Abstract
Purpose
Our previous works demonstrated the ability of metformin to revert resistance to gefitinib, a selective epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, in non-small-cell lung cancer (NSCLC) EGFR/LKB1 wild-type (WT) cell lines. However, the optimal dose of metformin to be used in non-diabetic patients still remains to be defined. The phase I–II trial METformin in Advanced Lung cancer (METAL) was designed to identify the maximum tolerated dose and to evaluate safety and activity of metformin combined with erlotinib in second-line treatment of patients with stage IV NSCLC, whose tumours harbour the WT EGFR gene.
Patients and methods
We report results from the safety run-in part designed to detect acute toxicities, to study pharmacokinetics and to identify the recommended phase II dose (RPD) to be used for the following phase of the study. In the run-in phase, metformin treatment was administered according to a dose escalation scheme and, subsequently, combined with erlotinib.
Results
Twelve patients were enrolled. Common adverse events were diarrhoea, decreased appetite, abdominal pain, vomiting and skin toxicity, mostly reversible with symptomatic medical treatment. Dose-limiting toxicities were vomiting and diarrhoea registered in the initial cohort receiving metformin 2000 mg plus erlotinib at 150 mg die, which was declared the maximum administered dose. Only one of nine patients treated at the next lower dose of 1500 mg of metformin plus erlotinib at 150 mg experienced G3 gastrointestinal toxicity. Metformin plasma-concentration profile confirmed the trend already observed in non-diabetic population. Glycemic profiles showed stability of the blood glucose level within the physiological range for non-diabetic subjects. At a follow-up of 30 weeks, six (50%) patients experienced a disease control (5 SD and 1 partial response).
Conclusions
The RP2D of metformin dose was defined at 1500 mg/day to be combined with erlotinib 150 mg.
Trial registration number
EudraCT number: 2014-000349-59.
Keywords: NSCLC, metformin, erlotinib, LKB1
Key messages.
What is already known about this subject?
A series of epidemiological evidences suggested a reduced incidence of cancer in patients treated with metformin compared with those taking other therapies.
In our preclinical model, the combination of metformin with an epidermal growth factor receptor (EGFR) inhibitor resulted synergistic in terms of inhibition of proliferation and induction of apoptosis, in particular in those cell lines harbouring wild-type LKB1 gene.
What does this study add?
This phase I/II dose-escalation study evaluated the combination of metformin and erlotinib in second line treatment of stage IV non-small-cell lung cancer EGFR wild type.
The safety profile was clinically acceptable and maximum tolerated dose was identified.
Preliminary activity data were obtained.
How might this impact on clinical practice?
This clinical experience demonstrated a therapeutic role of metformin in non-diabetic people affected by non-small-cell lung cancer.
The phase II of the trial, by enrolling a higher number of patients, will probably provide more information on the activity of the combination and more instruments for the identification of patient to be treated.
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide.1 Advances in the fields of cancer chemoprevention and therapy have the potential to reduce lung cancer-related mortality.2 Patients with type 1 or 2 diabetes often have clinical risk factors for the development of cancer. The correlation between diabetes and increased cancer risk has been supposed since 1910 by GD Maynard, and 100 years later a joint conference of the American Diabetes Association and the American Cancer Society led to a consensus that indicated an association between diabetes and the incidence of cancers.3 4 Among patients with diabetes, a higher relative risk has been observed for most cancers, including colon, lung, endometrium, rectum and breast. Since the first report of Evans et al, confirmed in a larger epidemiological study, a series of evidences suggested a protective role of metformin with reduced risk of cancer, compared with other antidiabetic treatments (sulfonylurea, insulin) in patients with diabetes.5 Notably, metformin mediates approximately a 30% reduction in lifetime of cancer risk in patients with diabetes.6–9 Experimental results show that metformin inhibits prostate and breast cancer cell growth in vitro, delays tobacco carcinogen-induced lung cancer onset in mice and delays spontaneous tumour development in mice.10
Metformin’s role as a chemopreventive drug in non-small-cell lung cancer (NSCLC) is still an object of debate. More preclinical data support its role as an adjuvant drug in the treatment of lung cancer, in combination with chemotherapy or targeted molecular drugs. This evidence led our group to examine the effects of combined treatment of metformin with gefitinib (ZD1839; Iressa), a reversible tyrosine kinase inhibitor (TKI) of the epidermal growth factor receptor (EGFR), in a panel of human NSCLC cell lines with a different sensitivity to the EGFR TKI, including an in vitro model of acquired resistance developed from the sensitive human lung adenocarcinoma CALU-3 cell line.11
The combination of metformin with gefitinib resulted synergistic in term of inhibition of proliferation and induction of apoptosis, in particular in those cell lines harbouring wild-type LKB1 gene. Of interest, such effects were shown also in NSCLC cell lines resistant to the EGFR TKI, suggesting that metformin could revert resistance to gefitinib. These effects were the consequence of the downregulation of key intracellular mediators of growth factor-activated cell survival and proliferation, such as phosphorylated MAPK (mitogen activated protein kinase), Akt and members of mammalian target of rapamycin (mTOR) pathway.11 However, the right dose of metformin to be tested in combination with other therapeutic drugs has still to be defined.
