period. A negative binomial regression model was used to examine the influence of respondent characteristics on the rate of discussions about, and prescription of, unfunded anticancer drugs.
Results
Of the 154 respondents (27% of 575 MOGA members), 92% had discussed and 68% had prescribed at least one unfunded anticancer drug in the last 3 months. Respondents reported discussing unfunded anticancer drugs with an average of 2.5 patients per month (95% CI 2.1 to 2.9), and prescribed them to an average of 0.9 patients per month (95% CI 0.7 to 1.2). The rate of discussing unfunded anticancer drugs was associated with being fully qualified (p=0.01), and being in a metropolitan practice (p=0.009), the rate of prescription was associated only with being in metropolitan practice (p=0.006). The concerns about discussing and prescribing unfunded anticancer drugs rated most important were as follows: ‘potential to cause financial hardship’ and ‘difficulty for patients to evaluate the benefits versus the costs’.
Conclusions
Australian medical oncologists frequently discuss and prescribe unfunded anticancer drugs, and are concerned about their patients having to face difficult decisions and financial hardship. Further research is needed to better understand the factors that affect how oncologists and patients value expensive, unfunded anticancer drugs.
Keywords: Anticancer drugs, Value, Out-of-pocket costs, Financial hardship
Key messages.
What is already known about this subject?
The price of new anticancer drugs has created challenges for patients and their oncologists.
Patients and oncologists have to grapple with determining the value of new anticancer drugs which can be difficult if the benefits are modest or uncertain, and the costs are high.
Patients who receive treatment with expensive anticancer drugs are often faced with a significant financial burden, which oncologists may spend time and effort trying to reduce.
What does this study add?
This study provides the first data estimating the frequency of discussion and prescription of expensive unfunded anticancer drugs by medical oncologists in a health system like Australia’s in which most anticancer drugs are publicly funded.
The study provides an insight into the attitudes and practices of Australian medical oncologists regarding unfunded anticancer drugs.
This study also highlights some important potential concerns and barriers to discussing and prescribing unfunded anticancer drugs in Australia.
How might this impact on clinical practice?
Even in publicly funded health systems, oncologists discuss and prescribe expensive unfunded anticancer drugs frequently and are concerned about causing their patients psychological distress and financial hardship.
Further research and education are needed to identify better ways of thinking and talking about unfunded anticancer drugs with patients, and to better understand the complex factors that contribute to how oncologists value expensive unfunded anticancer drugs, and their willingness to discuss and prescribe them.
Introduction
The number of drugs developed to treat cancer has greatly increased over recent years. Over 60 new anticancer drugs were approved by the USA Food and Drug Administration between 2005 and 2014, and a further 70 existing anticancer drugs had their indications expanded.1 The growth in the number of new anticancer drugs is welcomed by patients and oncologists as new treatments can increase response rates, delay progression, extend survival, improve the quality of life of patients with cancer and in some circumstances increase the chance of cure.
Along with the benefits of new anticancer drugs comes their considerable expense.2 The prices of new anticancer drugs have created challenges for payers, providers and patients.3 In predominantly publicly funded health systems like Australia’s, payers grapple with determining whether the benefits of a new anticancer drug are worth the extra cost. To be lawfully supplied in Australia, prescription drugs require marketing approval from the Therapeutic Goods Administration.4 If the drug is approved, pharmaceutical companies can submit applications to the Pharmaceutical Benefits Advisory Committee for drugs to be listed on the Pharmaceutical Benefits Scheme (PBS).5 Drugs listed on the PBS are provided to patients at a heavily subsidised price.
