Abstract
During the last decade, the treatment of advanced or metastatic renal cell carcinoma (RCC) was revolutionised with the advent of antiangiogenic drugs and tyrosine-kinase inhibitors. Several agents targeting the vascular endothelial growth factor (VEGF) pathway (sunitinib, bevacizumab, pazopanib, axitinib) or the mammalian target of rapamycin pathway (temsirolimus, everolimus) were since then progressively approved for first-line or later-line use in the treatment of patients with advanced RCC and became the new standard of care. As a result, the survival of patients with advanced RCC has significantly improved from a median overall survival of approximately 12 months in the cytokines era to more than 26 months with first-line VEGF inhibitors. During the two last years, the treatment of advanced RCC has witnessed a second revolution with the advent of immune checkpoint inhibitors, especially agents targeting the programmed cell death-1 receptor, as well as with the advent of new generation tyrosine-kinase receptor inhibitors. This article will review the new therapeutic options available for the treatment of advanced RCC, as well as the future potential molecular targets that are currently being investigated.
Keywords: Renal cell carcinoma New treatments Tyrosine-kinase inhibitor Immunotherapy Programmed cell death-1
Introduction
During the last decade, the treatment of advanced or metastatic renal cell carcinoma (RCC) was revolutionised with the advent of antiangiogenic drugs and tyrosine-kinase inhibitors (TKI). Consequently, cytokines treatment such as interferon-α and interleukin-2 (IL-2), which were the standard-of-care treatment until then, were quickly abandoned or restricted to very selected situations (like IL-2). Several agents targeting the vascular endothelial growth factor (VEGF) pathway (sunitinib, bevacizumab, pazopanib, axitinib) or the mammalian target of rapamycin (mTOR) pathway (temsirolimus, everolimus) were since then progressively approved for first-line or later-line use in the treatment of patients with advanced RCC and became the new standard of care.1–6 As a result, the survival of patients with advanced RCC has significantly improved from a median overall survival (OS) of approximately 12 months in the cytokines era to more than 26 months with first-line VEGF inhibitors.2 3 7
During the two last years, the treatment of advanced RCC has witnessed a second revolution with the advent of immune checkpoint inhibitors, especially agents targeting the programmed cell death-1 (PD-1) receptor as well as with the advent of new-generation TKIs. This article will review the new therapeutic options available for the treatment of advanced RCC, as well as the future potential molecular targets that are currently being investigated such as dual phosphatidylinositol-3 kinase (PI3k)-mTOR inhibitors, programmed cell death-1 ligand-1 (PD-L1) inhibitors and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) inhibitors and its combinations.
New VEGFR inhibitors and combinations
Several antiangiogenic drugs that target VEGF (bevacizumab) and its receptors (sorafenib, sunitinib, pazopanib, axitinib) are approved standard-of-care treatments options in first-line and second-line, after showing improved progression-free survival (PFS) in randomised phase III trials compared with interferon-α, placebo or other targeted agents.1–4 8 However, these antiangiogenic agents rarely cause maintained durable tumour responses and most patients will ultimately experience disease progression despite an initial period of response due to the development of treatment resistance. The increasingly improved knowledge on the molecular mechanisms behind resistance to VEGF receptor (VEGFR) inhibitors has led to the development of several new-generation antiangiogenic drugs with additional targeted mechanism of action such as cabozantinib and lenvatinib.
Cabozantinib
Cabozantinib, an oral TKI that targets cMet, VEGFRs and anexelekto (AXL), was tested in a randomised phase III trial (METEOR trial) compared with everolimus, in patients with advanced RCC that had progressed after VEGFR-targeted therapy.9 Given that upregulation of cMet and AXL has been described as a resistance mechanism to VEGFR inhibitors in preclinical models of RCC,10 the use of cabozantinib in the second-line setting had a robust biological rationale. In total, 658 patients were included and randomised to cabozantinib 60 mg once daily or everolimus 10 mg once daily. The primary endpoint was PFS. The secondary endpoints included OS, objective response rate (ORR) and safety. The study met its primary endpoint of showing improved PFS with cabozantinib. The median PFS was 7.4 months with cabozantinib and 3.8 months with everolimus (HR 0.58, 95% CI 0.45 to 0.75, p<0.001). The benefit in PFS with cabozantinib was observed in all prespecified subgroups regardless of the number of prior VEGFR inhibitors and Memorial Sloan Kettering Cancer Center (MSKCC) prognostic risk category. The ORR was significantly higher with cabozantinib than with everolimus (21% vs 5%, p<0.001).9 Importantly, the final mature OS results based on an unplanned second interim analysis published 1 year later showed an improvement in OS for the first time with a VEGFR inhibitor in advanced RCC.11 The median OS was 21.4 months (95% CI 18.7 to not estimable) with cabozantinib and 16.5 months (95% CI 14.7 to 18.8) with everolimus (HR 0.66, 95% CI 0.53 to 0.83, p=0.00026). cMet expression by immunohistochemistry was analysed as a potentially predictive biomarker for cabozantinib benefit. However, the cMet expression was not found to be associated with a differential outcome with cabozantinib as patients with both high and low cMet expression levels benefited from treatment. Regarding tolerability, the toxicity profile of cabozantinib was described as manageable and similar to what has already been described with other VEGFR inhibitors. Grade 3–4 adverse events (AEs) were observed in 71% of patients treated with cabozantinib and 60% of patients treated with everolimus. Treatment discontinuation because of an AE was similar in both treatment arms: 12% with cabozantinib and 11% with everolimus. The most common grade 3–4 AEs with cabozantinib were hypertension (15%), diarrhoea (13%), fatigue (11%), palmar-plantar erythrodysesthesia syndrome (8%), anaemia (6%) and hypomagnesaemia (5%).11 The main results of this clinical trial are summarised in table 1. Consequently, in April 2016 the Food and Drug Administration (FDA) approved the use of cabozantinib to treat patients with advanced RCC previously treated with at least one anticancer treatment including an antiangiogenic agent, becoming a new standard-of-care treatment option in that setting. The European Medicines Agency (EMA) subsequently granted approval in Europe.