Based on these preclinical considerations, the current phase I–II trial was designed to identify the maximum olerated ose (MTD) and to evaluate safety and activity of metformin combined with erlotinib in second-line treatment of patients with stage IV NSCLC whose tumours harbor the wild type EGFR gene. Here we report results from the safety run-in phase of the study.12
Methods
Study design
METAL trial is a multicentre, open label phase I–II study, designed to evaluate the safety and activity of metformin combined with erlotinib as second-line therapy in non-diabetic patients with stage IV NSCLC. This two-part trial consisted of a safety run-in part of metformin combined with erlotinib followed by a phase II part of metformin with erlotinib, as a second-line treatment in subjects with EGFR wild-type NSCLC.
The primary objective of the safety run-in part was to determine the MTD and the recommended phase II dose (RP2D) of metformin to combine with erlotinib. Secondary end points included the assessment of pharmacokinetics (PK), safety, tolerability and antitumour activity of the experimental treatment. Additional secondary end-points were to explore candidate markers or tumour characteristics predicting antitumour activity.
Eligibility
Study entry was limited to patients aged >18 years with histologically or cytologically confirmed NSCLC previously treated, with Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 1. Oxygen saturation >93% and adequate bone marrow, renal and liver function were required. Patients with asymptomatic, stable, treated brain metastases were eligible for trial participation. A non-platinum-based first-line therapy was allowed. Main exclusion criteria were: EGFR/ALK (anaplastic lymphoma kinase)-mutated histologically proven NSCLC, diabetes mellitus, clinically significant cardiovascular disease, previous exposure to EGFR TKIs, immunotherapy, hormonal therapy and radiotherapy within 2 weeks before entering the study. Institutional review board-approved informed consent was obtained for every patient before initiation of any trial-specific procedure or treatment.
Overall, this study was conducted in accordance with the principles of ‘good clinical practice’, and all applicable regulatory requirements.
Treatment
The run-in phase was designed to detect acute toxicities and activity of metformin in non-diabetic patients. Metformin treatment followed a dose escalation scheme, starting with a dose of 500 mg twice a day, orally with meals (day 0). At day 4, a metformin dose increase was planned at 500 mg three times a day. At day 8, metformin was increased to 1000 mg twice a day. At day 12, it was combined with erlotinib 150 mg daily.
An initial cohort of three patients (cohort 1) was planned to receive a maximum dose of metformin 2000 mg plus 150 mg of erlotinib. If dose-limiting toxicity (DLT) would not be observed up to 2 weeks of metformin and erlotinib combination treatment, an additional cohort of three patients would reach a total dose of 3000 mg of metformin (1000 + 2000 mg) on day 12 and erlotinib 150 mg was planned to be added to 3000 mg of metformin at day 16. On the contrary, if DLT had occurred in two or more patients treated at the 2000 mg dose of metformin level plus 150 mg of erlotinib, this dose would be declared the maximally administered dose (MAD) and three additional patients would be entered into the next lower dose level with metformin at 1500 mg and erlotinib at 150 mg.
After all, when patients in the second cohort had received 6 weeks of treatment with no safety concerns, other six additional patients will be enrolled in the same dose level to confirm the safety of the dose which will be used as RP2D. DLT is defined as any grade 3 or more non haematological or haematological toxicity or any treatment delay for more than 2 weeks due to trial treatment-related adverse effects.
