Abstract
Objective
Oral tyrosine kinase inhibitor has been shown to prolong progression-free survival (PFS) in epidermal growthfactor receptor (EGFR) mutation positive adenocarcinoma; however, the comparator arm has not included the current standard adenocarcinoma regimen (pemetrexed carboplatin induction followed by maintenance pemetrexed) and patients from Indian subcontinent. Hence, this study was carried out in Indian patients to compare gefitinib with the above-mentioned chemotherapy regimen.
Methods
This was an open-labelled, randomised, parallel group study comparing gefitinib (250 mg orally daily) with pemetrexed (500 mg/m2) and carboplatin (area under the curve 5) doublet intravenous induction chemotherapy regimen followed by maintenance pemetrexed (500 mg/m2) in patients with EGFR-activating mutation-positive stage IIIB or stage IV adenocarcinoma lung in the first-line setting. The primary endpoint for the study was PFS. 260 patients were required to demonstrate a 50% improvement in PFS of gefitinib over chemotherapy, with 80% power and 5% type 1 error. With an expected 5% dropout rate, the sample size was 290 patients.
Results
The median PFS in gefitinib arm was 8.4 months (95% CI 6.3 to 10.5 months) compared with 5.6 months (95% CI 4.2 to 7.0 months) in pemetrexed–carboplatin arm (HR: 95% CI 0.513 to 0.851; p −0.001). The impact of gefitinib on PFS was seen across all subgroups. There was no statistically significant difference in overall survival between the two arms. Haematologicalgrade3–4toxicities likeanaemia,neutropaenia and thrombocytopaenia were common in the pemetrexed–carboplatin arm while grade3–4 acneiform rash and diarrhoeawere common in the gefitinib arm.
Conclusion
The study confirms the superiority of gefitinib in prolonging PFS against the most active chemotherapy regimen of pemetrexed–carboplatin followed by maintenance pemetrexed in EGFR-mutated lung adenocarcinoma. The median PFS in Indian patients in gefitinib arm is similar to that reported in east Asians and Caucasians.
Keywords: EGFR mutation, NSCLC, Gefitinib, Pemetrexed, Lung cancer, Palliative
Key questions.
What is already known about this subject?
Activating mutations in the tyrosine kinase domain of epidermal growth factor receptors (EGFR) are important driver mutations in lung carcinoma.
Targeting these mutations with tyrosine kinase inhibitors (TKI) like gefitinib or erlotinib improves progression-free survival (PFS) but not overall survival in comparison with taxane and platinum or gemcitabine and platinum chemotherapy regimen.
In current era, in non-squamous lung cancer, pemetrexed with carboplatin followed by maintenance pemetrexed has emerged as the new standard of chemotherapy.
There is no existing comparison between the current standard, pemetrexed carboplatin/cisplatin (including maintenance pemetrexed) and gefitinib/erlotinib in EGFR-mutated lung cancer.
What does this study add?
The study provides evidence that gefitinib prolongs PFS against chemotherapeutic regimen of pemetrexed–carboplatin followed by maintenance pemetrexed in EGFR-mutated lung adenocarcinoma.
The overall survival is similar between the two regimens as per previously published studies.
How might this impact on clinical practice?
The results suggest that all patients with EGFR-activating mutation positive lung cancer should be treated with upfront gefitinib. Its is a better option than pemetrexed carboplatin regimen.
Background
Activating mutations in the tyrosine kinase domain of epidermal growth factor receptors (EGFRs) are important driver mutations in lung carcinoma.1 Tyrosine kinase inhibitors (TKIs), both reversible (gefitinib and erlotinib) and irreversible (afatinib), are available to block these receptors.2 Multiple studies done in East Asia, Europe and USA have shown that patients with EGFR-mutated tumours who are treated with TKIs as compared with platinum-based doublet chemotherapy have an improvement in progression-free survival (PFS) and objective response rate (ORR), but no improvement in overall survival (OS).3–7
The choice of the chemotherapy regimen for non-small-cell lung cancer (NSCLC) has changed from a common platinum-based regimen for all tumour types to a histology-directed approach. Pemetrexed with carboplatin is one of the most active chemotherapy combination regimens for patients with adenocarcinoma histology. The pemetrexed–cisplatin regimen prolonged OS as compared with gemcitabine–cisplatin in patients with adenocarcinoma and large cell carcinoma.8 Most of the trials that compared TKI with chemotherapy in EGFR-mutated adenocarcinoma used a regimen that included either gemcitabine or docetaxel with cisplatin or carboplatin.3–7 LUX-Lung 3 was the only study to compare TKI with the most effective pemetrexed–cisplatin combination regimen in patients with EGFR-mutated adenocarcinoma.7 The patients who were randomised to the chemotherapy arm in the LUX-Lung 3 study received up to six cycles of pemetrexed–cisplatin induction chemotherapy (maintenance was not permitted), while the patients randomised to TKI could receive afatinib until progression.
