Abstract
The treatment of cancer-associated venous thromboembolism (VTE) is difficult because cancer patients with VTE on anticoagulation are at an increased risk of bleeding compared with patients without VTE. This review summarises the evidence supporting the current standard of care and emerging treatment options. In difficult-to-treat subpopulations, where clinical data are often lacking, this review also provides the best clinical practice strategies based on the available data. The use of therapeutic doses of parenteral anticoagulants in patients with cancer-associated VTE for at least 3 to 6 months is supported by the current clinical data. After major cancer surgery, extended thromboprophylaxis for approximately 1 month following hospital discharge is also supported. In select populations of ambulatory cancer patients with solid tumours, or in patients with myeloma receiving immunomodulatory agents in combination with chemotherapy and/or corticosteroids, pharmacological prophylaxis could be considered. Although parenteral anticoagulants may not be tolerated by some patients, the data pertaining to the use of direct oral anticoagulants (DOACs) in cancer patients with VTE at this point can only be considered hypothesis generating. Clarity of the use of DOACs is awaiting the results of head-to-head trials between DOACs and parenteral anticoagulants. In addition, because of the lack of clinical trials, there are still unanswered questions on the optimal treatment regimens in subpopulations at increased risk of bleeding, including cancer patients with thrombocytopenia and those with brain metastases. For clinicians to balance the risk of recurrent thrombosis with the chance of bleeding, they need to assess the relevant clinical data. Current data support the use of parenteral anticoagulants in cancer patients with VTE, but many unanswered questions pertaining to the optimal regimens in special subpopulations and regarding the efficacy and safety of DOACs remain. To address this need, there are currently several clinical trials under way.
Keywords: venous thromboembolism, cancer, treatment, thromboprophylaxsis, thrombocytopenia, brain metastases
Introduction
The estimated annual incidence of venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), is 1 to 2 per 1000 person-years among the general population.1 However, the incidence of VTE is up to 6.5-fold higher in patients with cancer versus patients without cancer.2 3 Overall, cancer accounts for an estimated 18% of the total number of VTE cases, and VTE is a leading cause of death among patients with cancer.4 5 The survival rates are also lower, prognosis worse and healthcare costs higher in cancer patients with VTE compared with those without.6–11
Vitamin K antagonists (VKAs) with initial heparin treatment have long been considered the mainstay for the management of VTE.12 The treatment of cancer-associated VTE, however, is especially difficult because patients with cancer treated with a VKA are at an approximately threefold higher risk of VTE recurrence and up to a sixfold higher risk of bleeding versus patients without cancer but with VTE.13 14 Factors including potential drug–drug interactions with oncology regimens as well as vomiting, thrombocytopenia and renal dysfunction associated with cancer and its treatment can also complicate anticoagulation in patients with cancer.15 16 This review summarises the evidence supporting the current standard of care and emerging treatment options. In difficult-to-treat subpopulations where clinical data are often lacking, this review also provides the best clinical practice strategies based on the available data.
Parenteral anticoagulants
Treatment and secondary prevention
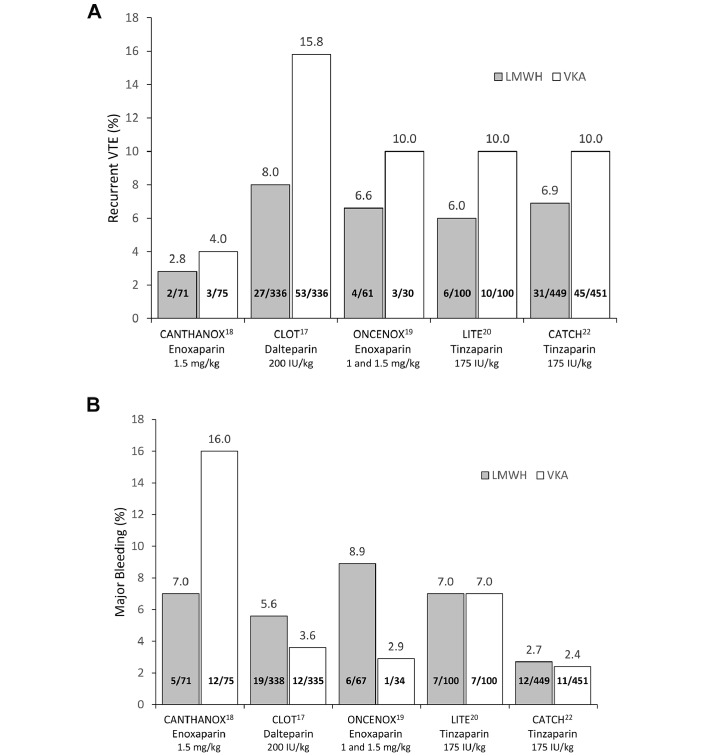
Numerous clinical trials have assessed the efficacy and safety of low-molecular-weight heparin (LMWH) for treatment and secondary prevention of cancer-associated VTE with generally favourable results (figure 1).17–23 The Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer study randomised 676 cancer patients with acute VTE to dalteparin (200 IU/kg/day) for 1 month followed by dose-reduced dalteparin (approximately 150 IU/kg/day) for 5 months, or to dalteparin (200 IU/kg/day) for 5 to 7 days and a VKA for 6 months (target international normalised ratio [INR] range 2.0–3.0).17 The risk of recurrent VTE was significantly lower with dalteparin versus VKA (HR 0.48, 95% CI 0.30 to 0.77, p=0.002), with no significant difference in the rate of major bleeding (6% vs 4%, respectively; p=0.27).17 In the Comparison of Acute Treatments in Cancer Hemostasis study, which assessed tinzaparin for the treatment of acute VTE with active cancer, 900 patients were randomised to tinzaparin (175 IU/kg/day) for 6 months or to tinzaparin (175 IU/kg/day) for 5 to 10 days and warfarin for 6 months (target INR of 2.0–3.0).22 Tinzaparin non-significantly reduced the risk of recurrent VTE (HR 0.65, 95% CI 0.41 to 1.03, p=0.07) and significantly reduced the risk of clinically relevant non-major bleeding (HR 0.58, 95% CI 0.40 to 0.84, p=0.004) but not major bleeding (HR 0.89, 95% CI 0.40 to 1.99, p=0.77) versus warfarin.22 Overall, in a recent meta-analysis, LMWH reduced the risk of recurrent VTE (relative risk [RR] 0.60, 95% CI 0.45 to 0.79, p <0.001) and had no effect on the risk of major bleeding (RR 1.07, 95% CI 0.66 to 1.73, p=0.08) versus VKA in cancer patients with acute VTE.24
Figure 1.
