Abstract
Background
The prognosis of locally advanced gastric cancer, such as clinical T4 disease, bulky nodal involvement, type 4 and large type 3 gastric cancer, remains unsatisfactory, even with D2 gastrectomy followed by adjuvant chemotherapy. One promising approach is neoadjuvant chemotherapy. Combination chemotherapy with S-1 and oxaliplatin (SOX) is recognised as a potentially promising regimen for gastric cancer. However, the use of neoadjuvant chemotherapy consisting of SOX for locally advanced gastric cancer has not been reported. The aim of this study was to determine the maximum tolerated dose (MTD) and recommended dose of preoperative chemotherapy combined with SOX for locally advanced gastric cancer.
Methods
Patients received two cycles of neoadjuvant chemotherapy with oxaliplatin on day 1, as well as S-1 (80 mg/m2/day, twice daily) for 14 days, repeated every 3 weeks. They then underwent gastrectomy with curative D2/3 lymph node dissection followed by adjuvant S-1 (80 mg/m2/day, twice daily) for 1 year. Escalation of oxaliplatin dose was planned (starting at level 0, oxaliplatin 100 mg/m2; level 1, 130 mg/m2).
Results
Six patients were enrolled. MTD was not reached at level 1. Oxaliplatin 130 mg/m2 in combination with S-1 80 mg/m2/day twice daily could be administered with acceptable toxicity. Peripheral neuropathy was observed in all patients but with no functional disorders. No treatment-related death was observed and the incidence of operative morbidity was tolerable. Resection with curative intent was undertaken in all patients with R0 resection performed in five (83%) and R1 in one. Two of the six patients had a pathological complete response (33%).
Conclusion
Neoadjuvant chemotherapy with an SOX regimen was feasible in patients with locally advanced gastric cancer. The recommended phase II dose was determined to be oxaliplatin 130 mg/m2 in combination with S-1 80 mg/m2/day, twice daily.
Keywords: gastric cancer, neoadjuvant chemotherapy, preoperative chemotherapy, S-1, oxaliplatin
Key questions.
What is already known about this subject?
Surgery with lymph node dissection is the primary treatment for patients with localised resectable gastric cancer.
However, the prognosis of locally advanced gastric cancer is poor.
One promising approach is neoadjuvant chemotherapy.
How might this impact on clinical practice?
Combination chemotherapy with S-1 and oxaliplatin (SOX) is recognised as a potentially promising regimen for gastric cancer.
The use of neoadjuvant chemotherapy consisting of SOX for locally advanced gastric cancer has not been reported, and the recommended doses (RDs) have not been established.
What does this study add?
This study shows that the RDs of neoadjuvant chemotherapy with SOX was defined as oxaliplatin at 130 mg/m2 in combination with S-1 at 80 mg/m2/day twice daily.
Background
Gastric cancer is the fifth most frequently diagnosed malignancy, and the third leading cause of cancer mortality worldwide.1 Surgery with lymph node dissection is the primary treatment for patients with localised resectable gastric cancer; however, recurrence rates are high, at about 40%–80% in advanced cases.2 Combined modality therapy should be considered for all patients with localised gastric cancer treated with curative intent. In Japan, standard therapy for resectable stage II or III gastric cancer is D2 gastrectomy followed by adjuvant S-1 for 1 year. However, the survival rate is not high enough (3-year survival rate at 76.2% for pathological stage IIIA and 64.2% for stage IIIB).3 In the Capectabine and Oxaliplatin Adjuvant Study in Stomach Cancer (CLASSIC) trial, D2 gastrectomy followed by adjuvant chemotherapy combined with capecitabine and oxaliplatin showed 3-year recurrence-free survival rate of 66% for pathological stage IIIA, 61% for stage IIIB and completion rate of the eight preplanned courses of adjuvant chemotherapy was only 67%.4 In Western countries, perioperative chemotherapy for localised gastric or gastro-oesophageal adenocarcinoma is regarded as a standard strategy and preoperative chemotherapy has been shown to be safe, and to induce downstaging and an improvement in R0 resection rate.5 However, prognosis remains extremely poor in patients with clinical T4 disease, clinical T3 disease in case of tumours invading the oesophagus, a special type of gastric cancer known as linitis plastica (or Borrman type 4) or the large ulcero-invasive type (type 3 with a maximum diameter≥8 cm), or bulky nodal involvement around the major branched arteries to the stomach.
The oral fluoropyrimidine S-1 is new oral anticancer drug that combines tegafur, a prodrug of fluorouracil, with 5-chloro-2,4-dihydropyrimidine, and potassium oxonate in a molar ratio of 1:0.4:1. Currently, platinum-based and fluoropyrimidine-based combinations are accepted worldwide as established first-line drug regimens for gastric cancer.6 Chemotherapy combined with S-1 plus oxaliplatin for advanced gastric cancer (G-SOX) has shown promising efficacy with acceptable toxicity.7–9 Although worldwide the dose of oxaliplatin for gastric cancer is 130 mg/m2 administered every 3 weeks, the recommended doses (RDs) of G-SOX for Japanese patients are unfortunately lower than this global standard. Furthermore, there has been no report of neoadjuvant chemotherapy with G-SOX for locally advanced gastric cancer.