The PARAMOUNT study demonstrated that in patients with non-squamous NSCLC who have non-progressive disease after induction chemotherapy, maintenance pemetrexed significantly prolongs PFS and OS (median PFS almost doubled from 2.6 to 4.3 months).9 Thus, the current standard first-line chemotherapy regimen for a patient with non-squamous NSCLC consists of pemetrexed–platinum induction followed by maintenance pemetrexed in patients with no evidence of disease progression (PD). As these results were not available when initial studies with oral TKI were carried out, the comparator arm in these studies would be considered as suboptimal in present era. To the best of our knowledge, no trial has compared the efficacy of an oral TKI with this current standard of pemetrexed–platinum induction followed by maintenance pemetrexed in EGFR-mutation-positive adenocarcinoma.
The Indian subcontinent contributes 23.6% of the world’s population and a significant number of patients with lung cancer. Although the initial trials in EGFR-mutation-positive lung cancer were done in the Asian population, patients from the Indian subcontinent were not represented in these studies.4 10 Hence, we planned this study to confirm the efficacy and safety of gefitinib in Indian patients with advanced EGFR-mutation-positive non-squamous lung cancer compared with pemetrexed with carboplatin induction chemotherapy followed by maintenance pemetrexed.
Methods
Study design
This was a single-centre, phase III, double-arm, parallel-group, open-labelled exploratory randomised study. The study was conducted in the outpatient department of the thoracic medical oncology unit in a tertiary care oncology centre in Mumbai, India.
Eligibility criteria
Adult patients (≥18 years) with pathologically confirmed adenocarcinoma of the lung were eligible for the study if they were previously untreated, had classic activating EGFR mutation (mutations in exons 18, 19 or 21), an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2 (at least ambulatory and capable of all self-care activities, may be unable to do any work-related activities and up and about more than half of the waking hours), locally advanced stage IIIB not amenable to local therapy or stage 4 (metastatic) disease, with measurable disease according to the RECIST criteria V.1.1 and with adequate organ function. We excluded patients with any of the following features: uncontrolled medical comorbidities, concurrent use of any other investigational agent, pregnancy, patients who had already received any palliative chemotherapy, biological therapy or immunotherapy, known severe hypersensitivity to carboplatin or pemetrexed, pre-existing idiopathic pulmonary fibrosis and a life expectancy of less than 12 weeks.
Intervention
After obtaining written informed consent, patients were randomly assigned in a 1:1 ratio to one of two arms: arm A (gefitinib) or arm B (pemetrexed–carboplatin). Block randomisation was done centrally by a neutral person who was not a part of the study team and was based in the clinical research secretariat in the hospital campus.
Patients in arm A received gefitinib 250 mg orally once daily. The first tablet of gefitinib was taken orally within 72 hours of randomisation and was to be continued daily until PD, intolerable toxicity or other prespecified criteria for discontinuation were met.
Patients in arm B received the doublet-combination chemotherapy regimen of carboplatin and pemetrexed. Pemetrexed was administered at 500 mg/m2 intravenously over 10 min on day 1, immediately followed by carboplatin dosed at area under the curve 5 (calculated by the Calvert formula) intravenously over 30 min. Patients received an appropriate high-risk antiemetic prophylaxis regimen, dexamethasone (8 mg orally daily, started from the day before chemotherapy, continued for 3 days) along with vitamin B12 (900 μg parenterally started 1 week prior to chemotherapy and repeated every 12 weeks) and folic acid (5 mg orally daily started at least 5 days prior to chemotherapy and continued daily until 2–3 weeks after the last cycle of pemetrexed) supplementation along with the chemotherapy.
The first chemotherapy cycle was administered within 10 days of randomisation. The regimen was repeated every 3 weeks for a total of six cycles. Patients who had non-PD following the completion of six cycles were offered maintenance pemetrexed, which was administered at a dose of 500 mg/m2 intravenously over 10 min every 3 weeks with an appropriate low risk antiemetic prophylaxis regimen, dexamethasone (8 mg orally daily, started from the day before chemotherapy, continued for 3 days), vitamin B12 and folic acid supplementation. Maintenance pemetrexed was continued until PD, intolerable toxicity or other prespecified criteria for discontinuation were met. The study design is depicted in online supplementary figure 1S
esmoopen-2017-000168supp001.pdf (189KB, pdf)
.