Incidence of (A) recurrent VTE and (B) major bleeding in select randomised clinical trials of LMWH for the treatment and secondary prevention of VTE in patients with cancer. CANTHANOX, Secondary Prevention Trial of Venous Thromboembolism With Enxoaparin; CATCH, Comparison of Acute Treatments in Cancer Hemostasis; CLOT, Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer; LITE, Long-Term Innohep Treatment Evaluation; LMWH, low-molecular-weight heparin; ONCENOX, Oncology and Enoxaparin; VKA, vitamin K antagonist; VTE, venous thromboembolism.
The risk of recurrence and bleeding associated with long-term LMWH treatment beyond 6 months presumably continues to be high in cancer patients with VTE. The Dalteparin Sodium for the Long-Term Management of Venous Thromboembolism in Cancer Patients study followed 334 cancer patients with newly diagnosed VTE treated with dalteparin for 12 months.25 All patients initially received dalteparin (200 IU/kg/day) for 4 weeks and dose-reduced dalteparin (approximately 150 IU/kg/day) during months 2 to 12.25 The incidence of new or recurrent VTE with LMWH was similar at 2 to 6 months and 7 to 12 months (3.4% [95% CI 1.6% to 6.1%] vs 4.1% [95% CI 1.8% to 8.0%], respectively).25 The incidence of major bleeding was also similar (1.1% [95% CI 0.6% to 1.9%] vs 0.7% [95% CI 0.3% to 1.4%]).25
Thromboprophylaxis in patients receiving chemotherapy
A number of studies have assessed LMWH prophylaxis in ambulatory patients with cancer receiving chemotherapy.26–32 In the Prospective, Randomised Trial of Simultaneous Pancreatic Cancer Treatment with Enoxaparin and Chemotherapy (PROSPECT-CONKO 004) study, 312 patients with advanced pancreatic cancer were randomised to receive first-line chemotherapy in an outpatient setting with or without enoxaparin. The risk of symptomatic VTE within the first 3 months was significantly lower with enoxaparin versus without enoxaparin (HR 0.12, 95% CI 0.03 to 0.52, p=0.001), and the risk of major bleeding did not differ (HR 1.4, 95% CI 0.35 to 3.72).26 Similarly, the SAVE-ONCO trial randomised 3212 patients on chemotherapy for metastatic or locally advanced solid tumours to receive prophylactic anticoagulation with semuloparin, an ultra-LMWH or placebo for the duration of chemotherapy.27 Semuloparin significantly reduced the risk versus placebo of symptomatic DVT, non-fatal PE or VTE-related death (HR 0.36, 95% CI 0.21 to 0.61, p<0.001), with no difference in the risk of major or clinically relevant non-major bleeding (HR 1.40, 95% CI 0.89 to 2.21).27 In another randomised placebo-controlled trial, a prophylactic course of nadroparin also reduced the incidence of VTE versus placebo in ambulatory patients receiving chemotherapy for metastatic or locally advanced cancer.28 Overall, in a Cochrane review of pharmacological thromboprophylaxis in ambulatory patients with cancer receiving chemotherapy, which included a total of 9861 patients, LMWH significantly reduced the risk of VTE compared with placebo or inactive control (RR 0.53, 95% CI 0.38 to 0.75) without significantly increasing the risk of major bleeding (RR 1.30, 95% CI 0.75 to 2.23).33
Perioperative thromboprophylaxis
Following surgery, VTE risk is elevated postdischarge in patients with cancer.34 In a prospective observational study, the incidence of clinically overt VTE in patients with cancer after surgery was approximately 1% to 3% depending on the type of surgery.35 Importantly, 40% of VTE events occurred after >21 days postsurgery, and 46% of the deaths postsurgery were due to VTE.35
Extended LMWH prophylaxis in patients with cancer beyond the first week postsurgery reduces the rate of VTE relative to short-term prophylaxis.36 37 In the Enoxaparin and Cancer II trial, following open-label prophylaxis with enoxaparin 40 mg for approximately 1 week, 501 patients who had undergone abdominal or pelvic surgery were randomly assigned to continued prophylaxis with enoxaparin or placebo for an additional 19 to 21 days.36 During the double-blind period, the incidence of VTE was significantly lower in patients with continued enoxaparin versus placebo (4.8% vs 12.0%, respectively; p=0.02).36 Similarly, a prospective, open-label trial compared the incidence of VTE in 427 patients with cancer who underwent abdominal surgery and were randomised to receive no further thromboprophylaxis after 7 days with dalteparin 5000 IU patients or prolonged administration of dalteparin for a further 21 days.37 The incidence of VTE was lower in the prolonged prophylaxis group versus the short-term prophylaxis group (7.3% vs 16.3%, respectively; RR reduction 55%, 95% CI 15% to 76%, p=0.012), and major bleeding events were similar in the two groups (0.5% vs 1.8%, respectively).37 However, in the Cancer, Bemiparin and Surgery Evaluation study, 4 weeks of one time per day bemiparin 3500 IU prophylactic treatment in patients with cancer undergoing abdominal or pelvic surgery did not significantly reduce the combined incidence of DVT, non-fatal PE and all-cause mortality relative to 1 week of bemiparin prophylaxis (RR reduction 24.4%, 95% CI −23.7% to 53.8%, p=0.26).38
In patients with cancer undergoing surgery, unfractionated heparin (UFH) appears to be as efficacious as LMWH in preventing VTE.39 40 In a meta-analysis of clinical trials evaluating the efficacy and safety of LMWH and UFH for thromboprophylaxis following cancer surgery, no differences were found in mortality with LMWH versus UFH treatment (RR 0.89, 95% CI 0.61 to 1.28) or in the risk of clinically suspected DVT (RR 0.73, 95% CI 0.23 to 2.28).40
Oral anticoagulants
Treatment and secondary prevention
LMWH monotherapy is generally considered the first-line treatment for cancer-associated VTE.41–47 Nevertheless, VKAs remain a common treatment strategy,48 49 possibly in part because of patients’ unease with long-term subcutaneous injections or limited access to LMWH.50
Unlike parenteral anticoagulants, direct oral anticoagulants (DOACs)—such as direct thrombin inhibitors (ie, dabigatran) and direct factor Xa inhibitors (ie, apixaban, rivaroxaban and edoxaban)—offer the convenience of oral administration, and as opposed to VKAs, they have more predictable pharmacodynamics and require no routine laboratory monitoring in most patients.51 As a class, DOACs have similar efficacy and are associated with less major bleeding than warfarin for the treatment of acute VTE.52 However, the efficacy and safety of DOACs in cancer patients with VTE have not been directly assessed in large, head-to-head trials with LMWH or VKA.