The aim of this study was to evaluate neoadjuvant chemotherapy consisting of SOX followed by D2/3 gastrectomy and adjuvant S-1 for locally advanced gastric cancer.
Methods
Eligibility criteria and patients
Eligibility criteria included the ability to take medication orally; aged 20 years or older; Eastern Cooperative Oncology Group performance status score of 0 or 1; histologically proven gastric adenocarcinoma; clinical T4 disease, or clinical T3 disease in the case of tumours invading the oesophagus, or of the schirrhous type (type 4) including large type 3 disease (over 8 cm), and those with bulky nodal involvement around major branched arteries to the stomach by abdominal CT and laparoscopy; resectable peritoneal dissemination (pathological CY1 or P1, except for clinical CY1 or P1); and adequate organ function, as defined by haemoglobin≥10 g/dL, absolute neutrophil count≥1.5×109/L, platelets count≥100×109/L, total bilirubin≤1.5 mg/dL, serum transaminases≤100 U/L and creatinine clearance≥60 mL/min. Exclusion criteria were evidence of chemotherapy, immunotherapy or radiotherapy for gastric cancer; prior myocardial infarction within 3 months; history of unstable angina pectoris, intestinal pneumonia, fibroid lung or severe emphysema; concurrent active malignancy; uncontrolled infection; severe mental disorder; and pregnancy or lactation.
The protocol was approved by the institutional review board of Kobe City Medical Center General Hospital, and the study followed the principles of the Declaration of Helsinki. All patients provided written, informed consent.
Neoadjuvant and adjuvant chemotherapy
Patients received two courses of neoadjuvant chemotherapy, consisting of S-1 (80 mg/m2, orally, days 1–14 followed by 1-week rest period) plus oxaliplatin on day 1 in a 3-week schedule followed by D2 or higher surgery. Patients with pathological R0/1 resection were administered S-1 (80 mg/m2, orally, days 1–28 followed by 2-week rest) for 1 year as adjuvant chemotherapy. The study was designed to determine the RD of neoadjuvant chemotherapy. The dose of S-1 was fixed and oxaliplatin was examined at doses of 100 mg/m2 (level 0) and 130 mg/m2 (level 1). A minimum of three patients were studied per dose level. If a dose-limiting toxicity (DLT) occurred in one of the first three patients assigned to a given dose level, three additional patients were assigned to the same dose level. The MTD was defined as the dose that induced a DLT during the neoadjuvant chemotherapy period in 50% or more of subjects. The RD was defined as one dose level below the MTD. If the MTD was not achieved, even at level 1, it was regarded as the RD. DLT was defined as any of the following adverse events occurring in the neoadjuvant chemotherapy period: (1) grade 4 neutropaenia lasting>4 days; (2) grade 4 thrombocytopaenia (<25×109/L); (3) febrile neutropaenia; (4) grade 3 or 4 non-haematological toxic effects; (5) a treatment delay longer than 14 days due to drug-related toxicity in the neoadjuvant chemotherapy period; or (6) treatment-related death. Neoadjuvant chemotherapy was administered triweekly until disease progression, unacceptable toxicity or withdrawal of consent for up to two cycles. The dose was modified for each patient based on haematological or non-haematological toxicity. Neoadjuvant chemotherapy was delayed if, on the planned day of treatment, neutrophils were <1.5×109/L, platelets were <75×109/L, total bilirubin was >2.0 mg/dL, or if persistent diarrhoea or stomatitis higher than grade 1 or peripheral sensory neuropathy higher than grade 2 was present. In patients with pharyngolaryngeal dysaesthesia, the duration of oxaliplatin infusion was prolonged from 2 to 6 hours. In the event of grade 4 non-haematological toxicities, treatment was definitively interrupted.
Surgery
Tumour resectability was assessed after completion of neoadjuvant chemotherapy. Resection criteria were as follows: (1) R0 resection was anticipated by D2 or extended D2 gastrectomy; (2) sufficient organ function; and (3) no active infection. Patients who fulfilled these criteria were subjected to surgery within 6 weeks after the last administration of neoadjuvant chemotherapy. Combined resection of adjacent organs was permitted when these procedures were indispensable for curative resection.
Study assessment
Pretreatment evaluation included a medical history; physical examination; complete blood cell count and serum chemistry tests; esophagogastroduodenoscopy; and chest, abdominal and pelvic CT scans. Clinical examination and biochemical tests were required before and during each cycle. All adverse events during chemotherapy were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE V.4.0). All surgery-related adverse events were evaluated by both CTCAE V.4.0 and the Clavien-Dindo classification.10
We planned to assess quality of life (QoL) every 3 weeks from the date of the first administration of neoadjuvant chemotherapy (baseline) to the date of operation, and then after operation every 3 months from the date of the first administration of the adjuvant chemotherapy for 1 year. Assessment was done using the Japanese version of the Functional Assessment of Cancer Therapy-Gastric instrument (FACT-Ga)11 and Functional Assessment of Cancer Therapy-Gynaecologic Oncology Group-Neurotoxicity (FACT-GOG-Ntx).12 The FACT-Ga is a 46-item questionnaire that measures both general health-related quality of life (HRQOL) (FACT-G) and additional HRQOL specifically related to the gastric cancer subscale. The FACT-GOG-Ntx is a 38-item questionnaire comprising two components: FACT-G and an 11-item Ntx subscale. The range of possible scores is 0 to 184 for the FACT-Ga and 0 to 152 for the FACT-GOG-Ntx, with higher scores indicating better HRQOL. The questionnaires were distributed to the patients by clinical research coordinators. Patients completed these questionnaires independently.