EGFR mutation testing
DNA was extracted from the formalin-fixed paraffin-embedded tumour blocks and amplified for exons 18, 19, 20 and 21 using a nested-PCR method with Taqman probes. Details of the procedure have been previously published by our group.11 12
Disease assessments
Patients were evaluated on the day 7 after the start of therapy and then on days 15, 21, 42, 63, 84, 105 and then subsequently every 2 months until progression. The initial visits at day 7 and day 15 were planned for evaluation of adverse events in pemetrexed–carboplatin in accordance with institutional policy. To have similarity between the two arms and avoid bias even in arm A, patients were seen on same days. At each visit, the patient’s history, physical examination, concurrent medications, adverse events and subjective response were documented. Adverse events were graded according to the common toxicity criteria for adverse events (CTCAE) V.4.02. Response assessment scans (axial imaging with contrast enhanced CT scans of the thorax and upper abdomen) were performed every 6 weeks for first 12 weeks and then every 8 weeks until objective PD was documented. Additional imaging was performed at the physician’s discretion based on the patient’s symptomatology or an abnormality in the blood test results. If a lesion was documented beyond thorax and abdomen in the baseline scan, then the body part harbouring the lesion was also included in the subsequent restaging scans except in the case of brain metastasis. In patients with stable brain metastasis at baseline who had received either surgical or radiation treatment for the brain metastasis, routine follow-up MRI of the brain was not performed. Response assessment was done according to RECIST criteria V.1.1.13
Follow-up postprogression
Survival follow-up began after the documentation of objective PD. Following disease progression on either of the arms, subsequent therapy was at the discretion of the treating physician. In general, patients who progressed on gefitinib were offered pemetrexed–carboplatin chemotherapy, and patients who progressed on the pemetrexed–carboplatin arm were offered gefitinib, as long as this therapy was deemed appropriate by the treating physician. After the documentation of PD, patients were followed up once every 8 weeks either by direct contact with the patient, the patient’s family, the patient’s family physician or via telephonic contact. Follow-up continued until the patient’s death, withdrawal of informed consent or final data cut-off and study closure.
End points
The primary end point of the study was PFS, which was defined as the time from randomisation to the first documentation of objective PD, change in treatment or death from any cause. Patients who did not have an event for PFS at the time of the data cut-off were censored at the date of their last objective tumour assessment. This included patients who lost to follow-up or who had withdrawn consent. The PFS for patients who did not undergo any repeat scans after their baseline scan were censored on day 0. Secondary end points included overall survival (OS) (defined as the time from randomisation to death from any cause), overall response rate (calculated as per RECIST), adverse events (graded as per CTCAE) and quality of life (scored on the European Organization for Research and Treatment of Cancer general and lung specific quality of life forms).
Sample size
We estimated that to demonstrate a 50% improvement in PFS for gefitinib as compared with pemetrexed–carboplatin, for an HR for progression of 0.48, with 80% power and 5% type 1 error, we needed to enrol 260 patients. With an expected dropout rate of 5%, we planned to enrol 290 patients. The baseline assumptions for our sample size estimation were taken from the IPASS study, which was the only randomised trial at the time of planning our study, comparing TKI with chemotherapy in a cohort of patients with lung adenocarcinoma, clinically enriched for a higher likelihood of harbouring an EGFR-activating mutation.10
Statistical analysis
The analysis for the primary end point that is, PFS was done by intention-to-treat method. Survival analysis, both PFS and OS, were estimated by the Kaplan-Meier method. The PFS between the two arms was compared using the log rank test. The COX regression analysis was used to estimate the HR with its 95% CI. As an unplanned post hoc exploratory analysis, we tested other factors for their impact on PFS, including age (below 65 years vs 65 years and over), gender (male vs female), smoking status (absent vs present), tobacco use (absent vs present), stage (3B vs 4), presence of brain metastasis, presence of liver metastasis, type of EGFR mutation (exon 19 vs exon 21) and ECOG PS (0–1 vs 2). These factors were then tested along with the study arm in a multivariate analysis. A p value of below 0.05 was considered significant. Forest plot was generated to depict the impact of gefitinib on PFS in the important subgroups. A similar analysis was done for OS. The ORR and toxicities were calculated by simple percentages and the difference in ORR and toxicities between the two arms was compared by Fisher’s exact test. In addition, descriptive statistics were performed. Continuous variables were expressed in terms of the median along with either range or IQR. Nominal or ordinal variables were expressed in terms of proportion with their respective 95% CI. Data cut-off was on 14 July 2016.