Before starting any study treatment, CT scans of the brain, chest and abdomen, 12-lead ECG, and a bone scan were required. Tumour response, based on investigator-assessed Response Evaluation Criteria in Solid Tumors (RECIST; V.1.1), was evaluated every 10 weeks. All patients who prematurely discontinued treatment for any reason were followed for survival. A CT scan with contrast of the brain, chest and abdomen and bone scan was done within 28 days prior to study entry (screening visit) and every 10 weeks.
In all enrolled patients, a fasting glucose monitoring with venous blood samples was performed at any visit. All patients were fully informed on the signs and symptoms of hypoglycaemia in order to perform promptly a glycemic self-monitoring by a blood glucose meter (Accu-Chek Aviva, Roche Diagnostics, Indianapolis, Indiana). All patients were thoroughly trained by experienced staff to the use of the blood glucose monitor. According to Wipple’s triad, an hypoglycaemic episode was defined as a blood glucose <50 mg/dL, presence of signs and or symptoms and, finally, prompt disappearance of these clinical phenomena by correction of hypoglycaemia.
Pharmacokinetic
Plasma-concentration timed profiles of metformin were determined in the run-in phase by timed withdrawals of peripheral venous blood at time 0, 2 hours (T2h) and 5 hours (T5h) after taking the first dose of metformin. The collected data were used for therapeutic drug monitoring (TDM) and for pharmacokinetics evaluation in non-diabetic patients. A 5 mL sample of peripheral blood for TDM was collected in tubes containing EDTA. In case of adverse event, a further TDM sampling was performed. Additional blood samples for TDM were carried out while increasing the dose of metformin and in case of adverse events.
Next Generation Sequencing analysis
Tumour samples were analysed with the Ion AmpliSeq Colon and Lung Cancer Panel (Life Technologies) using Ion Torrent semiconductor sequencing. The panel contains primer pairs to analyse over 500 known mutations and eventually novel mutations in 87 hotspot regions of the following 22 genes: ALK, EGFR, ERBB2, ERBB4, FGFR1, FGFR2, FGFR3, MET, DDR2, KRAS, PIK3CA, BRAF, AKT1, PTEN, NRAS, MAP2K1, STK11, NOTCH1, CTNNB1, SMAD4, FBXW7, TP53.
Statistical analysis
The final sample size in the phase I part of the study depends on the number of DLTs observed at the different dose levels.
Results
Twelve patients were enrolled in the safety run-in part of the study. Patient characteristics and baseline demographics were summarised in table 1.
Table 1.
Patient characteristics
| Characteristics | |
| Age (years) | |
| Median | 65.8 |
| Range | 59–75 |
| Sex | |
| Male | 10 (83%) |
| Female | 2 (16%) |
| ECOG performance status | |
| 0 | 5 (42%) |
| 1 | 7 (58%) |
| Stage at diagnosis | |
| IIIB | 3 (25%) |
| IV | 9 (75%) |
| Histological type | |
| Squamous | 3 (25%) |
| Adenocarcinoma | 8 (66%) |
| NOS | 1 (8%) |
| Smoking status | |
| Former smoker | 6 (50%) |
| Smoker | 5 (41%) |
| Never smoker | 1 (8%) |
ECOG, Eastern Cooperative Oncology Group; NOS, not otherwise specified.
The median age was 65.8 (range 59–75) and 58% of patients had ECOG PS 1. The majority of patients were smokers and former smokers (91%), with adenocarcinoma histology (66%) and presented with IV stage at diagnosis (75%).
An initial cohort of three patients (cohort 1) received metformin at maximum dose of 2000 mg plus erlotinib at 150 mg die. As grade 3 toxicity occurred in two of three patients treated at the same dose level, the dose escalation was stopped and this dose level was declared the MAD (table 2).
Table 2.
DLTs registered during the first 2 weeks of combined treatment of metformin and erlotinib
| Dose level | Dose of metformin (mg) | Dose of erlotinib (mg) | Patients (n) | DLT (patients, n) |
| 1 | 2000 | 150 | 3 | Grade 3 vomiting (1), Grade 3 diarrhoea (1) |
| 2 | 1500 | 150 | 9 | None |
DLT, dose-limiting toxicity.
Grade 3 toxicity was represented by gastrointestinal disorders such as vomiting and diarrhoea, concomitantly with grade 1–2 abdominal pain and loss of appetite.