Surgical specimens were evaluated pathologically, with each case independently assessed by two histopathologists (YI and CI). Pathological response was scored using the Becker criteria13 and Japan criteria.14 15 Surgical specimens were pathologically evaluated and graded according to the proportion of the tumour affected by degeneration or necrosis. The Becker criteria was graded as follows: grade 1, complete (0% residual tumour; grade 1a) or subtotal tumour regression (<10% residual tumour per tumour bed; grade 1b); grade 2, partial tumour regression (10%–50% residual tumour per tumour bed); and grade 3, minimal or no tumour regression (>50% residual tumour per tumour bed). The Japan criteria was graded as follows: grade 0, none of the tumour affected; grade 1a, <1/3 affected; grade 1b, ≥1/3 and<2/3 affected; grade 2, ≥2/3 affected; and grade 3, no residual tumour. Peritoneal lavage cytology (CY) is diagnosed from either ascites or peritoneal lavage and was classified as CY1 (positive) and CY0 (negative).
Endpoints
The primary endpoint in this study was the MTD and RD of this neoadjuvant G-SOX regimen. Secondary endpoints included pathological response rate (pRR), relapse-free survival (RFS), overall survival (OS), curative resection rate (R0, R0/1 resection rate), relative dose intensity, completion rate of neoadjuvant/adjuvant chemotherapy and safety. Dose intensity was calculated as the ratio of the actual to planned dose intensity in milligrams per square metre per week. Safety and efficacy analyses were both conducted in an intention-to-treat population, defined as all patients enrolled in the study who received at least one dose of chemotherapy. RFS was defined as the time from the date of the first administration of neoadjuvant chemotherapy to the first documentation of disease relapse or death. OS was determined from the date of the first administration of neoadjuvant chemotherapy to the date of death or last confirmation of survival. All statistical analyses were conducted using the SPSS software package (SPSS 22.0, Chicago, Illinois, USA).
This trial was registered with the University Hospital Medical Information Network (No. UMIN000015181).
Results
Patients
From October 2014 to January 2015, six patients were enrolled. Characteristics of the enrolled patients are listed in table 1. All patients were chemo-naïve and had a good performance status. The clinical stage IIA case was a clinical T3 tumour invading the oesophagus with clinical N0, M0.
Table 1.
Patient characteristics (n=6)
| Variable | n | % | |
| Age, years | Median | 68 | |
| Range | 45–73 | ||
| Sex | Male | 3 | 50 |
| Female | 3 | 50 | |
| ECOG PS | 0 | 6 | 100 |
| 1 | 0 | 0 | |
| Primary tumour location | U (E+) | 3 (2) | 50 (33) |
| M | 1 | 17 | |
| L | 2 | 33 | |
| Histology | Intestinal | 3 | 50 |
| Diffuse | 3 | 50 | |
| Clinical T stage | T3 | 3 | 50 |
| T4 | 3 | 50 | |
| Clinical N stage | N0 | 1 | 17 |
| N1 | 4 | 67 | |
| N2 | 1 | 17 | |
| TNM stage | IIA | 1 | 17 |
| IIB | 2 | 33 | |
| IIIA | 2 | 33 | |
| IIIB | 1 | 17 | |
| IIIC | 0 | 0 | |
| Macroscopic type | 0-–IIc | 1 | 17 |
| 1 | 0 | 0 | |
| 2 | 3 | 50 | |
| 3 | 1 | 17 | |
| 4 | 1 | 17 |
ECOG, Eastern Cooperative Oncology Group; E+, esophageal invasion positive; PS, performance status .
Neoadjuvant chemotherapy and toxicities
Three patients were enrolled at dose level 0 (S-1 80 mg/m2/day, oxaliplatin 100 mg/m2). No patient had a DLT at level 0, and hence three more patients were enrolled at dose level 1 (S-1 80 mg/m2/day, oxaliplatin 130 mg/m2). Finally, as no patient had a DLT at level 1, the RD for phase II study was determined to be S-1 80 mg/m2/day and oxaliplatin 130 mg/m2.
The worst toxicities through the neoadjuvant chemotherapy period are listed in table 2. All patients completed the two courses of neoadjuvant chemotherapy, and toxicity was assessable in all patients. Grade 3 or more toxicities occurred in only one patient (17%) as anaemia but recovered without transfusion. Peripheral neuropathy was observed in all patients but with no functional disorders. No treatment-related death was observed. The average percentage of dose intensity delivered during the neoadjuvant chemotherapy was 85.9% for S-1 and 92.6% for oxaliplatin.