Study oversight
This was an investigator-initiated study and was approved by the institutional ethics committee. The study was planned, conceptualised and written by the principal investigator. The study was registered with Clinical Trial Registry of India (CTRI/2015/08/006113). The study was conducted in accordance with good clinical practice guidelines, the Declaration of Helsinki and relevant national (Indian Council for Medical Research) and institutional (Tata Memorial Hospital Institutional Ethics Committee and Clinical Research Secretariat Guidelines) for human experimentation. The study was monitored by the institutional data safety and monitoring committee on 21 March 2013. The data were analysed and interpreted by all the investigators. The first draft of the manuscript was written by the lead author and the corresponding author. All authors reviewed, modified and approved the final draft. All authors had unrestricted access to the data, vouch for completeness and accuracy of the data and verify that the draft results are in accordance with the study plan reported in the protocol.
Results
Baseline characteristics
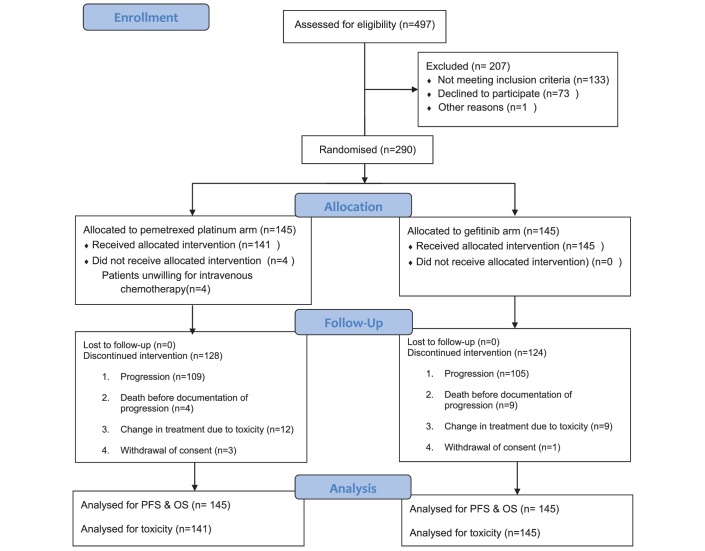
Between February 2012 and April 2016, 290 patients were randomised, 145 to each arm. The details of patient allocation and events are depicted in the CONSORT diagram in figure 1. The baseline characteristics are shown in table 1. The patients’ baseline characteristics were well balanced between the two arms except for gender distribution, with a higher proportion of female patients in the arm A (p value 0.001). The median age of the entire patient cohort was 54 years (range 26–80 years). The overall proportion of important subgroups included 43.4% female patients (126/290), 20.7% ex-smokers (60/290), 38.3% tobacco chewers (111/290) and 94.1% had ECOG PS 0–1 (273/290).
Figure 1.
Consort diagram.
Table 1.
Baseline details of patients in both arms
| Variable | Arm A (gefitinib arm) | Arm B (pemetrexed–platinum arm) |
| Mean age | 54.44 years | 53.12 years |
| Elderly status (≥65 years) | ||
| Gender | ||
| Male | 67 (46.2%) | 97 (66.9%) |
| Female | 78 (53.8%) | 48 (33.1%) |
| Smoking status | ||
| Never smoker | 113 (77.9%) | 117 (80.7%) |
| Ex-smoker | 32 (22.1%) | 28 (19.3%) |
| Tobacco habit status | ||
| Present | 52 (35.9%) | 59 (40.7%) |
| Absent | 93 (64.1%) | 86 (59.3%) |
| ECOG PS | ||
| PS-0 | 3 (2.1%) | 2 (1.4%) |
| PS-1 | 132 (91.0%) | 136 (93.8%) |
| PS-2 | 10 (6.9%) | 7 (4.8%) |
| Stage | ||
| 3 | 2 (1.4%) | 3 (2.1%) |
| 4 | 143 (98.6%) | 142 (97.9%) |
| Presence of brain metastasis | 22 (15.7%) | 23 (15.9%) |
| Presence of liver metastasis | 35 (24.1%) | 40 (27.6%) |
| EGFR mutation status Exon 18 (G719C point mutation) | 4 (2.8%) | 2 (1.4%) |
| Exon 19 | 76 (52.4%) | 92 (63.4%) |
| Exon 21 | 65 (44.8%) | 51 (35.2%) |
ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor.
Treatment delivery and toxicity
In arm A, 27 patients (18.6%) required interruptions in gefitinib. The median number of interruptions was 1 (IQR 1–3). The median time for which gefitinib was on hold during a single interruption was 11 days (IQR 7–24 days). The median cumulative length of drug interruption considering all periods of interruption was 14 days (IQR 7–27 days). The reasons for drug interruption included toxicity in 22 patients (81.5%) and non-compliance in 5 patients (18.5%). Toxicities that led to drug interruptions were skin (nine patients), liver (nine patients), diarrhoea (three patients) and myelosuppression (one patient). One patient developed grade 4 thrombocytopaenia from gefitinib; the patient was then treated with off-trial dose reductions. However, grade 4 thrombocytopaenia persisted even after dose reduction to 250 mg once a week, and hence the gefitinib was discontinued. Nine patients (6.2%) required a change in therapy due to intolerable toxicity.