Even in Australia, where most prescription medicines are subsidised, the rising cost of anticancer drugs creates problems for patients.6 For example, it is often months, and sometimes years, from the publication of positive trial results to the listing of a drug on the PBS.7 If a new anticancer drug is not listed on the PBS, then accessing it may require patients to pay its full cost, which can amount to thousands of dollars per month.8 9 In some instances, pharmaceutical companies provide access to these drugs via compassionate access programmes or cost-sharing programmes; however, these still may involve substantial out-of-pocket costs. This means that the cost of treatment can become a major factor in decision making for cancer patients.10 Many patients will forego high-cost treatments altogether, or discontinue them early if the expense becomes prohibitive.11
A recent survey of medical oncologists from the USA indicated that the vast majority felt obliged to offer all available treatment options to their patients regardless of their cost.12 Discussing expensive treatment options can be difficult for oncologists who do not wish to impose a financial hardship on their patients, especially if the incremental benefit of the treatment is modest or uncertain.13 Oncologists may also spend time and effort trying to find ways to reduce or avoid this financial hardship for their patients, for example, by seeking alternative sources of funding.14 As the cost of anticancer drugs continues to rise, oncologists and their patients will increasingly have to face the difficulty of determining their value.15
Unfunded anticancer drugs are those that are not subsidised by the government and therefore entail substantial out-of-pocket costs to patients. A study of the practices and attitudes of Australian medical oncologists regarding disclosure of expensive unfunded anticancer drugs in 2006 found that almost half of the responding oncologists reported prescribing at least one unfunded anticancer drug.16 Since then, the available number of unfunded anticancer drugs has grown. A recent study at a single Australian institution found that almost half of the anticancer drug treatment protocols contained a drug that was not PBS listed.17 The extent to which Australian medical oncologists currently discuss and prescribe unfunded anticancer drugs is unknown. The aim of this study was to examine the current practices, attitudes and beliefs of Australian medical oncologists regarding the discussion and prescription of unfunded anticancer drugs.
Methods
We performed a cross-sectional survey of medical oncologists and medical oncology trainees throughout Australia. We asked respondents to recall their discussions about, and prescriptions of, unfunded anticancer drugs over the last 3 months. Based on previous definitions used in Australian studies, we defined unfunded anticancer drugs as any prescription anticancer drug that:
was not listed on the PBS;
was not used as part of a clinical trial or free access programme;
may require the patient to pay substantially more than standard pharmacy dispensing fees.8 16
Respondents also provided details about their personal and practice characteristics and their attitudes and opinions about issues related to unfunded anticancer drugs, including concerns about discussing and prescribing them.
The survey instrument was developed by three medical oncologists. A focus group of five medical oncologists was used to assess face validity, content validity and clarity, before wider distribution. The final version of the survey is available online (supplementary material).
esmoopen-2017-000170supp001.pdf (413.7KB, pdf)
Potential participants were members of the Medical Oncology Group of Australia (MOGA). MOGA is the peak national body representing medical oncologists in Australia. All members of MOGA (medical oncologists and medical oncology trainees) were invited to participate by email in August 2014. The email included a brief explanation of the survey, a participant information statement and a hypertext link to the survey. The survey took approximately 15 min to complete, and respondents were not offered any inducements to complete the survey. A reminder email was sent to all potential participants 1 month after the initial invitation. The study was approved by the University of Sydney Human Research Ethics Committee (Project number: 2014/173). All responses to the survey were anonymous and non-identifiable.
The analysis set comprised respondents who answered at least one question about the discussion or prescription of unfunded anticancer drugs. A negative binomial regression model was used to examine the influence of personal and professional characteristics on the rate of discussion and prescription of unfunded anticancer drugs. Characteristics were first tested individually, and then after adjusting for clinical workload (hours per week on average spent in outpatient clinics). Backwards elimination was used to develop a multivariable model comprising statistically significant, independent predictors adjusting for clinical workload.
Results
We received evaluable responses from 154 of the 575 (27%) medical oncologists in the MOGA database, including 142 who answered all questions. The characteristics of the 154 respondents are summarised in table 1. Qualified oncologists outnumbered trainees by 4:1, but more than half the respondents were 40 years or younger, and only 40% had more than 10 years’ experience in oncology practice. The numbers of females and males were similar. Most respondents practiced in public clinics in metropolitan areas.
Table 1.