Table 2.
Maximum toxicity per patient during neoadjuvant chemotherapy (n=6)
| Adverse event | NCI CTC grade | |||||
| Haematologic | 1 | 2 | 3 | 4 | All, % | 3/4, % |
| Leucopenia | 3 | 0 | 0 | 0 | 50 | 0 |
| Neutropaenia | 0 | 0 | 0 | 0 | 0 | 0 |
| Anaemia | 1 | 0 | 1 | 0 | 33 | 17 |
| Thrombocytopaenia | 1 | 0 | 0 | 0 | 17 | 0 |
| Creatinine increased | 2 | 0 | 0 | 0 | 33 | 0 |
| Non-haematologic | ||||||
| Anorexia | 4 | 0 | 0 | 0 | 67 | 0 |
| Constipation | 1 | 0 | 0 | 0 | 17 | 0 |
| Diarrhoea | 1 | 1 | 0 | 0 | 33 | 0 |
| Dysgeusia | 1 | 0 | 0 | 0 | 17 | 0 |
| Fatigue | 4 | 0 | 0 | 0 | 67 | 0 |
| Febrile neutropaenia | - | - | 0 | 0 | 0 | 0 |
| Fever | 1 | 0 | 0 | 0 | 17 | 0 |
| Hiccups | 1 | 0 | 0 | 0 | 17 | 0 |
| Mucositis | 2 | 0 | 0 | 0 | 33 | 0 |
| Nausea/vomiting | 3 | 0 | 0 | 0 | 50 | 0 |
| Peripheral sensory neuropathy | 6 | 0 | 0 | 0 | 100 | 0 |
| Skin hyperpigmentation | 1 | 0 | 0 | 0 | 17 | 0 |
| Watering eyes | 2 | 0 | 0 | 0 | 33 | 0 |
NCI CTC, National Cancer Institute Common Toxicity Criteria.
Surgery
All six patients who completed chemotherapy underwent surgery, with a median time from starting the neoadjuvant G-SOX to surgery of 62 days (range, 53–69). Surgical findings are shown in table 3. Resection with curative intent was undertaken in all six patients, consisting of R0 resection in five and R1 in one due to positive peritoneal cytology. Thus, the proportion of R0 resections was 83% and the proportion of R0/1 resection was 100%.
Table 3.
Surgical findings in all enrolled patients (n=6)
| n | % | ||
| Type of surgery | Total gastrectomy | 4 | 67 |
| Distal gastrectomy | 2 | 33 | |
| Dissection | D2 | 6 | 100 |
| Combined resection | Gall bladder | 4 | 67 |
| Spleen | 2 | 33 | |
| Pancreas | 1 | 17 | |
| None | 1 | 17 | |
| Peritoneal cytology | Negative | 5 | 83 |
| Positive | 1 | 17 | |
| Operation time, min | Median | 251.5 | |
| range | 167–363 | ||
| Blood loss, mL | Median | 360 | |
| range | 57–2194 |
Operative morbidity was observed in two of the six patients, including pancreatic fistula (grade 2 in both CTCAE V.4.0 and Clavien-Dindo classification) and splenic vein thrombosis (grade 1 in both CTCAE V.4.0 and Clavien-Dindo classification) (table 4). There was no operative mortality. No patients required re-operation for morbidity. Median hospitalisation duration was 10 days (range, 9–45).
Table 4.
Surgical complication (n=6)
| n | % | |
| Pancreatic fistula | 2* | 33 |
| Splenic vein thrombosis | 1 | 17 |
*One patient had both pancreatic fistula and splenic vein thrombosis.
Pathological response
Two specimens had complete tumour regression (grade 1a in Becker criteria and grade 3 in Japan criteria). Two specimens had <10% vital tumour (grade 1b), one specimen had grade 2 regression (10%–50% residual tumour) and one specimen had >50% remaining residual tumour, corresponding to grade 3 in the Becker criteria. In the Japan criteria, three specimens had grade 2 (≥2/3 affected) and one had grade 1a (<1/3 affected). From the above results, pathological complete response (pCR) rate was 33%, and pRR was 83% according to the Becker criteria and Japan criteria. Four patients (67%) achieved a pathological downstaging after neoadjuvant G-SOX therapy. Table 5 shows the pathological findings in all resected patients.
Table 5.
Pathological findings in all resected patients (n=6)
| Case | Lauren | cT | cN | cM | cStage | ypT | ypN | yM | ypStage | JGCA criteria | Becker criteria |
| 1 | Diffuse | 3 | 1 | 0 | IIB | - | 0 | 0 | - | 3 | 1a |
| 2 | Intestinal | 3 | 0 | 0 | IIA | 1b | 0 | 0 | IA | 2 | 1b |
| 3 | Diffuse | 3 | 1 | 0 | IIB | - | 0 | 0 | - | 3 | 1a |
| 4 | Diffuse | 4a | 2 | 0 | IIIB | 4a | 2 | 1 | IV | 1a | 3 |
| 5 | Intestinal | 4a | 1 | 0 | IIIA | 1a | 0 | 0 | IA | 2 | 1b |
| 6 | Intestinal | 4a | 1 | 0 | IIIA | 3 | 3a | 0 | IIIB | 2 | 2 |
JGCA, Japanese Gastric Cancer Association.