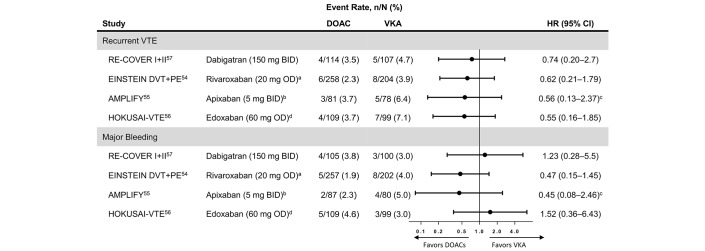
In the phase 3 clinical trials for DOACs versus VKAs in acute treatment of VTE, only 6% of patients had active cancer.53 In addition, the patients with cancer in these trials were not representative of patients at risk for cancer-associated VTE (eg, only approximately 15% to 30% had metastatic cancer and only approximately 30% were receiving chemotherapy).54–57 Furthermore, the criteria for defining cancer status at baseline in these studies differed from the clinical trials assessing LMWH for cancer-associated VTE.24 Therefore, the results of the cancer subgroup analyses of these trials can only be considered hypothesis generating. Nevertheless, subgroup analyses demonstrated a similar risk of recurrent VTE and major bleeding for dabigatran, rivaroxaban, edoxaban and apixaban compared with LMWH/warfarin54–57 (figure 2). Overall, in a network meta-analysis of all phase 3 trials, the risk of recurrent VTE in patients with cancer in the pooled DOAC group tended to be lower than that in the pooled VKA group (RR 0.65, 95% CI 0.38 to 1.09, p=0.10), without an increased risk of major bleeding (RR 0.72, 95% CI 0.39 to 1.35, p=0.31).24
Figure 2.
Forest plot of the HRs for DOACs vs warfarin for (A) new or recurrent VTE and (B) major bleeding based on the published subanalyses of the patients with active cancer at baseline included in the major DOAC phase 3 clinical trials for VTE. BID, two times per day; DOAC, direct oral anticoagulant; OD, one time per day; VKA, vitamin K antagonist; VTE, venous thromboembolism. aRivaroxaban 15 mg BID for the first 21 days followed by 20 mg OD. bApixaban 10 mg BID for 7 days followed by 5 mg BID. cRelative risk. dPatients with a creatinine clearance of 30 to 50 mL/min, a bodyweight of <60 kg or who were receiving concomitant treatment with select P-glycoprotein inhibitors received edoxaban 30 mg OD.
A small prospective study assessed the safety of dabigatran (dosed according to creatinine clearance) versus acenocoumarol in the secondary prevention of VTE in 46 patients with cancer and DVT.58 In this study, dabigatran had a safety and tolerability profile in patients with cancer consistent with those reported in the larger clinical trial population.58
Since direct comparisons between DOACs and LMWH in patients with cancer are not presently available, DOACs cannot be considered for routine treatment of VTE in these patients. However, there are currently ongoing head-to-head trials assessing the efficacy and safety of DOACs versus LMWH monotherapy for the treatment of VTE in patients with cancer, including for apixaban (ClinicalTrials.gov: NCT02585713), edoxaban (ClinicalTrials.gov: NCT02073682; Hokusai-VTE Cancer) and rivaroxaban (ClinicalTrials.gov: NCT02583191; CONKO_011/ AIO-SUP-0115/Ass and ISRCTN Registry: 86712308; Select-D).59–61 These studies should provide clarity about the role of DOACs in the treatment of cancer-associated VTE.
Thromboprophylaxis
Data on the use of oral anticoagulants for the prevention of VTE in ambulatory patients with cancer are limited. In a double-blind randomised trial in women receiving chemotherapy for metastatic breast cancer, 315 patients were randomly assigned to receive placebo or a very-low-dose warfarin (1 mg) for 6 weeks, after which dose-adjusted warfarin (INR 1.3–1.9) treatment was continued until 1 week after the end of chemotherapy.62 The incidence of VTE was significantly lower in the low-dose warfarin group versus placebo (7 events vs 1 event; p=0.031).62 Major bleeding occurred in 2 placebo patients and 1 warfarin patient.62
A secondary analysis of the of the Multicenter, Randomised, Parallel Group Efficacy and Safety Study for the Prevention of Venous Thromboembolism in Hospitalised Acutely Ill Medical Patients Comparing Rivaroxaban with Enoxaparin (MAGELLAN) trial and a phase 2 study are currently the only clinical data available pertaining to the efficacy of DOACs for thromboprophylaxis in patients with active cancer.63 The MAGELLAN trial compared a prophylactic treatment of rivaroxaban for 35 days with enoxaparin administered for 10 days followed by placebo in hospitalised, medically ill patients.63 Among the subgroup of patients with cancer, the incidence of VTE with rivaroxaban (9.9%) was similar to enoxaparin/placebo (7.4%) and the incidence of bleeding was higher (5.4% vs 1.4%).63 Phase 2 pilot study assessed the safety of apixaban (5, 10 or 20 mg one time per day) versus placebo for preventing VTE in 125 patients with metastatic cancer receiving chemotherapy.64 In this study, the incidence of recurrent VTE was 1.1% with apixaban compared with 13.8% with placebo.64 There is an ongoing trial to directly evaluate the efficacy and safety of a prophylactic treatment of rivaroxaban versus placebo in ambulatory patients with cancer receiving chemotherapy (ClinicalTrials.gov: NCT02555878; CALLISTO).61
Special populations at increased risk of bleeding
Patients with chemotherapy-induced thrombocytopenia
Up to 24% of patients with solid tumours treated with chemotherapy develop clinically significant thrombocytopenia.65 66 As these patients are at an increased risk of major bleeding during chemotherapy,67 it is challenging to find the balance between the risk of bleeding and thrombosis.68 Furthermore, treatment decisions in these patients are complicated by the lack of large randomised studies.68 In the clinical trials assessing the efficacy and safety of LMWH for the treatment of VTE, patients were generally excluded or treatment was interrupted if they had low platelet counts (ie, <30×109–100×109/L).17–23 Therefore, the available evidence about the use of anticoagulants in these patients comes primarily from small retrospective studies and case reports.
In a case series of 5 patients with thrombocytopenia and haematological malignancies treated with enoxaparin for concomitant VTE, platelet transfusions were given for platelet counts <20×109/L, and enoxaparin dose was reduced for platelet counts <50×109/L.69 There were 2 major bleeding events (1 fatal) and 1 minor bleed; there were no incidences of recurrent or new VTE.69 Conversely, there were no major bleeds reported in a small retrospective study of 10 patients with haematological malignancies and thrombocytopenia undergoing intensive chemotherapy who received enoxaparin as thromboprophylaxis or for catheter-related central venous thrombosis.70 As in the case series, during the period of severe thrombocytopenia, the enoxaparin dose was generally reduced.70 Similarly, in another study of 4 patients receiving chemotherapy for leukaemia who had VTE and a mean platelet count <60×109/L, enoxaparin was not associated with haemorrhagic complications, and there were no incidences of recurrent VTE.71 In the absence of direct evidence, treatment decisions in this patient population may be determined on a case-by-case basis.