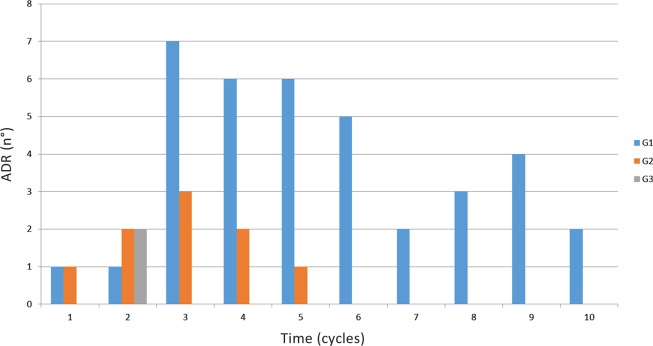
Based on design of safety run-in-part, we decided that three additional patients would be treated at the next lower dose level (1500 mg of metformin) and the cohort of metformin at 3000 mg was not performed. Only in one case grade 3 gastrointestinal toxicity (nausea) occurred with the assumption of 1500 mg metformin and erlotinib. Six additional patients were treated at the same dose level to confirm the safety data. Toxicity in this cohort of patients was mostly represented by G1 gastrointestinal toxicity such as nausea, vomiting, diarrhoea, abdominal pain and loss of appetite, and, in almost all cases, the symptomatic medical treatment led to complete symptoms resolution (figure 1)(table 3).
Figure 1.
Toxicity (grade 1–3) in all patients and dose levels stratified by cycle. ADR, adverse drug reaction.
Table 3.
Toxicity (grade 1–3) in all cycles stratified by dose level.
| Dose level | Vomiting | Nausea | Diarrhoea | Abdominal pain | Loss of appetite | Skin toxicity | ||||||||||||
| G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | |
| 1 | 2 (16.6) | 1 (8.3) | 1 (8.3) | 6 (50) | 1 (8.3) | 0 (0) | 3 (25) | 1 (8.3) | 1 (8.3) | 3 (25) | 1 (8.3) | 0 (0) | 6 (50) | 0 (0) | 0 (0) | 1 (8.3) | 0 (0) | 0 (0) |
| 2 | 1 (8.3) | 0 (0) | 0 (0) | 3 (25) | 0 (0) | 0 (0) | 2 (16.6) | 1 (8.3) | 0 (0) | 2 (16.6) | 1 (8.3) | 0 (0) | 4 (33.3) | 0 (0) | 0 (0) | 2 (16.6) | 2 (16.6) | 1 (8.3) |
Data presented as n (%).
In particular, during the dose escalation phase, no toxicity with metformin 1000 mg/day was verified, while two cases of G2 gastrointestinal toxicity occurred with metformin 1500 mg/day.
Only in one patient G3 skin toxicity associated with erlotinib administration was recorded. The first episode of G3 skin rash appeared after 4 weeks of combined treatment, regressed after discontinuation of treatment for 7 days (patient and investigator's decision).
Metformin plasma-concentration timed profiles confirmed the trend previously reported for healthy non-diabetic patients13 with a rapid increase at T2h and a subsequent decrease at T5h. Serum concentrations at day 4, 8 and 12, after about 8 hours from metformin assumption, remained in the therapeutic range of 1–2mg/L, in accordance with literature data.
Moreover, self-monitoring blood glucose (at fasting and 2 hours after meals performed by blood glucose meter, every three–five days) showed physiological blood glucose homeostasis, both during the dose escalation phase of metformin and during the combination with erlotinib. During entire study, none of the patients had hypoglycaemic events, and none experienced glycemic values less than 60 mg/dL.
CT scan of brain, chest and abdomen and bone scan were performed every 10 weeks. In particular, radiological assessments performed at 10 weeks showed disease progression with appearance of new hepatic lesions in three patients; full-body CT scan performed at 20 weeks, in additional, revealed three patients with progression of disease in lung, brain and adrenal glands, respectively (figure 2).
Figure 2.
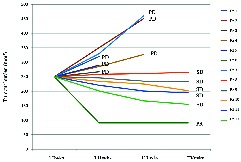
Best tumour response. wks, weeks.
After 30 weeks of combination treatment, stable disease in five patients and a partial response in one patient, according to RECIST criteria, were recorded. At a follow-up of 20 months, one patient is still on treatment (figure 2).