Adjuvant chemotherapy and toxicities
Of the six patients, only one patient did not receive postoperative chemotherapy, owing to the patient’s request. Therefore, five patients received adjuvant chemotherapy, with a median time from surgery to the start the adjuvant chemotherapy of 42 days (range, 25–64). Regarding safety assessment, the worst toxicities of adjuvant chemotherapy with S-1 are summarised in table 6.
Table 6.
Maximum toxicity per patient during adjuvant S-1 monotherapy (n=5)
| Adverse event | NCI CTC grade | |||||
| Haematologic | 1 | 2 | 3 | 4 | All, % | 3/4, % |
| Leucopenia | 1 | 1 | 0 | 0 | 40 | 0 |
| Neutropaenia | 0 | 2 | 1 | 0 | 60 | 20 |
| Anaemia | 3 | 1 | 1 | 0 | 100 | 20 |
| Thrombocytopaenia | 2 | 0 | 0 | 0 | 40 | 0 |
| Non-haematologic | ||||||
| Anorexia | 1 | 2 | 0 | 0 | 60 | 0 |
| Alopecia | 2 | 0 | 0 | 0 | 40 | 0 |
| Constipation | 2 | 0 | 0 | 0 | 40 | 0 |
| Diarrhoea | 2 | 0 | 0 | 0 | 40 | 0 |
| Dysgeusia | 2 | 0 | 0 | 0 | 40 | 0 |
| Dysphagia | 2 | 0 | 0 | 0 | 40 | 0 |
| Fatigue | 4 | 0 | 0 | 0 | 80 | 0 |
| Febrile neutropaenia | - | - | 0 | 0 | 0 | 0 |
| Nausea/vomiting | 1 | 0 | 0 | 0 | 20 | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 0 | 1 | 0 | 0 | 20 | 0 |
| Peripheral sensory neuropathy | 1 | 0 | 0 | 0 | 20 | 0 |
| Skin hyperpigmentation | 2 | 0 | 0 | 0 | 40 | 0 |
| Watering eyes | 1 | 0 | 0 | 0 | 20 | 0 |
NCI CTC, National Cancer Institute Common Toxicity Criteria.
Quality of life
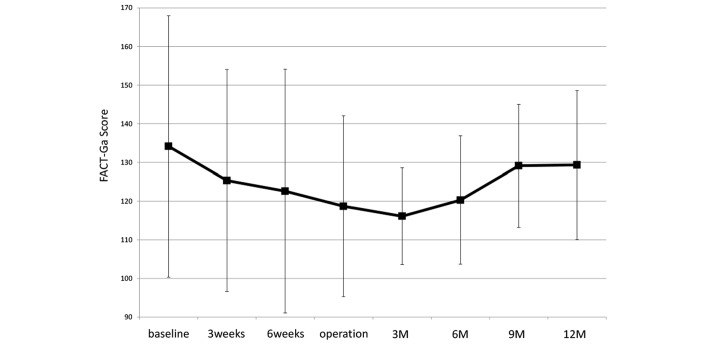
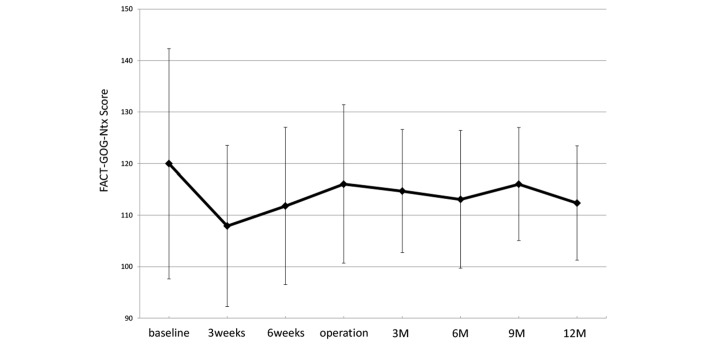
All patients were assessed for QoL using the FACT-Ga and FACT-GOG-Ntx V.4. The time course of mean FACT-Ga and FACT-GOG-Ntx scores are shown in figures 1 and 2.
Figure 1.
Time course of the mean FACT-Ga Quality of Life scale after initiation of neo G-SOX therapy: 3 weeks, 3 weeks after first administration of neoadjuvant chemotherapy consisting of SOX; 6 weeks, 6 weeks after first administration of neoadjuvant chemotherapy consisting of SOX; 3M, 3 months after operation; 6M, 6 months after operation; 9M, 9 months after operation; and 12M, 12 months after operation. FACT-Ga, Functional Assessment of Cancer Therapy-Gastric instrument; G-SOX, S-1 plus oxaliplatin for advanced gastric cancer.