Patients with brain metastases
Patients with malignant brain tumours are at an estimated 3% to 60% increased risk of VTE during the postoperative period.72 A prophylactic anticoagulant regimen is effective in preventing VTE in patients undergoing neurosurgery.73 However, the prophylactic use of anticoagulation in patients with malignant brain tumours undergoing surgery is controversial with some, but not all,74–76 studies suggesting a possible increased risk of intracranial haemorrhage.77 78
In a retrospective cohort study of 293 cancer patients with brain metastases, there were no differences in the 1 year cumulative incidence of significant intracranial haemorrhage in patients receiving therapeutic doses of enoxaparin compared with those not receiving anticoagulation (21% vs 22%; p=0.87).79 To date, the PRODIGE study is the largest randomised trial to assess the use of LMWH thromboprophylaxis in these patients.78 In the study, 186 patients with newly diagnosed malignant glioma were randomised to either dalteparin (5000 IU) or placebo for a total of 12 months starting within 4 weeks of surgery.78 This study was prematurely closed for recruitment because of lower-than-anticipated recruitment and because of the expiration of the study drug.78 During the first 6 months, there was a non-significant trend toward decreased incidence of VTE with LMWH versus placebo (HR 0.51, 95% CI 0.19 to 1.4, p=0.17).78 However, during this time, there were 3 major bleeds (all intracranial, 1 fatal) with LMWH and none with placebo. Over the full 12 months of the study, there were a total of 5 major bleeds on LMWH versus 1 on placebo (HR 4.2, 95% CI 0.48 to 36).78 Overall, at present, there is no consistent clinical evidence to indicate long-term primary pharmacological thromboprophylaxis in this high-risk patient population.
Summary of treatment guidelines
Treatment and secondary prevention
Parenteral anticoagulants are considered first-line therapy for the treatment and prevention of VTE in patients with active cancer. Treatment guidelines—including from the American College of Chest Physicians, the American Society of Clinical Oncology (ASCO), the British Committee for Standards in Haematology (BCSH), the European Society of Medical Oncology, the National Comprehensive Cancer Network and the International Clinical Practice Guidelines—recommend LMWH for the short-term and long-term management of VTE in patients with cancer.41–47 Due to of the lack of clinical trial data, most guidelines do not recommend the use of DOACs for the acute or long-term management of VTE. However, ASCO and BCSH guidelines state that oral anticoagulants could be considered for long-term treatment of VTE in patients with cancer based on patient preference or when LMWH is not available.42 46 47 The International Clinical Practice Guidelines state that DOACs can be considered for early maintenance (10 days to 3 months) and long-term therapy (>3 months) in patients with VTE and stable cancer not receiving anticancer therapy.45 In general, treatment guidelines recommend that anticoagulation should be continued for at least 3 to 6 months as long as there is clinical evidence of active malignant disease in cancer patients with established VTE.41–47
Thromboprophylaxis
Routine thromboprophylaxis is not recommended in outpatients with cancer,42 46 47 especially those deemed at low risk for VTE.80 However, in select populations of cancer patients with solid tumours or in patients with myeloma receiving immunomodulatory agents, prophylaxis could be considered.41–47 80 81 In patients with myeloma taking immunomodulatory agents, the European Myeloma Network recommends that the LMWH prophylaxis be continued for at least 4 months, after which patients may be switched to aspirin.81
For the prevention of VTE in patients with cancer in an inpatient setting or perioperatively, treatment guidelines recommend parenteral anticoagulants for primary thromboprophylaxis.41–47 80 81 After major cancer surgery (abdominal and pelvic cancer surgery), extended thromboprophylaxis for approximately 1 month is recommended following hospital discharge42 43 46 because VTE is frequently observed in these patients more than 3 weeks after discharge.35
Patients with chemotherapy-induced thrombocytopenia
In the absence of clinical trials specifically in cancer patients with thrombocytopenia, treatment guidelines are generally based on the platelet count exclusion thresholds used in the clinical trials assessing anticoagulants in patients with cancer. In general, the guidelines recommend that a full anticoagulant dose be used in patients with cancer with platelet counts >50 x 109 L.42 44–47 82 Anticoagulants may be considered in patients with platelet counts <50 x 109 L on a case-by-case basis, considering risk of bleeding and risk associated with VTE.42 44–47 However, ASCO, BCSH and International Society on Thrombosis and Haemostasis (ISTH) state that anticoagulation is contraindicated in patients with severe thrombocytopenia (platelet counts <20 x 109/L [ASCO] or 25 x 109/L [BCSH and ISTH]).46 47 82 BCSH and ISTH also recommend the use of platelet transfusions to allow for full-dose anticoagulation or a 50% dose reduction in patients with platelet counts between 25 x 109 and <50 x 109/L.47 82
Patients with brain metastases
There are limited data available on the use of anticoagulants in patients with brain metastases who develop VTE. That said, ASCO and International Clinical Practice Guidelines note that anticoagulants are not absolutely contraindicated in patients with brain tumours per se.42 45 46 However, an ISTH guidance statement indicates that outpatient pharmacological thromboprophylaxis is not recommended in patients with a diagnosis of primary brain tumour.80
Treatment practices