For 6 of 12 (50%) patients, formalin-fixed paraffin-embedded tumour tissues were collected and analysed with Ion AmpliSeq Colon and Lung Cancer Panel. A 2% sensitivity threshold has been set for this panel following the results of a validation study.14 High-quality DNA was extracted from all six samples, thus multiple gene mutation assessment was possible in all these cases. Next Generation Sequencing (NGS) analysis did not evidence the presence of significant mutations or deletions in the panel of genes tested.
Conclusions
The initial interest in metformin as an anticancer agent has come from clinical and epidemiological research, which has provided an empiric basis for its evaluation in the clinical setting.
Beneficial effects of metformin have been strongly observed in the treatment of polycystic ovarian syndrome, non-alcoholic fatty liver disease and premature puberty.15–17 These clinical experiences demonstrated both a therapeutic role of metformin forward diabetes and confirmed also in non-diabetic people the low incidence of side effects, in particular hypoglycaemia, with the same rate of gastrointestinal effects observed in diabetic subjects. Considering the mechanism of action, the clinical safety, the well characterised pharmacodynamics profile and the low cost of metformin make it an ideal candidate for development as an anticancer agent.
On this basis, hypothesising a great clinical potentiality, we designed this study with an association of metformin plus erlotinib, two drugs with possible gastrointestinal side effects, to assess the MTD of metformin. However, a number of issues need further consideration before the development of metformin as a cancer therapy.
First, considering that the majority of preclinical studies with metformin have been conducted with doses of metformin higher than those achieved in patients with diabetes, the usual antidiabetic dose of metformin may not reflect the antitumour efficacy of the drug. In this view, our study has demonstrated that doses higher than 1500 may be too toxic for non-diabetic patients when combined with erlotinib.
Probably, the strategy to divide the metformin dose into three daily administrations may have reduced the incidence of these side effects of metformin, as occurs in patients with diabetes.
However, it has been shown that the positive charge of metformin could promote its accumulation within the mitochondrial matrix by 1 000-fold (>20 mmol/L). Indeed, metformin accumulates in tissues at concentrations several fold higher than those in blood,18–20 indicating that concentrations of metformin similar to those used in pre-clinical models (1–10 mmol/L) might be attained also at lower doses.
The RP2D of metformin achieved in the run-in phase of the METAL study demonstrated to be well tolerated, mostly showing G1–2 gastrointestinal and skin toxicity. Treatment of adverse events was manageable and toxicity resulted reversible.
As demonstrated in previous pharmacokinetic studies metformin treatment did not alter glycemic profile in patients with cancer, thus rendering the drug fit also for non-diabetic patients.
Although on a small number of patients, preliminary activity of the combination of metformin and erlotinib was very encouraging. Median progression-free survival (mPFS) was found at 20 weeks of treatment, and by the time of this work writing, one patient is still on treatment (20 months on treatment). In this setting of patients, historical data from the TAILOR (Tarceva Italian Lung Optimization tRial) study21 refer the mPFS in the EGFR TKI-treated patients as 2.4 months (10 weeks).
Metformin may exert these effects by activation of AMP-activated protein kinase, which while inhibiting protein synthesis and gluconeogenesis, results in the inhibition of the mTOR, a downstream effector of growth factor signalling, commonly activated in malignant cells and associated with resistance to anticancer drugs.
The combination with erlotinib represents a synergistic inhibition of this pathway together with the blockade of the MAPK signalling.
The goal is to define the setting of patients, which may benefit from the combination of metformin and TKIs through the identification of predictors of response to treatment and biomarkers. Our preclinical work highlighted the role of WT LKB1 as predictor of response by such combination.11 NGS analysis on 50% of enrolled patients did not evidenced the presence of any significant alteration on LB1 and other genes, which confirm our previous observation; however, the absence of negative control leaves open the question of biomarkers predicting benefit from such combination.
The phase II of the trial, by enrolling a higher number of patients, will probably provide more informations on the activity of the combination and more instruments for the identification of patient to be treated.
Acknowledgments
The METAL study is sponsored by the Department of Clinical and Experimental Medicine and Surgery “F. Magrassi and A. Lanzara”, Second University of Naples.