Patients randomised to arm B received a median of six cycles of pemetrexed–carboplatin (range 0–6 cycles). Following the completion of six cycles of induction pemetrexed–carboplatin chemotherapy, 64 patients (44.1%) were eligible for maintenance pemetrexed. Six patients refused maintenance. A median of six cycles of maintenance pemetrexed was delivered (range 1–26 cycles). Forty patients (27.6%) had a delay in chemotherapy. The reasons for delay were toxicity in 30 patients and logistic difficulties in 10 patients. Dose reductions were required in pemetrexed alone in five patients and in both pemetrexed and carboplatin in seven patients. The median reduction in the doses of both pemetrexed and carboplatin was 20%. In three patients, following the initial dose reduction, subsequent chemotherapy cycles could be delivered at the reduced dose. In 12 patients (8.3%), treatment had to be changed due to toxicity.
The worse grade 3–4 toxicities are shown in table 2. The cumulative incidence of adverse events of any grade in arm A and arm B was 88.3% and 91.7%, respectively (p value 0.434). The pattern of toxicity differed between the two treatment arms. Acneiform rash and diarrhoea were common in arm A while haematological toxicities like anaemia, neutropaenia and thrombocytopaenia predominated in arm B (table 2). There was no therapy-related death in either arm.
Table 2.
All grade adverse events in both arms. Only the worst grade toxicity is shown
| Variable | Pemetrexed arm (n=141) | Gefitinib arm (n=145) |
| Haematological | ||
| Anaemia | 111 (78.7%) | 77 (53.1%) |
| Neutropaenia | 53 (37.6%) | 4 (2.8%) |
| Thrombocytopaenia | 57 (40.4%) | 10 (6.9%) |
| Biochemical | ||
| Raised SGOT | 57 (40.4%) | 77 (53.1%) |
| Raised SGPT | 72 (51.1%) | 79 (54.5%) |
| Clinical | ||
| Skin rash | 40 (28.4%) | 101 (69.7%) |
| Loose motions | 39 (27.7%) | 67 (46.2%) |
| Vomiting | 34 (24.1%) | 19 (13.1%) |
| Febrile neutropaenia | 12 (8.5%) | 1 (0.7%) |
SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic aspartate aminotransferase.
Outcomes
Response rate
The best ORR in evaluable patients was 63.5% in arm A (87, n=137) and 45.3% in arm B (59, n=130) (p –0.003, Fisher’s exact test, two-sided p value). The details of ORR are shown in online supplementary table S1.
Progression-free survival
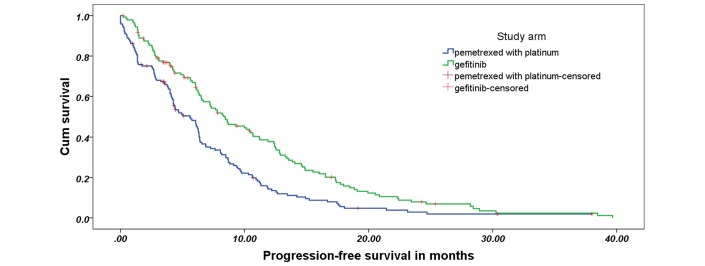
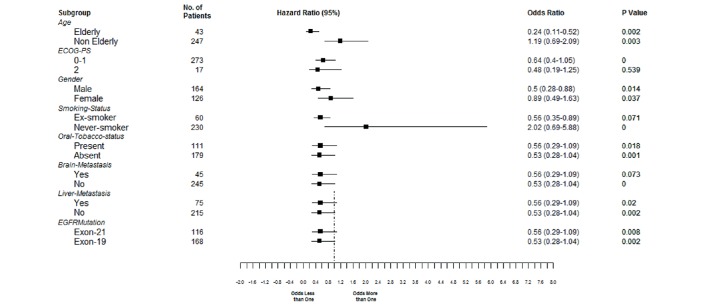
The median duration of follow-up at the time of analysis was 14.2 months; 257 events that fulfilled the criteria for PD had taken place. The median PFS in arm A was 8.4 months (95% CI 6.3 to 10.5 months) compared with 5.6 months (95% CI 4.2 to 7.0 months) in arm B (HR: 0.66, 95% CI 0.513 to 0.851; p −0.001) (figure 2). The impact of various factors on PFS and the adjusted HRs on multivariate analysis are shown in the supplementary table S2. The superiority of gefitinib on PFS was seen in all patient subgroups, as depicted in the forest plot in figure 3.