A significant number of patients with cancer-associated VTE may not be managed according to established treatment recommendations.83 Of the 275 physicians responding to a survey of members of various German oncology and haematology clinics, only approximately 75% reported treating acute VTE in cancer patients with LMWH. In this survey, only 55% of specialists reported continuing LMWH treatment for 3 to 6 months, of whom most reported a dose reduction to 50% to 75% of the initial dose during this period.84 However, in an international survey of 141 physicians, the long-term use of LMWH monotherapy to treat cancer-associated VTE was higher among thrombosis specialists versus other physician specialists and among European physicians versus physicians from the USA.85
In a retrospective analysis of health insurance claims in the USA collected between 2009 and 2014, warfarin was the most commonly prescribed anticoagulant in cancer patients with acute VTE (warfarin, 50%; LMWH, 40%; other anticoagulants, 10%).86 Of note, LMWH was prescribed more often in patients with cancer types associated with a high risk of VTE (eg, pancreatic and stomach cancers). Similarly, in a retrospective cohort study of hospitalised patients with PE between 1998 and 2008, LMWH was prescribed in only 13.7% of patients with cancer.87 In both studies, the relatively low use of LMWH occurred even though LMWH monotherapy is considered the first-line treatment for cancer-associated VTE. However, in a cross-sectional study of patients with newly diagnosed acute VTE in Canada in 2013, approximately 85% of patients with cancer were prescribed LMWH monotherapy for the subsequent treatment of VTE.88
Pharmacological thromboprophylaxis may also be underused in patients with cancer at high risk for VTE.89–91 In a cross-sectional study of 775 hospitalised cancer patients with VTE admitted between January and June 2013, only 50.6% of patients received pharmacological prophylaxis during their hospital stay.90 However, 31.9% of patients were deemed to have at least 1 relative contraindication for an anticoagulant, including active bleeding (5.5%) and severe thrombocytopenia (20.8%).90 Among patients not contraindicated for an anticoagulant, 74.2% received pharmacological thromboprophylaxis.90 Patients with cancer undergoing cancer-specific therapy were significantly less likely to receive thromboprophylaxis versus patients with cancer admitted for other reasons (OR 0.37, 95% CI 0.22 to 0.61, p <0.001).90 In addition, 79% of patients eligible for thromboprophylaxis and classified as being at a high VTE risk by their Padua Prediction Score were prescribed prophylaxis; 63% of those considered as low risk also received prophylaxis.90
Conclusions
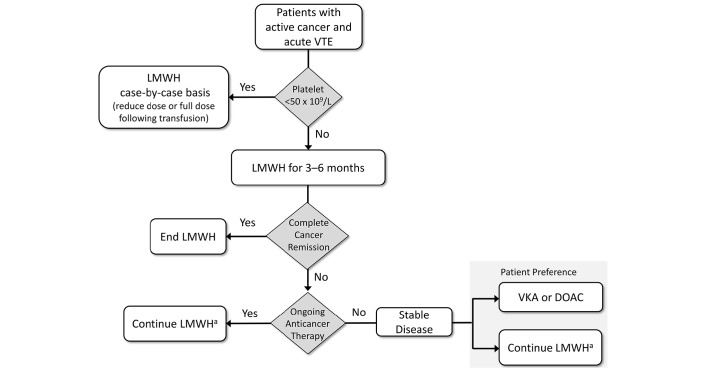
Many of the treatment suggestions for the management of cancer-associated VTE are derived from retrospective studies or are extrapolated from studies on patients without cancer because of a lack of randomised controlled trials focused on specific subpopulations.92 Therefore, treatment recommendations may differ slightly based on differences in treatment practices between nations and on how much weight is put on the available clinical trial data or lack thereof. Nevertheless, there is a general consensus (figure 3).
Figure 3.
Treatment and secondary prevention strategy diagram for VTE in patients with active cancer based on the treatment guidelines for cancer-associated VTE. DOAC, direct oral anticoagulant; LMWH, low-molecular-weight heparin; VKA, vitamin K antagonist; VTE, venous thromboembolism. aThere is an evidence gap regarding which dose of LMWH to choose for extended therapy beyond 6 months; in clinical practice, some experts continue with full therapeutic dose and others reduce to a prophylactic dose.
Cancer-associated VTE should be treated for at least 3 to 6 months with a therapeutic dose of LMWH. In patients with complete remission, treatment can be stopped after 6 months. In patients with active cancer (eg, presence of metastatic disease and/or ongoing anticancer treatment), treatment with LMWH at prophylactic doses or oral anticoagulants, depending on patient preference, can be continued beyond 6 months. In patients with cancer with platelet counts <50 x 109 L or in patients with brain tumours, anticoagulants should be considered on a case-by-case basis. After major cancer surgery (abdominal and pelvic cancer surgery), extended thromboprophylaxis for approximately 1 month is recommended following hospital discharge. In select populations of ambulatory cancer patients with solid tumours or in patients with myeloma receiving immunomodulatory agents in combination with chemotherapy and/or corticosteroids, primary pharmacological thromboprophylaxis (primarily LMWH) could also be considered.29
In practice, for clinicians to balance the risk of recurrent thrombosis with the chance of bleeding, they need to assess the relevant clinical data. Unfortunately, because of the lack of clinical trials, there are still unanswered questions on the optimal treatment regimens; which may affect adherence to treatment recommendations. Therefore, treatment decisions are often made on a case-by-case basis per individual bleeding and thrombotic risk. In addition, anticoagulant options are currently limited to parenteral anticoagulants, which may not be tolerated by some patients. To address the lack of data, several clinical trials are under way, including those assessing the relative efficacy and safety of DOACs in the treatment and prevention of cancer-associated VTE. DOACs may provide more convenient anticoagulation compared with LMWH, when their efficacy and safety are proven in patients with cancer.
Acknowledgments
Assistance in medical writing and editorial support was provided by Stefan Kolata, PhD, of AlphaBioCom, LLC (King of Prussia, PA), and funded by Daiichi Sankyo, Inc. (Parsippany, NJ).
Footnotes
Contributors: All authors fulfil ICMJE authorship criteria.