Footnotes
Funding: This work has been supported by Associazione Italiana Per La Ricerca Sul Cancro (AIRC)-Project MFAG 2013-N.14392. The authors have no other relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA: A Cancer Journal for Clinicians 2009;59:225–49. 10.3322/caac.20006 [DOI] [PubMed] [Google Scholar]
- 2. Bunn PA. Worldwide overview of the current status of lung cancer diagnosis and treatment. Arch Pathol Lab Med 2012;136:1478–81. 10.5858/arpa.2012-0295-SA [DOI] [PubMed] [Google Scholar]
- 3. Maynard GD. A statistical study in cancer death-rates. Biometrika 1910;7:276–304. 10.1093/biomet/7.3.276 [DOI] [Google Scholar]
- 4. Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010;33:1674–85. 10.2337/dc10-0666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330:1304–5. 10.1136/bmj.38415.708634.F7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Libby G, Donnelly LA, Donnan PT, et al. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 2009;32:1620–5. 10.2337/dc08-2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mazzone PJ, Rai H, Beukemann M, et al. The effect of metformin and thiazolidinedione use on lung cancer in diabetics. BMC Cancer 2012;12:410 10.1186/1471-2407-12-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murtola TJ, Tammela TL, Lahtela J, et al. Antidiabetic medication and prostate cancer risk: a population-based case-control study. Am J Epidemiol 2008;168:925–31. 10.1093/aje/kwn190 [DOI] [PubMed] [Google Scholar]
- 9. Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle 2009;8:909–15. 10.4161/cc.8.6.7933 [DOI] [PubMed] [Google Scholar]
- 10. Memmott RM, Mercado JR, Maier CR, et al. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer Prev Res 2010;3:1066–76. 10.1158/1940-6207.CAPR-10-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morgillo F, Sasso FC, Della Corte CM, et al. Synergistic effects of metformin treatment in combination with gefitinib, a selective EGFR tyrosine kinase inhibitor, in LKB1 wild-type NSCLC cell lines. Clin Cancer Res 2013;19:3508–19. 10.1158/1078-0432.CCR-12-2777 [DOI] [PubMed] [Google Scholar]
- 12. Fasano M, Della Corte CM, Capuano A, et al. A multicenter, open-label phase II study of metformin with erlotinib in second-line therapy of stage IV non-small-cell lung cancer patients: treatment rationale and protocol dynamics of the METAL trial. Clin Lung Cancer 2015;16:57–9. 10.1016/j.cllc.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 13. Sambol NC, Chiang J, O'Conner M, et al. Pharmacokinetics and pharmacodynamics of metformin in healthy subjects and patients with noninsulin-dependent diabetes mellitus. J Clin Pharmacol 1996;36:1012–21. 10.1177/009127009603601105 [DOI] [PubMed] [Google Scholar]
- 14. Normanno N, Petraroli R, Rico A, et al. Abstract 36: The OncoNetwork Consortium: A European Collaborative Research study on the development of an Ion AmpliSeq gene panel targeting hot spots in colon and lung cancers. Cancer Res 2013;73((Suppl)):36. 10.1158/1538-7445.AM2013-36 [DOI] [Google Scholar]
- 15. Lord JM, Flight IH, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ 2003;327:951–3. 10.1136/bmj.327.7421.951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marchesini G, Brizi M, Bianchi G, et al. Metformin in non-alcoholic steatohepatitis. Lancet 2001;358:893–4. 10.1016/S0140-6736(01)06042-1 [DOI] [PubMed] [Google Scholar]
- 17. Ibáñez L, Ong K, Valls C, et al. Metformin treatment to prevent early puberty in girls with precocious pubarche. J Clin Endocrinol Metab 2006;91:2888–91. 10.1210/jc.2006-0336 [DOI] [PubMed] [Google Scholar]
- 18. Wilcock C, Bailey CJ. Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica 1994;24:49–57. 10.3109/00498259409043220 [DOI] [PubMed] [Google Scholar]
- 19. Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 2000;348(Pt 3):607–14. 10.1042/bj3480607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carvalho C, Correia S, Santos MS, et al. Metformin promotes isolated rat liver mitochondria impairment. Mol Cell Biochem 2008;308:75–83. 10.1007/s11010-007-9614-3 [DOI] [PubMed] [Google Scholar]
- 21. Garassino MC, Martelli O, Broggini M, et al. TAILOR trialists. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013;14:981–8. 10.1016/S1470-2045(13)70310-3 [DOI] [PubMed] [Google Scholar]