Figure 2.
Progression free survival curve.
Figure 3.
Forest plot for progression-free survival. Value below 1 suggests benefit from gefitinib. ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor.
Overall survival
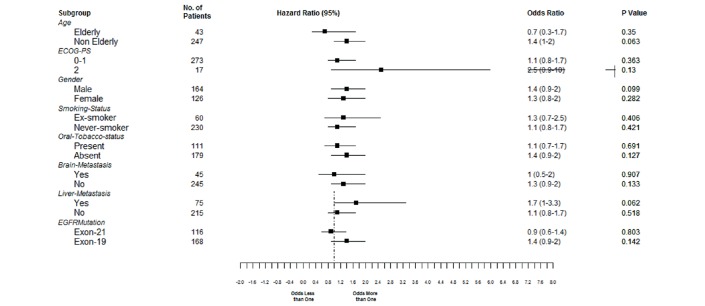
One hundred and sixty-three patients have died. The median OS in arm A was 18 months (95% CI 15.2 to 20.8), while it was 22.6 months (95% CI 18.6 to 26.6) in arm B (HR: 0.78, 95% CI 0.56 to 1.09, p value 0.133). The impact of various factors on OS and the adjusted HRs on multivariate analysis are shown in online supplementary table S3. Figure 4 shows the forest plot for OS.
Figure 4.
Forest plot for overall survival. Value below 1 suggest benefit from gefitinib.
Post–first-line treatment
Two hundred and fourteen patients developed objective PD, 105 in arm A and 109 in arm B. One hundred and two patients out of 109 patients (93.6%) in arm B received second-line treatment, which consisted of gefitinib in all patients. In contrast, only 67 out of 105 patients in arm A who developed PD (63.8%) received second-line treatment, which consisted of pemetrexed–platinum (58 patients), weekly paclitaxel (6 patients), single-agent pemetrexed (1 patient) and other therapies (2 patients). The proportion of patients who received second-line treatment was statistically higher in arm B (p –0.000). The reasons why 45 patients who had PD did not receive second-line treatment are shown in table 3.
Table 3.
Reasons for not change of treatment postprogression
| Brain metastasis | Asymptomatic progression | Patients not willing | Poor performance status or clinical situation not permitting | Total | |
| Pemetrexed–platinum | 1 | 0 | 2 | 4 | 7 |
| Gefitinib | 8 | 7 | 7 | 16 | 38 |
Discussion
Our study achieved its primary end point and proved that first-line treatment of EGFR-mutated lung adenocarcinoma with gefitinib as compared with pemetrexed–carboplatin followed by maintenance pemetrexed led to a significant prolongation in PFS with an HR of 0.66. To the best of our knowledge, this is the first and largest study comparing gefitinib with pemetrexed–carboplatin doublet chemotherapy in the era of histology-directed therapy and maintenance therapy. Studies published earlier with reversible TKIs have either compared them with a taxane–platinum combination or gemcitabine–platinum combination.3–7 The results of our study confirm the fact that genotype-directed treatment of classic activating EGFR mutation positive lung adenocarcinoma improves PFS as compared with the most active chemotherapy regimen. The HR achieved in this study is similar to that reported in previous studies across the globe.3–7
The ORR, PFS and OS with oral TKI differ between studies reported from Asian regions and Europe.3–7 The reason for this difference may be ethnic differences. Indian patients are ethnically different from both East Asians and Caucasians. The results of our study confirm that irrespective of ethnicity, patients with EGFR mutation positive tumours are sensitive to TKIs and have a significantly longer PFS when treated with first-line TKI. The PFS and OS in Indian patients is similar to that reported across the globe (table 4).
Table 4.
Comparison of median PFS and OS reported in the current study with that reported in literature.
| Author | n | Region | Arms | PFS in months | OS in months | Second line received* |
| Rosell et al3 | 173 | Europe | Erlotinib | 9.7 | 19.3 | NR |
| Docetaxel or gemcitabine with either cisplatin or carboplatin | 5.2 | 19.5 | 76% | |||
| Zhou et al4 | 165 | China | Erlotinib | 13.1 | 22.8 | NR |
| Gemcitabine with carboplatin | 4.6 | 27.2 | NR | |||
| Maemondo et al5 | 230 | Japan | Gefitinib | 10.8 | 30.5 | 67.5% |
| Paclitaxel/carboplatin | 5.4 | 23.6 | 94.6% | |||
| Mitsudomi et al6 | 177 | Japan | Gefitinib | 9.2 | 30.9 | NR |
| Docetaxel/cisplatin | 6.3 | NR | NR | |||
| Sequist et al7 | 345 | East Asia, Europe, Australia | Afatinib | 11.1 | NR | NR |
| Pemetrexed/cisplatin | 6.9 | NR | NR | |||
| Current study | 290 | India | Gefitinib | 8.4 | 18 | 63.8% |
| Pemetrexed carboplatin followed by pemetrexed maintenance | 5.6 | 22.6 | 93.6% |
*The percentage of patients who received second line among patients who were eligible for same.