Personal and professional characteristics of 154 responding oncologists
| Characteristic | n (%) |
| Age | |
| ≤30 | 4 (3) |
| 31–40 | 81 (52) |
| 41–50 | 34 (22) |
| 51–60 | 23 (15) |
| >60 | 12 (8) |
| Role | |
| Qualified oncologist | 30 (81) |
| Trainee oncologist | 124 (19) |
| Sex | |
| Female | 72 (47) |
| Male | 82 (53) |
| Years worked in medical oncology | |
| ≤10 | 91 (59) |
| 11–20 | 29 (19) |
| >20 | 34 (22) |
| Hours spent in outpatient clinics per week | |
| ≤20 | 89 (58) |
| >20 | 65 (42) |
| Hours spent in research per week | |
| ≤20 | 129 (84) |
| >20 | 25 (16) |
| Practice type | |
| Mostly public | 105 (68) |
| Mostly private | 21 (14) |
| Other* | 28 (18) |
| Practice location | |
| Mostly metropolitan | 121 (79) |
| Mostly regional/rural | 26 (17) |
| Other† | 7 (5) |
*Includes respondents with an equal mix of public and private practice, or no clinical practice.
†Includes respondents with an equal mix of metropolitan and regional/rural practice, or no clinical practice.
In a recent 3-month period, respondents currently in practice reported discussing unfunded anticancer drugs with an average of 2.5 patients per month (95% CI: 2.1 to 2.9), and prescribing unfunded anticancer drugs to an average of 0.9 patients per month (95% CI 0.7 to 1.2). Almost all of the respondents had discussed (99%) or prescribed (93%) an unfunded anticancer drug at some time in the past. Ninety-two per cent of respondents had discussed, and 68% had prescribed, an unfunded anticancer drug in the last 3 months. Sixty-nine per cent thought they were currently prescribing more unfunded anticancer drugs than they had 5 years ago, and 77% thought they would be prescribing more unfunded anticancer drugs in 10 years’ time than they are now.
Respondents reported discussing or prescribing unfunded anticancer drugs more often to patients considering palliative treatment (88% of respondents) versus adjuvant/curative treatment (12%); patients considering last-line treatment (68%) versus first-line treatment (32%); and patients younger than 70 (96%) versus older than 70 (4%). Respondents reported that, on average, patients or their support person initiated about 15% of discussions about unfunded anticancer drugs. Respondents reported that, on average, they recommended against treatment with an unfunded anticancer drug in about 27% of discussions.
Associations between the characteristics of respondents, and the rate of both discussion and prescription of unfunded anticancer drugs, are summarised in table 2. Working in private practice and working in a metropolitan practice were each associated with an increased rate of both discussion and prescription in univariable analyses adjusting for clinical workload. Metropolitan practice was the only variable significantly associated with both discussion and prescription rate in multivariable analysis. Being a qualified medical oncologist, rather than a trainee, was also significantly associated with the rate of discussion, but not with the rate of prescription, in both univariable and multivariable analyses.
Table 2.
Associations between respondents’ characteristics and the rates of discussion and prescription of unfunded anticancer drugs
| Outcome/Characteristic | Univariable analysis (adjusted for clinical workload) | Multivariable analysis (adjusted for clinical workload) | ||||
| Rate ratio | 95% CI | p Value | Rate ratio | 95% CI | p Value | |
| Discussion rate | ||||||
| Role | ||||||
| Qualified oncologist | 1.60 | 1.08 to 2.37 | 0.020 | 1.67 | 1.13 to 2.46 | 0.01 |
| Trainee oncologist (reference group) | – | – | ||||
| Sex | ||||||
| Male | 1.29 | 0.92 to 1.81 | 0.14 | – | ||
| Female (reference group) | ||||||
| Age (per decade) | 1.14 | 0.97 to 1.35 | 0.11 | – | ||
| Years worked in medical oncology (per decade) | 1.09 | 0.92 to 1.28 | 0.32 | – | ||
| Practice type | ||||||
| Mostly private/other | 1.66 | 1.17 to 2.36 | 0.005 | – | ||
| Mostly public (reference group) | ||||||
| Practice location | ||||||
| Mostly metropolitan | 1.61 | 1.08 to 2.39 | 0.02 | 1.68 | 1.14 to 2.48 | 0.009 |
| Mostly regional/rural/other (reference group) | ||||||
| Prescription rate | ||||||
| Role | ||||||
| Qualified oncologist | 1.63 | 0.94 to 2.83 | 0.08 | – | ||
| Trainee oncologist (reference group) | ||||||
| Sex | ||||||
| Male | 0.89 | 0.56 to 1.40 | 0.61 | – | ||
| Female (reference group) | ||||||
| Age (per decade) | 1.22 | 0.98 to 1.51 | 0.08 | – | ||
| Years worked in medical oncology (per decade) | 1.21 | 0.97 to 1.51 | 0.09 | – | ||
| Practice type | ||||||
| Mostly private/other | 1.70 | 1.03 to 2.80 | 0.04 | – | ||
| Mostly public (reference group) | ||||||
| Practice location | ||||||
| Mostly metropolitan | 2.20 | 1.25 to 3.87 | 0.006 | 2.20 | 1.25 to 3.87 | 0.006 |
| Mostly regional/rural and other (reference group) | ||||||
The median maximum out-of-pocket cost respondents estimated that their patients had paid, or would be expected to pay, for a course of an unfunded anticancer drug, typically lasting a few months, was A$7500 (around US$5800; €5400); range: A$200 to A$100 000. The frequency with which various methods were used to cover the costs of unfunded anticancer drugs is summarised in figure 1. Respondents estimated that 60% of prescriptions were partially subsidised through a pharmaceutical company access programme, and that approximately 30% of the prescriptions were fully paid for by their patients.