Competing interests: CA received honoraria for lectures from Sanofi, Pfizer/BMS, Daiichi Sankyo, Boehringer Ingelheim and Bayer. PWK has served as a consultant for Daiichi Sankyo, Bayer, Boehringer Ingelheim, CSL Behring and Ablynx and has received investigator-initiated research grants from Daiichi Sankyo, Bayer, Leo Pharma, Pfizer and CSL Behring. GA has received personal fees from Boehringer Ingelheim, Sanofi, Daiichi Sankyo and Bayer.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1. Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol 2012;25:235–42. 10.1016/j.beha.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 2. Walker AJ, Card TR, West J, et al. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer 2013;49:1404–13. 10.1016/j.ejca.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 3. Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000;160:809–15. [DOI] [PubMed] [Google Scholar]
- 4. Heit JA, O'Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med 2002;162:1245–8. 10.1001/archinte.162.11.1245 [DOI] [PubMed] [Google Scholar]
- 5. Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007;5:632–4. 10.1111/j.1538-7836.2007.02374.x [DOI] [PubMed] [Google Scholar]
- 6. Chew HK, Wun T, Harvey D, et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med 2006;166:458–64. 10.1001/archinte.166.4.458 [DOI] [PubMed] [Google Scholar]
- 7. Chew HK, Wun T, Harvey DJ, et al. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol 2007;25:70–6. 10.1200/JCO.2006.07.4393 [DOI] [PubMed] [Google Scholar]
- 8. Sørensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846–50. 10.1056/NEJM200012213432504 [DOI] [PubMed] [Google Scholar]
- 9. Alcalay A, Wun T, Khatri V, et al. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol 2006;24:1112–8. 10.1200/JCO.2005.04.2150 [DOI] [PubMed] [Google Scholar]
- 10. Lyman GH, Eckert L, Wang Y, et al. Venous thromboembolism risk in patients with cancer receiving chemotherapy: a real-world analysis. Oncologist 2013;18:1321–9. 10.1634/theoncologist.2013-0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohoon KP, Ransom JE, Leibson CL, et al. Direct Medical costs attributable to cancer-associated venous thromboembolism: a population-based longitudinal study. Am J Med 2016;129:1000.e15–25. 10.1016/j.amjmed.2016.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kearon C, Kahn SR, Agnelli G, et al. ; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 2008;133:454S–545. 10.1378/chest.08-0658 [DOI] [PubMed] [Google Scholar]
- 13. Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002;100:3484–8. 10.1182/blood-2002-01-0108 [DOI] [PubMed] [Google Scholar]
- 14. Hutten BA, Prins MH, Gent M, et al. Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: a retrospective analysis. J Clin Oncol 2000;18:3078–83. 10.1200/JCO.2000.18.17.3078 [DOI] [PubMed] [Google Scholar]
- 15. Kearon C, Akl EA, Comerota AJ, et al. ; American College of Chest Physicians. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141:e419S–94S. 10.1378/chest.11-2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sahni V, Choudhury D, Ahmed Z. Chemotherapy-associated renal dysfunction. Nat Rev Nephrol 2009;5:450–62. 10.1038/nrneph.2009.97 [DOI] [PubMed] [Google Scholar]
- 17. Lee AY, Levine MN, Baker RI, et al. CLOT Investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146–53. 10.1056/NEJMoa025313 [DOI] [PubMed] [Google Scholar]
- 18. Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med 2002;162:1729–35. 10.1001/archinte.162.15.1729 [DOI] [PubMed] [Google Scholar]
- 19. Deitcher SR, Kessler CM, Merli G, et al. ONCENOX Investigators. Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period . Clin Appl Thromb Hemost 2006;12:389–96. 10.1177/1076029606293692 [DOI] [PubMed] [Google Scholar]
- 20. Hull RD, Pineo GF, Brant RF, et al. LITE Trial Investigators. Self-managed long-term low-molecular-weight heparin therapy: the balance of benefits and harms. Am J Med 2007;120:72–82. 10.1016/j.amjmed.2006.03.030 [DOI] [PubMed] [Google Scholar]
- 21. Romera A, Cairols MA, Vila-Coll R, et al. A randomised open-label trial comparing long-term sub-cutaneous low-molecular-weight heparin compared with oral-anticoagulant therapy in the treatment of deep venous thrombosis. Eur J Vasc Endovasc Surg 2009;37:349–56. 10.1016/j.ejvs.2008.11.030 [DOI] [PubMed] [Google Scholar]
- 22. Lee AY, Kamphuisen PW, Meyer G, et al. CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA 2015;314:677–86. 10.1001/jama.2015.9243 [DOI] [PubMed] [Google Scholar]
- 23. Hull RD, Pineo GF, Brant RF, et al. LITE Trial Investigators. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med 2006;119:1062–72. 10.1016/j.amjmed.2006.02.022 [DOI] [PubMed] [Google Scholar]
- 24. Posch F, Königsbrügge O, Zielinski C, et al. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res 2015;136:582–9. 10.1016/j.thromres.2015.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Francis CW, Kessler CM, Goldhaber SZ, et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: the DALTECAN Study. J Thromb Haemost 2015;13:1028–35. 10.1111/jth.12923 [DOI] [PubMed] [Google Scholar]
- 26. Pelzer U, Opitz B, Deutschinoff G, et al. Efficacy of prophylactic low-molecular weight heparin for ambulatory patients with advanced pancreatic cancer: outcomes from the CONKO-004 trial. J Clin Oncol 2015;33:2028–34. 10.1200/JCO.2014.55.1481 [DOI] [PubMed] [Google Scholar]
- 27. Agnelli G, George DJ, Kakkar AK, et al. SAVE-ONCO Investigators. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer . N Engl J Med 2012;366:601–9. 10.1056/NEJMoa1108898 [DOI] [PubMed] [Google Scholar]
- 28. Agnelli G, Gussoni G, Bianchini C, et al. PROTECHT Investigators. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol 2009;10:943–9. 10.1016/S1470-2045(09)70232-3 [DOI] [PubMed] [Google Scholar]
- 29. Palumbo A, Cavo M, Bringhen S, et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. J Clin Oncol 2011;29:986–93. 10.1200/JCO.2010.31.6844 [DOI] [PubMed] [Google Scholar]
- 30. Maraveyas A, Waters J, Roy R, et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer 2012;48:1283–92. 10.1016/j.ejca.2011.10.017 [DOI] [PubMed] [Google Scholar]
- 31. Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood 2012;119:933–9. 10.1182/blood-2011-03-344333 [DOI] [PubMed] [Google Scholar]
- 32. Verso M, Gussoni G, Agnelli G. Prevention of venous thromboembolism in patients with advanced lung cancer receiving chemotherapy: a combined analysis of the PROTECHT and TOPIC-2 studies. J Thromb Haemost 2010;8:1649–51. 10.1111/j.1538-7836.2010.03901.x [DOI] [PubMed] [Google Scholar]
- 33. Di Nisio M, Porreca E, Otten HM, et al. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev 2014;8:CD008500. 10.1002/14651858.CD008500.pub3 [DOI] [PubMed] [Google Scholar]