NR, not reported; OS, overall survival; PFS, progression-free survival.
The superiority of TKI has been speculated to be a function of continuous treatment until PD as opposed to cytotoxic chemotherapy that is administered for a fixed number of cycles rather than treatment until PD. The results of our study disproves this hypothesis. In prior studies reported with reversible or irreversible TKIs, the comparator chemotherapy arms were restricted to a maximum of four to six cycles of platinum doublet chemotherapy; maintenance therapy was not permitted.3–7 However, in our study, the comparator arm consisted of induction chemotherapy with six cycles of pemetrexed and carboplatin, followed by continuation maintenance pemetrexed in patients with non-PD. Forty-four per cent of the patients in arm B received continuous treatment. This proportion is lower than that reposted in prior studies of continuous pemetrexed maintenance (57.4%–67.3%)) (PARAMOUNT trial- AVAPERL trial).9 14 However, an important difference between our trial and prior maintenance trials was that in our trial, patients were considered for maintenance pemetrexed after six cycles, while in prior studies, induction chemotherapy consisted of four cycles of doublet chemotherapy. In our study, at completion of four cycles of pemetrexed and carboplatin, 70.1% of patients would have been eligible for maintenance pemetrexed. This figure is similar to that reported in other maintenance studies.9 14 In keeping with the results reported by Park et al15, who reported that six cycles of chemotherapy improved time to progression as compared with four cycles, we planned the comparator arm to consist of six cycles of chemotherapy followed by maintenance chemotherapy, since PFS was the primary end point of our study.
Afatinib has been compared with six cycles of pemetrexed cisplatin in the LUX-Lung 3 study, and the study yielded similar results to our current study, that is, afatinib led to a longer PFS than pemetrexed–platinum.7 Afatinib is an irreversible TKI and by virtue of its mechanism of action should have a better efficacy than reversible TKIs like gefitinib. The evidence of such improved efficacy is seen in the LUX-Lung s7 study.16
Patients treated with gefitinib had a numerically inferior median OS than patients in the pemetrexed–carboplatin arm, which was opposite to the findings of PFS. Similar findings have been reported in prior studies like the OPTIMAL study in which patients treated with erlotinib had a significantly longer PFS (median 13.1 months) as compared with gemcitabine–carboplatin (4.6 months); however, the OS was not statistically different-median OS of 22.8 months for the patients treated with erlotinib versus 27.2 months for the chemotherapy-treated patients.4 There is no study that has shown that oral TKI prolongs OS as compared with chemotherapy in EGFR mutation positive patients. The reasons for the apparent lack of effect of TKI on OS may be due to patient crossover and differential treatment received following disease progression in both arms. In our study, a significant proportion of patients in the gefitinib arm did not receive second-line treatment due to various reasons including poor general condition at progression, asymptomatic progression or brain metastasis as the sole site of progression. Our study also highlights the importance of close follow-up in these patients. Patients on treatment in the gefitinib arm were followed up post day 105, every 2 months while patients on pemetrexed–carboplatin arm were followed up at 3 weekly intervals. This close follow-up may have enabled timely interventions in the patients on the pemetrexed–carboplatin arm. Even asymptomatic progression needs intervention in these patients. Both physicians and patients need to be sensitised to the fact that these patients can deteriorate rapidly and if therapy is not started at the time of asymptomatic PD, they may not be fit for further treatment by the time of the next follow-up. This contradicts the current literature which is largely based on retrospective evidence that suggests that asymptomatic progression or progression in sanctuary sites like brain which can be treated with radiation may not necessitate a change in treatment.17–19
The interesting and peculiar finding is the PFS and OS in tobacco chewers versus non-tobacco chewers. Though the relationship of EGFR mutation with smoking status is well known, the impact of tobacco chewing in non-smokers on EGFR mutation status is unknown. In our study though, oral tobacco use was neither an independent prognostic factor affecting PFS or OS.