Figure 1.
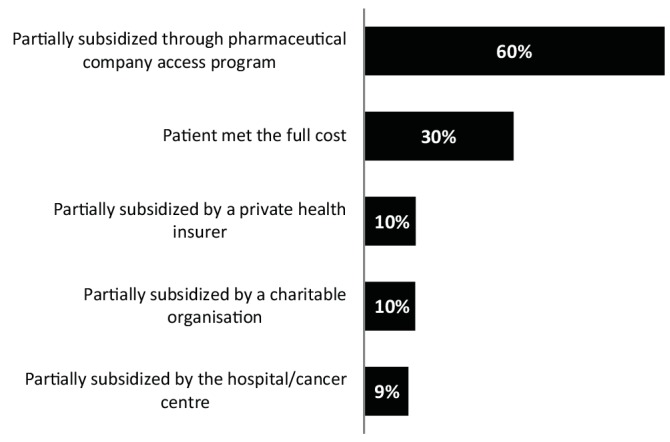
Estimated frequency of methods used to cover the cost of unfunded anticancer drugs (more than one method possible for each prescription).
Out-of-pocket costs to patients were reported to influence the willingness to prescribe an unfunded anticancer drug, ‘somewhat’ or ‘a lot’ by 83% (118/143) of respondents, and not at all by 6% (9/143) of respondents. Eleven per cent (16/143) of respondents felt it was never appropriate for a patient to pay for treatment with an unfunded anticancer drug. Just over half the respondents (55%, 78/142) said they were comfortable discussing out-of-pocket costs with patients.
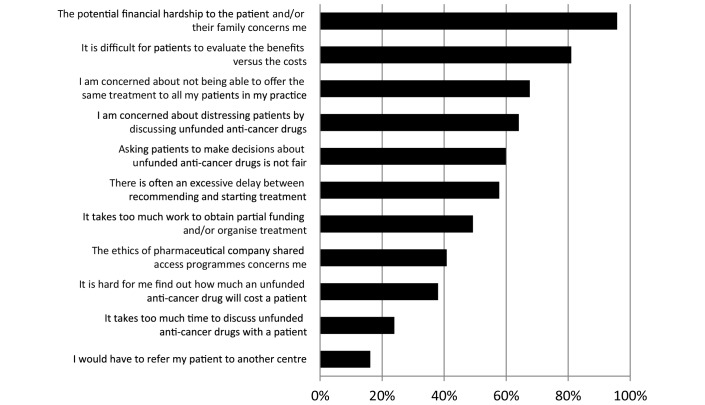
The concerns we presented about discussing and prescribing unfunded anticancer drugs that were rated most important by respondents were ‘potential to cause financial hardship’ and ‘difficulty for patients to evaluate the benefits versus the costs’ (figure 2). Concerns about the ‘time it takes to discuss unfunded anticancer drugs with a patient’ and ‘the need to refer a patient to another centre’ were rated least important.
Figure 2.