- 34. Merkow RP, Bilimoria KY, McCarter MD, et al. Post-discharge venous thromboembolism after cancer surgery: extending the case for extended prophylaxis. Ann Surg 2011;254:131–7. 10.1097/SLA.0b013e31821b98da [DOI] [PubMed] [Google Scholar]
- 35. Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg 2006;243:89–95. 10.1097/01.sla.0000193959.44677.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bergqvist D, Agnelli G, Cohen AT, et al. ENOXACAN II Investigators. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002;346:975–80. 10.1056/NEJMoa012385 [DOI] [PubMed] [Google Scholar]
- 37. Rasmussen MS, Jorgensen LN, Wille-Jørgensen P, et al. FAME Investigators. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: a multicenter randomized open-label study. J Thromb Haemost 2006;4:2384–90. 10.1111/j.1538-7836.2006.02153.x [DOI] [PubMed] [Google Scholar]
- 38. Kakkar VV, Balibrea JL, Martínez-González J, et al. CANBESURE Investigators. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. J Thromb Haemost 2010;8:1223–9. 10.1111/j.1538-7836.2010.03892.x [DOI] [PubMed] [Google Scholar]
- 39. ENOXACAN Study Group. Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double-blind randomized multicentre trial with venographic assessment. Br J Surg 1997;84:1099–103. [PubMed] [Google Scholar]
- 40. Akl EA, Terrenato I, Barba M, et al. Low-molecular-weight heparin vs unfractionated heparin for perioperative thromboprophylaxis in patients with cancer: a systematic review and meta-analysis. Arch Intern Med 2008;168:1261–9. 10.1001/archinte.168.12.1261 [DOI] [PubMed] [Google Scholar]
- 41. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: chest guideline and expert panel report. Chest 2016;149:315–52. 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 42. Lyman GH, Bohlke K, Falanga A. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Oncol Pract 2015;11:e442–4. 10.1200/JOP.2015.004473 [DOI] [PubMed] [Google Scholar]
- 43. Mandalà M, Labianca R; European Society for Medical Oncology. Venous thromboembolism (VTE) in cancer patients. ESMO clinical recommendations for prevention and management. Thromb Res 2010;125:S117–9. 10.1016/S0049-3848(10)70028-1 [DOI] [PubMed] [Google Scholar]
- 44. NCCN Clinical Practical Guidelines in Oncology. Cancer-associated venous thromboembolic disease version 1.2014: National Comprehensive Care Network. 2014. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed 11 Mar 2016). [DOI] [PubMed]
- 45. Farge D, Bounameaux H, Brenner B, et al. International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol 2016;17:e452–66. 10.1016/S1470-2045(16)30369-2 [DOI] [PubMed] [Google Scholar]
- 46. Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2013;31:2189–204. 10.1200/JCO.2013.49.1118 [DOI] [PubMed] [Google Scholar]
- 47. Watson HG, Keeling DM, Laffan M, et al. Guideline on aspects of cancer-related venous thrombosis. Br J Haematol 2015;170:640–8. 10.1111/bjh.13556 [DOI] [PubMed] [Google Scholar]
- 48. Delate T, Witt DM, Ritzwoller D, et al. Outpatient use of low molecular weight heparin monotherapy for first-line treatment of venous thromboembolism in advanced cancer. Oncologist 2012;17:419–27. 10.1634/theoncologist.2011-0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mahé I, Sterpu R, Bertoletti L, et al. Long-term anticoagulant therapy of patients with venous thromboembolism. What are the practices? PLoS One 2015;10:e0128741 10.1371/journal.pone.0128741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wittkowsky AK. Barriers to the long-term use of low-molecular weight heparins for treatment of cancer-associated thrombosis. J Thromb Haemost 2006;4:2090–1. 10.1111/j.1538-7836.2006.02073.x [DOI] [PubMed] [Google Scholar]
- 51. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism: a systematic review. Ann Intern Med 2012;157:796–807. 10.7326/0003-4819-157-10-201211200-00532 [DOI] [PubMed] [Google Scholar]
- 52. van der Hulle T, Kooiman J, den Exter PL, et al. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost 2014;12:320–8. 10.1111/jth.12485 [DOI] [PubMed] [Google Scholar]
- 53. van Es N, Coppens M, Schulman S, et al. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood 2014;124:1968–75. 10.1182/blood-2014-04-571232 [DOI] [PubMed] [Google Scholar]
- 54. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PE): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol 2014;1:e37–46. 10.1016/S2352-3026(14)70018-3 [DOI] [PubMed] [Google Scholar]
- 55. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost 2015;13:2187–91. 10.1111/jth.13153 [DOI] [PubMed] [Google Scholar]
- 56. Raskob GE, van Es N, Segers A, et al. Hokusai-VTE Investigators. Edoxaban for venous thromboembolism in patients with cancer: results from a non-inferiority subgroup analysis of the Hokusai-VTE randomised, double-blind, double-dummy trial. Lancet Haematol 2016;3:e379–87. 10.1016/S2352-3026(16)30057-6 [DOI] [PubMed] [Google Scholar]
- 57. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost 2015;114:150–7. 10.1160/TH14-11-0977 [DOI] [PubMed] [Google Scholar]
- 58. Mazilu L, Parepa IR, Suceveanu AI, et al. P221 Venous thromboembolism: secondary prevention with dabigatran vs. acenocumarol in patients with paraneoplastic deep vein thrombosis. Results from a small prospective study in Romania. Cardiovasc Res 2014;103:S39. 10.1093/cvr/cvu082.154 [DOI] [Google Scholar]
- 59. van Es N, Di Nisio M, Bleker SM, et al. Edoxaban for treatment of venous thromboembolism in patients with cancer. Rationale and design of the Hokusai VTE-cancer study. Thromb Haemost 2015;114:1268–76. 10.1160/TH15-06-0452 [DOI] [PubMed] [Google Scholar]
- 60. Young A, Dunn J, Chapman O, et al. , eds SELECT-D: Anticoagulation therapy in selected cancer patients at risk of recurrence of venous thromboembolism In: ASCO annual meeting proceedings; 2014. [DOI] [PubMed] [Google Scholar]
- 61. Bach M, Bauersachs R. Spotlight on advances in VTE management: CALLISTO and EINSTEIN CHOICE. Thromb Haemost 2016;116:S24–32. 10.1160/TH16-06-0486 [DOI] [PubMed] [Google Scholar]
- 62. Levine M, Hirsh J, Gent M, et al. Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet 1994;343:886–9. 10.1016/S0140-6736(94)90008-6 [DOI] [PubMed] [Google Scholar]
- 63. Cohen AT, Spiro TE, Spyropoulos AC et al. MAGELLAN Investigators ; . Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013;368:513–23. 10.1056/NEJMoa1111096 [DOI] [PubMed] [Google Scholar]
- 64. Levine MN, Gu C, Liebman HA, et al. A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. J Thromb Haemost 2012;10:807–14. 10.1111/j.1538-7836.2012.04693.x [DOI] [PubMed] [Google Scholar]