Our trial was not without drawbacks. Our study was performed at a single institution. Patients in the chemotherapy arm did not receive bevacizumab. However, the use of bevacizumab with chemotherapy in adenocarcinoma is not accepted worldwide as the treatment of choice and a similar practice is followed at our institution. Gefitinib was chosen as the oral TKI rather than erlotinib or afatinib. Theoretically, erlotinib and afatinib may be more effective than gefitinib in the first-line setting, although evidence is still unclear on the same.
Conclusion
This study confirms that gefitinib prolongs PFS compared with the most active chemotherapy regimen of pemetrexed–carboplatin in EGFR-mutated NSCLC patients. The median PFS and OS in Indian patients with gefitinib is similar to east Asians and Caucasians.
Footnotes
Contributors: VP, KP : Concept, design, patient management, data entry, data analysis, data interpretation, first draft and final draft.
VN : Concept, design, patient management, design entry, design analysis, data interpretation, first draft and final draft.
AJ : Patient management, data interpretation, first draft and final draft.
AC: Mutation analysis, first draft and final draft.
AB : Statistical analysis, first draft and final draft.
RV: Pathology reports,first draft and final draft.
SG, SM : Patient management, first draft and final draft.
AR, AK, NP, AC, AG, VT : Data entry, first draft and final draft.
AM, AJ : Radiology, first draft and final draft.
NP: Nuclear medicine, first draft and final draft.
Funding: TRAC, intramural grant from Tata Memorial Hospital.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Correction notice: This article has been corrected since it was published Online First. Estimated glomerular filtration rate has been updated to read epidermal growth factor receptor.
References
- 1.Pao W, Chmielecki J, Rational CJ. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer 2010;10:760–74. 10.1038/nrc2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer 2015;88:74–9. 10.1016/j.lungcan.2015.01.026 [DOI] [PubMed] [Google Scholar]
- 3.Rosell R, Carcereny E, Gervais R, et al. ; Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46. 10.1016/S1470-2045(11)70393-X [DOI] [PubMed] [Google Scholar]
- 4.Zhou C, Wu YL, Chen G, et al. . Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42. 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 5.Maemondo M, Inoue A, Kobayashi K, et al. ; North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–8. 10.1056/NEJMoa0909530 [DOI] [PubMed] [Google Scholar]
- 6.Mitsudomi T, Morita S, Yatabe Y, et al. ; West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–8. 10.1016/S1470-2045(09)70364-X [DOI] [PubMed] [Google Scholar]
- 7.Sequist LV, Yang JC, Yamamoto N, et al. . Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–34. 10.1200/JCO.2012.44.2806 [DOI] [PubMed] [Google Scholar]
- 8.Scagliotti GV, Parikh P, von Pawel J, et al. . Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543–51. 10.1200/JCO.2007.15.0375 [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares L, de Marinis F, Dediu M, et al. . Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 2012;13:247–55. 10.1016/S1470-2045(12)70063-3 [DOI] [PubMed] [Google Scholar]
- 10.Mok TS, Wu YL, Thongprasert S, et al. . Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–57. 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 11.Chougule A, Prabhash K, Noronha V, et al. . Frequency of EGFR mutations in 907 lung adenocarcioma patients of Indian ethnicity. PLoS One 2013;8:e76164 10.1371/journal.pone.0076164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noronha V, Prabhash K, Thavamani A, et al. . EGFR mutations in Indian lung cancer patients: clinical correlation and outcome to EGFR targeted therapy. PLoS One 2013;8:e61561 10.1371/journal.pone.0061561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 14.Barlesi F, Scherpereel A, Rittmeyer A, et al. . Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J Clin Oncol 2013;31:3004–11. 10.1200/JCO.2012.42.3749 [DOI] [PubMed] [Google Scholar]
- 15.Park JO, Kim SW, Ahn JS, et al. . Phase III trial of two versus four additional cycles in patients who are nonprogressive after two cycles of platinum-based chemotherapy in non small-cell lung cancer. J Clin Oncol 2007;25:5233–9. 10.1200/JCO.2007.10.8134 [DOI] [PubMed] [Google Scholar]
- 16.Park K, Tan EH, O’Byrne K, O’Byrne K, et al. . Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577–89. 10.1016/S1470-2045(16)30033-X [DOI] [PubMed] [Google Scholar]
- 17.Lo PC, Dahlberg SE, Nishino M, et al. . Delay of treatment change after objective progression on first-line erlotinib in epidermal growth factor receptor-mutant lung cancer. Cancer 2015;121:2570–7. 10.1002/cncr.29397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weickhardt AJ, Scheier B, Burke JM, et al. . Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807–14. 10.1097/JTO.0b013e3182745948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu HA, Sima CS, Huang J, et al. . Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol 2013;8:346–51. 10.1097/JTO.0b013e31827e1f83 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2017-000168supp001.pdf (189KB, pdf)