Percentage of responding oncologists who rated as important the specified concerns about discussing and prescribing unfunded anticancer drugs.
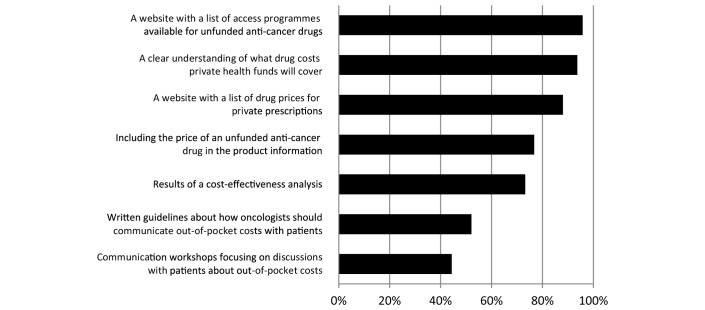
We presented a list of possible aids to facilitate discussions about unfunded anticancer drugs (figure 3). Those rated most useful by respondents were ‘a website with a list of access programmes available for unfunded anticancer drugs’ and ‘a clear understanding of what drug costs private health funds will cover’. Those rated least useful were ‘written guidelines about how oncologists should communicate out-of-pocket costs with patients’ and ‘communication workshops focusing on discussions with patients about out-of-pocket costs’.
Figure 3.
Percentage of responding oncologists who rated as useful the specified aids to facilitate discussion about unfunded anticancer drugs.
Discussion
This survey indicates that in a health system which is predominantly publicly funded, responding medical oncologists discussed unfunded anticancer drugs with approximately three patients per month, prescribed them to approximately one patient per month and that nearly all had prescribed an unfunded anticancer drug. This is much higher than a comparable study published 10 years ago which found that <50% of Australian medical oncologists had prescribed an unfunded anticancer drug.16
The median maximum out-of-pocket costs that respondents recalled their patients had paid, or were expected to pay, for a course of an unfunded anticancer drug was A$7500. This is a considerable amount of money in conjunction with other costs of care and may be unaffordable for many patients. Costs were likely to be much higher for the 30% of patients who were meeting the full cost of the drugs. Recommendations against treatment with an unfunded anticancer drug occur in over a quarter of discussions about them in our study but we did not ascertain whether the high cost was the main reason for recommending against their use. It is likely to be a factor given that the potential to cause financial hardship was the concern rated most highly by respondents when discussing or prescribing unfunded anticancer drugs. Other factors such as the type of evidence used to make recommendations, the strength of that evidence or the incremental benefit over less costly options may also be important, but we did not ascertain these in our study. Patient factors and disease factors are also likely to have a significant role as respondents reported that it was more likely they would discuss and prescribe unfunded anticancer drugs to patients who were younger, had incurable disease or were considering last-line therapy. It would be interesting to explore how much willingness to discuss or prescribe an unfunded anticancer drug in the last-line setting correlates with the level of discomfort of the oncologist in discussing palliative care.
The proportion of respondents (16% (23/142)) that reported being uncomfortable with discussing the out-of-pocket costs of unfunded anticancer drugs was lower than anticipated. An Australian study published in 2008 reported that discussing high-cost drugs was one of the most difficult communication issues for Australian oncologists.13 However, it may be that levels of discomfort have decreased over time as the frequency of these discussions has increased. Like their North American counterparts, most Australian oncologists think that out-of-pocket costs affect their willingness to prescribe an unfunded anticancer drug, a finding that warrants further research.11 18
Respondents reported that approximately six of ten occasions they prescribed unfunded anticancer drugs involved partial subsidies via pharmaceutical company access programmes. Although these programmes allow earlier access to novel therapies that are not yet PBS listed, their widespread use raises a number of ethical dilemmas reported to be a concern by 41% of respondents, such as their use as a medical marketing tool, the changing requirements of the programmes depending on commercial imperatives and the favouring of patients able to afford substantial contributions associated with the cost-sharing programmes.19 Pharmaceutical companies are not permitted to advertise these programmes directly to patients in Australia, and this may explain why over a third of respondents reported it was hard for them to find the costs of unfunded anticancer drugs. This may also be why respondents judged that the most useful aids for clinical practice would be better access to information about the costs and methods of accessing unfunded anticancer drugs. This lack of knowledge or resources about the costs of care was rated the most important barrier to cost discussions by US oncologists in a recent survey.12
Respondents in predominantly metropolitan practices were more likely to discuss and prescribe unfunded anticancer drugs than those in predominantly regional or rural practices. Possible explanations include differences in treatment preferences of oncologists and/or patients in regional or rural areas, and greater barriers to accessing unfunded drugs (or information about them) in these areas. The lower likelihood that medical oncology trainees discussed unfunded anticancer drugs is likely to reflect their lower confidence and level of responsibility, but also supports the need for education about discussing unfunded anticancer drugs.