Figure 2.
Time course of the mean FACT-GOG-NTx Quality of Life scale after initiation of neo G-SOX therapy: 3 weeks, 3 weeks after first administration of neoadjuvant chemotherapy consisting of SOX; 6 weeks, 6 weeks after first administration of neoadjuvant chemotherapy consisting of SOX; 3M, 3 months after operation; 6M, 6 months after operation; 9M, 9 months after operation; and 12M, 12 months after operation. FACT-GOG-Ntx, Functional Assessment of Cancer Therapy-Gynaecologic Oncology Group-Neurotoxicity; G-SOX, S-1 plus oxaliplatin for advanced gastric cancer.
Survival
With a median follow-up period of 20 months (range, 18.5–23.5), no patient has died or experienced any recurrence.
Discussion
This is the first report of the feasibility and activity of neoadjuvant chemotherapy consisting of SOX in patients with locally advanced gastric cancer. RDs of neoadjuvant chemotherapy with SOX were defined as oxaliplatin at 130 mg/m2 in combination with S-1 at 80 mg/m2/day, twice daily.
Surgery with systematic node dissection and postoperative adjuvant chemotherapy using S-1 or capecitabine plus oxaliplatin is the standard treatment for potentially curable advanced gastric cancer in Asia including Japan.3 4 However, even extended surgical procedures and postoperative adjuvant chemotherapies have not sufficiently improved the survival of patients with subgroup of gastric cancer such as clinical T4 disease, clinical T3 disease with tumours invading the oesophagus, Borrman type 4 or large type 3, or bulky nodal involvement around the major branched arteries to the stomach. This is due to the high rate of metastatic disease at the time of surgery and to the high recurrence rate even after curative resection. In treating type 4 or large type 3 T4 gastric cancer, the primary tumour often involves the spleen or pancreas, and necessitates organ resection. Even without involvement, splenectomy is often carried out in consideration of the high frequency of nodal metastasis to the splenic hilum. Recovery from such surgery is often prolonged, and a delay or cancellation of adjuvant chemotherapy is not rare. This situation therefore warrants the need for a new strategy, such as preoperative or perioperative chemotherapy. A phase II study to evaluate the safety and efficacy of preoperative chemotherapy with cisplatin plus S-1 (CS) followed by radical surgery in patients with type 4 or large type 3 gastric cancer (JCOG0210) showed an excellent percentage of completion of protocol treatment, which comprised two courses of preoperative chemotherapy and R0/1 resection (73.5%).16 However, the 3-year OS which was secondary endpoint of the study was 24.5% (95% CI, 13.6% to 37.1%) and thus the lower limit of the 95% CI was lower than the prespecified threshold (15%). Therefore, a standard treatment strategy has not yet established in Asia. On the contrary, due to the results of the UK Medical Research Council Adjuvant Gastric Infusional Chemothrapy (MAGIC) trial5 and Randomized ECF for Advanced and Locally Advanced Esophagogastric Cancer 2 (REAL-2) trial,17 epirubicin, cisplatin and 5-FU (ECF) and epirubicin, oxaliplatin and capecitabine are considered to be standard perioperative chemotherapies in Western counties. The REAL-2 trial showed that the non-nephrotoxic platinum compound oxaliplatin was as effective as cisplatin, and was associated with lower incidences of grade 3 or 4 neutropaenia, alopecia, renal toxicity and thromboembolism. Furthermore, phase III study comparing G-SOX with CS as first-line chemotherapy for advanced gastric cancer has demonstrated that G-SOX is as effective as CS with favourable safety profile.9 The present trial was therefore conducted on these bases.
Allowing for the small number of patients in this study, the safety of neo G-SOX appeared to be promising. Grade 3 or more toxicities occurred in only one patient (17%) as anaemia but recovered without transfusion, and all patients could be treated by radical surgery, with a median time from the start of neoadjuvant G-SOX to surgery of 62 days. The frequently cited multicentre MAGIC trial investigated the efficacy of a perioperative chemotherapy regimen consisting of three perioperative and three postoperative cycles of ECF in patients with gastric adenocarcinoma and oesophagogastric junction. The most common grade 3 or higher adverse events with the preoperative ECF regimen in that trial were granulocytopenia (23.8%) and leucopenia (11.5%).5 Preoperative chemotherapy requires a relatively low toxicity regimen, because the target tumours are resectable or marginally resectable.