- 65. Hitron A, Steinke D, Sutphin S, et al. Incidence and risk factors of clinically significant chemotherapy-induced thrombocytopenia in patients with solid tumors. J Oncol Pharm Pract 2011;17:312–9. 10.1177/1078155210380293 [DOI] [PubMed] [Google Scholar]
- 66. Dutcher JP, Schiffer CA, Aisner J, et al. Incidence of thrombocytopenia and serious hemorrhage among patients with solid tumors. Cancer 1984;53:557–62. [DOI] [PubMed] [Google Scholar]
- 67. Elting LS, Rubenstein EB, Martin CG, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol 2001;19:1137–46. 10.1200/JCO.2001.19.4.1137 [DOI] [PubMed] [Google Scholar]
- 68. Ibrahim RB, Skewes MD, Kuriakose P. ‘Sailing in troubled waters’: a review of the use of anticoagulation in adult cancer patients with thrombocytopenia. Blood Coagul Fibrinolysis 2016;27:615–30. 10.1097/MBC.0000000000000539 [DOI] [PubMed] [Google Scholar]
- 69. Lim MS, Enjeti AK. Safety of anticoagulation in the treatment of venous thromboembolism in patients with haematological malignancies and thrombocytopenia: report of 5 cases and literature review. Crit Rev Oncol Hematol 2016;105:92–9. 10.1016/j.critrevonc.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 70. Herishanu Y, Misgav M, Kirgner I, et al. Enoxaparin can be used safely in patients with severe thrombocytopenia due to intensive chemotherapy regimens. Leuk Lymphoma 2004;45:1407–11. 10.1080/10428190410001663671 [DOI] [PubMed] [Google Scholar]
- 71. Imberti D, Vallisa D, Anselmi E, et al. Safety and efficacy of enoxaparin treatment in venous thromboembolic disease during acute leukemia. Tumori 2004;90:390–3. [DOI] [PubMed] [Google Scholar]
- 72. Marras LC, Geerts WH, Perry JR. The risk of venous thromboembolism is increased throughout the course of malignant glioma: an evidence-based review. Cancer 2000;89:640–6. [DOI] [PubMed] [Google Scholar]
- 73. Iorio A, Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: a meta-analysis. Arch Intern Med 2000;160:2327–32. 10.1001/archinte.160.15.2327 [DOI] [PubMed] [Google Scholar]
- 74. Schmidt F, Faul C, Dichgans J, et al. Low molecular weight heparin for deep vein thrombosis in glioma patients. J Neurol 2002;249:1409–12. 10.1007/s00415-002-0855-5 [DOI] [PubMed] [Google Scholar]
- 75. Perry SL, Bohlin C, Reardon DA, et al. Tinzaparin prophylaxis against venous thromboembolic complications in brain tumor patients. J Neurooncol 2009;95:129–34. 10.1007/s11060-009-9911-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Robins HI, O'Neill A, Gilbert M, et al. Effect of dalteparin and radiation on survival and thromboembolic events in glioblastoma multiforme: a phase II ECOG trial. Cancer Chemother Pharmacol 2008;62:227–33. 10.1007/s00280-007-0596-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dickinson LD, Miller LD, Patel CP, et al. Enoxaparin increases the incidence of postoperative intracranial hemorrhage when initiated preoperatively for deep venous thrombosis prophylaxis in patients with brain tumors. Neurosurgery 1998;43:1074–9. 10.1097/00006123-199811000-00039 [DOI] [PubMed] [Google Scholar]
- 78. Perry JR, Julian JA, Laperriere NJ, et al. PRODIGE: a randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J Thromb Haemost 2010;8:1959–65. 10.1111/j.1538-7836.2010.03973.x [DOI] [PubMed] [Google Scholar]
- 79. Donato J, Campigotto F, Uhlmann EJ, et al. Intracranial hemorrhage in patients with brain metastases treated with therapeutic enoxaparin: a matched cohort study. Blood 2015;126:494–9. 10.1182/blood-2015-02-626788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Khorana AA, Otten HM, Zwicker JI, et al. Prevention of venous thromboembolism in cancer outpatients: guidance from the SSC of the ISTH. J Thromb Haemost 2014;12:1928–31. 10.1111/jth.12725 [DOI] [PubMed] [Google Scholar]
- 81. Terpos E, Kleber M, Engelhardt M, et al. European Myeloma Network guidelines for the management of multiple myeloma-related complications. Haematologica 2015;100:1254–66. 10.3324/haematol.2014.117176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Carrier M, Khorana AA, Zwicker J, et al. Management of challenging cases of patients with cancer-associated thrombosis including recurrent thrombosis and bleeding: guidance from the SSC of the ISTH. J Thromb Haemost 2013;11:1760–5. 10.1111/jth.12338 [DOI] [PubMed] [Google Scholar]
- 83. Mahé I, Chidiac J, Helfer H, et al. Factors influencing adherence to clinical guidelines in the management of cancer-associated thrombosis. J Thromb Haemost 2016;14:2107–13. 10.1111/jth.13483 [DOI] [PubMed] [Google Scholar]
- 84. Matzdorff A, Ledig B, Stuecker M, et al. Practice patterns for prophylaxis and treatment of venous thromboembolism in German cancer patients. Oncol Res Treat 2016;39:194–201. 10.1159/000444734 [DOI] [PubMed] [Google Scholar]
- 85. Kleinjan A, Aggarwal A, Van de Geer A, et al. A worldwide survey to assess the current approach to the treatment of patients with cancer and venous thromboembolism. Thromb Haemost 2013;110:959–65. 10.1160/TH13-05-0414 [DOI] [PubMed] [Google Scholar]
- 86. Khorana AA, Yannicelli D, McCrae KR, et al. Evaluation of US prescription patterns: are treatment guidelines for cancer-associated venous thromboembolism being followed? Thromb Res 2016;145:51–3. 10.1016/j.thromres.2016.07.013 [DOI] [PubMed] [Google Scholar]
- 87. Kleinjan A, Hutten BA, Di Nisio M, et al. Anticoagulant treatment of cancer patients with pulmonary embolism in the real world. actual use of low-molecular-weight heparin in cancer. Neth J Med 2014;72:467–72. [PubMed] [Google Scholar]
- 88. Dault R, Vanasse A, Blais L, et al. Patterns and predictors of use of anticoagulants for the treatment of venous thromboembolism following approval of rivaroxaban. Clin Appl Thromb Hemost 2016;22 765 71. 10.1177/1076029615611249 [DOI] [PubMed] [Google Scholar]
- 89. Kakkar AK, Levine M, Pinedo HM, et al. Venous thrombosis in cancer patients: insights from the FRONTLINE survey. Oncologist 2003;8:381–8. 10.1634/theoncologist.8-4-381 [DOI] [PubMed] [Google Scholar]
- 90. Zwicker JI, Rojan A, Campigotto F, et al. Pattern of frequent but nontargeted pharmacologic thromboprophylaxis for hospitalized patients with cancer at academic medical centers: a prospective, cross-sectional, multicenter study. J Clin Oncol 2014;32:1792–6. 10.1200/JCO.2013.53.5336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Deitcher SR. Primary prevention of venous thromboembolic events (VTE) in cancer patients: an American survey study. J Clin Oncol 2004;22:8086. 10.1200/jco.2004.22.14_suppl.8086 [DOI] [Google Scholar]
- 92. Lee AY, Peterson EA. Treatment of cancer-associated thrombosis. Blood 2013;122:2310–7. 10.1182/blood-2013-04-460162 [DOI] [PubMed] [Google Scholar]