The main strengths of our study are that it reflects the contemporary practice and attitudes of Australian medical oncologists and contributes to the growing evidence that the high cost of new anticancer drugs is a major concern and influence on the practice of medical oncologists. It also provides the first information about the frequency of their discussions about, and prescription of, unfunded anticancer drugs. The main limitation of our study is the response rate of 27%, typical of physician surveys, and similar to a US study about communicating the costs of therapy.20 21 The age, gender and proportion of trainees among responders were similar to that of the general MOGA membership, but this does not ensure that our respondents’ answers would accurately reflect those of non-responders. Recall bias is another limitation because the study relied on medical oncologists’ recollections of past practice. Responding oncologists may have overestimated the frequency of their discussions and prescriptions. However, even so, it is clear that the issue of unfunded anticancer drugs is sufficiently common and important to warrant further research and attention.
This study shows that Australian medical oncologists frequently discuss and prescribe unfunded anticancer drugs, and that they are concerned about causing their patients psychological distress and financial hardship. Our study suggests that medical oncologists would value better information about the costs to their patients and methods for accessing unfunded anticancer drugs. Research and education are needed to identify and implement better ways of thinking and talking about unfunded anticancer drugs with patients, and to better understand the complex factors that contribute to how oncologists value expensive unfunded anticancer drugs, and their willingness to discuss and prescribe them.
Footnotes
Contributors: All authors have made substantial contributions to the conception and design of the work, and have analysed and/or interpreted the data. All authors contributed to revision of the manuscript and have given approval for the final version to be published. As the corresponding author, I agree to be responsible for the content as guarantor. No contributor has been omitted from the list of authors. The specific work undertaken by each author is outlined below.
DJK: conceived and developed the research proposal and methods, collected and analysed the data, interpreted the findings and drafted and revised the manuscript.
LM: contributed to the research proposal, interpretation of findings and revision of the manuscript.
AM: contributed to the research proposal and methods, analysed the data, interpreted the findings and revised the manuscript.
DF: contributed to the research proposal, interpretation of the findings and revision of the manuscript.
MRS: conceived the research proposal and methods and contributed to analysis of the data, interpretation of the findings and revision of the manuscript.
Funding: DJK has been supported by a National Health and Medical Research Council Postgraduate Scholarship and a Sydney Catalyst Top-Up Research Scholar Award.
Competing interests: None declared.
Ethics approval: University of Sydney Human Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Unpublished data are available on request by contacting the corresponding author.
References
- 1. Masters GA, Krilov L, Bailey HH, et al. Clinical cancer advances 2015: annual report on progress against cancer from the American Society of clinical oncology. J Clin Oncol 2015;33:786–809. 10.1200/JCO.2014.59.9746 [DOI] [PubMed] [Google Scholar]
- 2. Sullivan R, Peppercorn J, Sikora K, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol 2011;12:933–80. 10.1016/S1470-2045(11)70141-3 [DOI] [PubMed] [Google Scholar]
- 3. Berry SR, Evans WK, Strevel EL, et al. Variation and consternation: access to unfunded cancer drugs in Canada. J Oncol Pract 2012;8:35–9. 10.1200/JOP.2011.000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Australian Government Department of Health. Therapeutic goods administration. TGA Basics. https://www.tga.gov.au/tga-basics (accessed February 25, 2017). [Google Scholar]
- 5. Australian Government Department of Health: guidelines for preparing a submission to the pharmaceutical benefits advisory committee-version 5.0. https://pbac.pbs.gov.au/content/information/files/pbac-guidelines-version-5.pdf (accessed September 14, 2016).