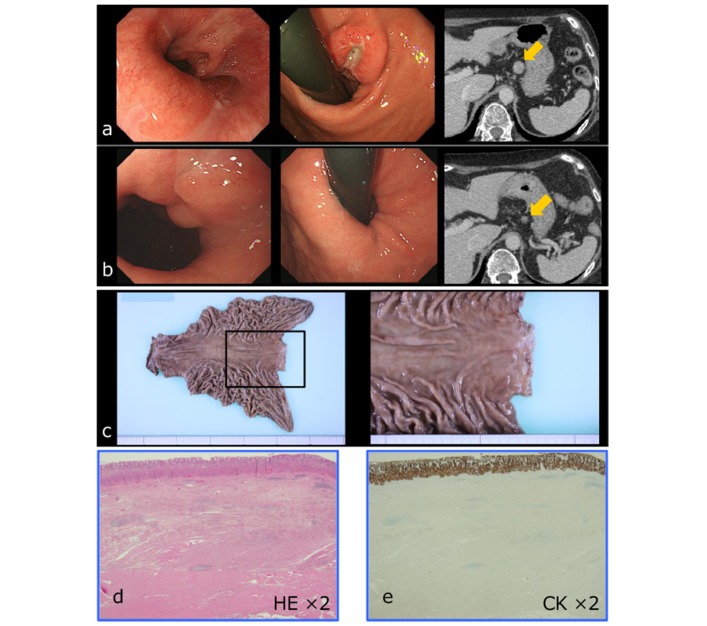
Although efficacy was not the primary endpoint of this study, antitumour activity (pCR rate 33%, pRR 83%, pathological downstaging 67%) is highly promising. Although the number of patients was small, these results might confirm the efficacy of the G-SOX regimen in the treatment of gastric cancer. Figure 3 shows the pCR case.
Figure 3.
Pathological complete response case. (a) Pretreatment findings; (b) after two courses of neo G-SOX; (c) surgically extracted specimen after neo G-SOX; (d) haematoxylin-–eosin (HE) staining of the resected specimen; and (e)cytokeratin (CK) staining of the resected specimen. G-SOX, S-1 plus oxaliplatin for advanced gastric cancer.
In the potentially curative setting, improved survival is associated with resection accompanied by perioperative chemotherapy. However, little is known about the effects of neoadjuvant chemotherapy on QoL. In particular, oxaliplatin is associated with a commonly occurring acute neuropathy that is characterised by distal or perioral paraesthesias. A correlation has been identified between the severities of acute and chronic neuropathies,18 and oxaliplatin-induced neurotoxicity may continue after the chemotherapy and interfere with patients’ daily activities. Evaluation of the time course of symptoms during and after oxaliplatin therapy is essential for physicians and patients to thoroughly understand the clinical features of peripheral neuropathy. In this study, we planned two cycles of oxaliplatin administration and evaluated oxaliplatin-induced neuropathy using the FACT-Ga and FACT-GOG-Ntx assessments. FACT-GOG-Ntx, which is a specific scale for neurotoxicity-related QoL, showed that sensory neuropathy caused a deterioration in QoL immediately after the initiation of neo G-SOX, but that QoL recovered after the neoadjuvant chemotherapy.
Several limitations related to the design of this study warrant mention. We initially planned a dose-escalation design up to oxaliplatin 130 mg/m2, which is regarded as a global standard dose.8 19 20 The dose of oxaliplatin did not reach the MTD, and the question therefore remains whether the oxaliplatin doses could have been further escalated in patients with locally advanced gastric cancer. Furthermore, the optimum number of neoadjuvant chemotherapy administrations is unknown. Thrombocytopenia is a highly characteristic toxicity of chemotherapy with SOX. Previous phase II trials in patients with colorectal cancer using S-1 at 80 mg/m2/day plus oxaliplatin at 130 mg/m2 reported a high frequency of treatment discontinuation due to prolonged thrombocytopenia.21 22 A previous phase II study of the G-SOX regimen for advanced gastric cancer showed a median onset of thrombocytopenia at after 42 days and a nadir platelet count at 113 days. Furthermore, median time from this nadir to grade 0 or the platelet count at treatment initiation was 15 days and the duration of thrombocytopenia was 21 days.7 Two courses (6 weeks) of a neoadjuvant G-SOX regimen is theoretically consistent with the onset of thrombocytopenia (42 days). If the planned number of neoadjuvant chemotherapy courses is more than two, the platelet count might deteriorate below the permissible range for gastrectomy and consequently prolong the planned operation. A high response rate and relatively low toxicity are required for preoperative chemotherapy, because target tumours are resectable or marginally resectable and the patient must receive potentially curative surgery after chemotherapy. Accordingly, we planned this study with two courses (6 weeks) as neoadjuvant chemotherapy and toxicities were acceptable.
In conclusion, this phase I study demonstrates that RDs of neoadjuvant chemotherapy consisted of G-SOX was S-1 80 mg/m2/day in combination with oxaliplatin 130 mg/m2. This regimen demonstrated sufficient activity to warrant phase II testing, and a phase II study of neoadjuvant G-SOX in patients with locally advanced gastric cancer is now ongoing as the Neo G-SOX PII study (No. UMIN000018661).
Acknowledgments
The authors would like to thank the patients and families who participated in this study. The authors also thank Rie Tamaki, Saori Tokuhara and Chiori Taniguchi for their support.
Footnotes
Contributor: HS planned, analysed and submitted the study.
AM recruited patients of this study.
MK recruited patients of this study.
TK recruited patients of this study.
YO recruited patients of this study.
YH recruited patients of this study.
HY is a member of study steering committee.
YI evaluated the pathological results.
CI evaluated the pathological results.
KM is a study statistician.
HH is a member of study steering committee.