- 6. Australian Government Department of Health: Australian Statistics on Medicines 2014. http://www.pbs.gov.au/statistics/asm/2014/australian-statistics-on-medicines-2014.pdf (accessed September 14, 2016).
- 7. Lewis JR, Lipworth W, Kerridge I, et al. Dilemmas in the compassionate supply of investigational Cancer drugs. Intern Med J 2014;44:841–5. 10.1111/imj.12530 [DOI] [PubMed] [Google Scholar]
- 8. Kaser E, Shaw J, Marven M, et al. Communication about high-cost drugs in oncology--the patient view. Ann Oncol 2010;21:1910–4. 10.1093/annonc/mdq068 [DOI] [PubMed] [Google Scholar]
- 9. Australian Government Department of Health. Pharmaceutical Benefits Scheme. PBS Frequently Asked Questions http://www.pbs.gov.au/info/general/faq (accessed February 25, 2017). [Google Scholar]
- 10. Stump TK, Eghan N, Egleston BL, et al. Cost concerns of patients with Cancer. J Oncol Pract 2013;9:251–7. 10.1200/JOP.2013.000929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berry SR, Bell CM, Ubel PA, et al. Continental divide? the attitudes of US and Canadian oncologists on the costs, cost-effectiveness, and health policies associated with new cancer drugs. J Clin Oncol 2010;28:4149–53. 10.1200/JCO.2010.29.1625 [DOI] [PubMed] [Google Scholar]
- 12. Altomare I, Irwin B, Zafar SY, et al. Physician experience and attitudes toward addressing the cost of cancer care. J Oncol Pract 2016;12:247–8. 10.1200/JOP.2015.007401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dimoska A, Girgis A, Hansen V, et al. Perceived difficulties in consulting with patients and families: a survey of australian cancer specialists. Med J Aust 2008;189:612–5. [DOI] [PubMed] [Google Scholar]
- 14. Chan KK, Wong B, Siu LL, et al. Less than ideal: how oncologists practice with limited drug access. J Oncol Pract 2012;8:190–5. 10.1200/JOP.2011.000337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mileshkin L, Schofield PE, Jefford M, et al. To tell or not to tell: the community wants to know about expensive anticancer drugs as a potential treatment option. J Clin Oncol 2009;27:5830–7. 10.1200/JCO.2009.22.7793 [DOI] [PubMed] [Google Scholar]
- 16. Thomson J, Schofield P, Mileshkin L, et al. Do oncologists discuss expensive anti-cancer drugs with their patients? Ann Oncol 2006;17:702–8. 10.1093/annonc/mdj136 [DOI] [PubMed] [Google Scholar]
- 17. Mellor JD, Van Koeverden P, Yip SW, Swk Y, et al. Access to anticancer drugs: many evidence-based treatments are off-label and unfunded by the Pharmaceutical Benefits Scheme. Intern Med J 2012;42:1224–9. 10.1111/j.1445-5994.2012.02751.x [DOI] [PubMed] [Google Scholar]
- 18. Neumann PJ, Palmer JA, Nadler E, et al. Cancer therapy costs influence treatment: a national survey of oncologists. Health Aff 2010;29:196–202. 10.1377/hlthaff.2009.0077 [DOI] [PubMed] [Google Scholar]
- 19. Azad A, Franco M. Co-funded expanded access programmes for new oncology drugs: creating a two-tier system for australian cancer patients? Intern Med J 2013;43:843–4. 10.1111/imj.12126 [DOI] [PubMed] [Google Scholar]
- 20. Schrag D, Hanger M. Medical oncologists' views on communicating with patients about chemotherapy costs: a pilot survey. J Clin Oncol 2007;25:233–7. 10.1200/JCO.2006.09.2437 [DOI] [PubMed] [Google Scholar]
- 21. Martins Y, Lederman RI, Lowenstein CL, et al. Increasing response rates from physicians in oncology research: a structured literature review and data from a recent physician survey. Br J Cancer 2012;106:1021–6. 10.1038/bjc.2012.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2017-000170supp001.pdf (413.7KB, pdf)