HK is a member of study steering committee.
MK is a member of study steering committee.
TK is a member of study steering committee.
SK is a member of study steering committee.
AT advised the study design.
All investigators finally approved the manuscript.
Competing interests: MK has received honoraria from Chugai Pharma, Takeda Pharmaceutical, Yakult, Taiho Pharma and Merck Serono. Takeshi Kato has received honoraria and/or research funding from Chugai Pharma, Takeda Pharmaceutical, Eli-Lilly, Taiho Pharma, Bayer and Merck Serono. AT has received honoraria from Chugai Pharma, Takeda Pharmaceutical, Eli-Lilly, Taiho Pharma, Bayer, Bristol-Myers Squibb Japan, Daiichi Sankyo and Merck Serono. All remaining authors have declared no conflict of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Reference
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–386. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2. Gallo A, Cha C. Updates on esophageal and gastric cancers. World J Gastroenterol 2006;12:3237–42. 10.3748/wjg.v12.i20.3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sakuramoto S, Sasako M, Yamaguchi T, et al. ; ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810–20. 10.1056/NEJMoa072252 [DOI] [PubMed] [Google Scholar]
- 4. Bang Y-J, Kim Y-W, Yang H-K, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. The Lancet 2012;379:315–21. 10.1016/S0140-6736(11)61873-4 [DOI] [PubMed] [Google Scholar]
- 5. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11–20. 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 6. Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006;24:2903–9. 10.1200/JCO.2005.05.0245 [DOI] [PubMed] [Google Scholar]
- 7. Koizumi W, Takiuchi H, Yamada Y, et al. Phase II study of oxaliplatin plus S-1 as first-line treatment for advanced gastric cancer (G-SOX study). Ann Oncol 2010;21:1001–5. 10.1093/annonc/mdp464 [DOI] [PubMed] [Google Scholar]
- 8. Kim GM, Jeung HC, Rha SY, et al. A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer 2012;48:518–26. 10.1016/j.ejca.2011.12.017 [DOI] [PubMed] [Google Scholar]
- 9. Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol 2015;26:141–8. 10.1093/annonc/mdu472 [DOI] [PubMed] [Google Scholar]
- 10. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–96. 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 11. Garland SN, Pelletier G, Lawe A, et al. Prospective evaluation of the reliability, validity, and minimally important difference of the functional assessment of cancer therapy-gastric (FACT-Ga) quality-of-life instrument. Cancer 2011;117:1302–12. 10.1002/cncr.25556 [DOI] [PubMed] [Google Scholar]
- 12. Calhoun EA, Welshman EE, Chang CH, et al. Psychometric evaluation of the functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer 2003;13:741–8. 10.1111/j.1525-1438.2003.13603.x [DOI] [PubMed] [Google Scholar]
- 13. Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003;98:1521–30. 10.1002/cncr.11660 [DOI] [PubMed] [Google Scholar]
- 14. Ninomiya Y, Yanagisawa A, Kato Y, et al. Histological indications of a favorable prognosis with far-advanced gastric carcinomas after preoperative chemotherapy. J Cancer Res Clin Oncol 1999;125:699–706. 10.1007/s004320050337 [DOI] [PubMed] [Google Scholar]
- 15. Association JGC. Japanese Classificatin of Gastric Carcinoma. 14 th edn Tokyo: Kanehara, Inc, 2010. [Google Scholar]
- 16. Iwasaki Y, Sasako M, Yamamoto S, et al. ; Gastric Cancer Surgical Study Group of Japan Clinical Oncology Group. Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol 2013;107:741–5. 10.1002/jso.23301 [DOI] [PubMed] [Google Scholar]
- 17. Cunningham D, Starling N, Rao S, et al. ; Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36–46. 10.1056/NEJMoa073149 [DOI] [PubMed] [Google Scholar]
- 18. Pachman DR, Qin R, Seisler DK, et al. Clinical course of oxaliplatin-induced neuropathy: results from the randomized phase III trial N08CB (Alliance). J Clin Oncol 2015;33:3416–22. 10.1200/JCO.2014.58.8533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bang YJ, Kim YW, Yang HK, et al. ; CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315–21. 10.1016/S0140-6736(11)61873-4 [DOI] [PubMed] [Google Scholar]
- 20. Park YH, Lee JL, Ryoo BY, et al. Capecitabine in combination with oxaliplatin (XELOX) as a first-line therapy for advanced gastric cancer. Cancer Chemother Pharmacol 2008;61:623–9. 10.1007/s00280-007-0515-7 [DOI] [PubMed] [Google Scholar]
- 21. Yamada Y, Tahara M, Miya T, et al. Phase I/II study of oxaliplatin with oral S-1 as first-line therapy for patients with metastatic colorectal cancer. Br J Cancer 2008;98:1034–8. 10.1038/sj.bjc.6604271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zang DY, Lee BH, Park HC, et al. Phase II study with oxaliplatin and S-1 for patients with metastatic colorectal cancer. Ann Oncol 2009;20:892–6. 10.1093/annonc/mdn721 [DOI] [PubMed] [Google Scholar]