Table 1.
Clinical trials leading to newly approved drugs for advanced renal cell carcinoma
| Name of the study | Type of study | Setting | n | Primary endpoint | Treatment arms | Median OS | Median PFS | ORR | Most common grade 3–4 AEs |
| METEOR trial | Phase III randomised open-label | Second-line or later-line after ≥1 VEGFR inhibitor | 658 | PFS | Cabozantinib (n=330) |
21.4 months | 7.4 months | 21% | Hypertension (15%), diarrhoea (13%), fatigue (11%), PPES (8%) |
| Everolimus (n=328) |
16.5 months (HR 0.66, p=0.00026) |
3.8 months (HR 0.58, p <0.001) | 5% (p<0.001) |
Anaemia (16%), fatigue (7%), hyperglycaemia (5%) | |||||
| Phase II randomised open-label | Second-line or later-line after ≥1 VEGFR inhibitor | 153 | PFS | Lenvatinib plus everolimus (n=51) |
25.5 months | 14.6 months | 43% | Diarrhoea (20%), fatigue (14%), hypertension (14%), vomiting (8%), anaemia (8%) | |
| Lenvatinib (n=52) |
19.1 months (HR 0.75, p=0.32)* |
7.4 months (HR 0.66, p=0.12)* |
27% (p=0.10)* |
Proteinuria (19%), hypertension (17%), diarrhoea (12%), fatigue (8%) | |||||
| Everolimus (n=50) |
15.4 months (HR 0.51, p=0.024)* |
5.5 months (HR 0.40, p=0.0005)* |
6% (p<0.0001)* |
Anaemia (12%), dyspnoea (8%), hyperglycaemia (8%), hypertriglyceridaemia (8%) | |||||
| CheckMate 025 trial | Phase III randomised open-label | Second-line or third-line after ≥1 VEGFR inhibitor | 821 | OS | Nivolumab (n=406) |
25.0 months | 4.6 months | 25% | Fatigue (2%), anaemia (2%), diarrhoea (1%) |
| Everolimus (n=397) |
19.6 months (HR 0.73, p=0.002) |
4.4 months (HR 0.88, p=0.11) |
5% (p<0.001) |
Anaemia (8%), hypertriglyceridaemia (5%), hyperglycaemia (4%) |
*As compared with combination arm (lenvatinib plus everolimus).
AE, adverse events; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PPES, palmar-plantar erythrodysesthesia syndrome; VEGFR, vascular endothelial growth factor receptor.
High expression of cMet and AXL in patients with advanced RCC has been associated with poor prognosis.11 12 Therefore, because cabozantinib targets receptor tyrosine kinases including cMet and AXL, its use was also studied as first-line treatment in patients with advanced RCC with unfavourable prognostic signs. A randomised phase II trial (the cabozantinib versus sunitinib or CABOSUN trial) compared cabozantinib with sunitinib as first-line therapy in 157 patients with intermediate and poor risk advanced RCC as per the International Metastatic Renal Cell Carcinoma Database Consortium criteria.13 The primary endpoint was PFS while the secondary endpoints included OS, ORR and safety. Importantly, treatment with cabozantinib significantly prolonged PFS compared with that in patients treated with sunitinib. The median PFS was 8.2 months with cabozantinib and 5.6 months with sunitinib (HR 0.69, 95% CI 0.48 to 0.98, p=0.012). Cabozantinib was also associated with a greater ORR of 46% vs 18% with sunitinib. However, the median OS did not differ significantly between treatment arms (26.4 months with cabozantinib versus 23.5 months with sunitinib, HR 0.87, 95% CI 0.55 to 1.4). Cabozantinib’s safety profile was similar to what was described in the METEOR trial, with most common grade 3–4 AEs including hypertension (28%), diarrhoea (10%), palmar-plantar erythrodysesthesia syndrome (8%) and fatigue (6%). Of note, around 20% of patients in both arms had to discontinue treatment due to AEs.13 Consequently the authors conclude that cabozantinib represents a potential new treatment option for patients with untreated RCC. However, this affirmation should be taken with caution given the fact that the CABOSUN trial was a phase II study, with a small sample size and especially because the sunitinib arm significantly underperformed compared with the data from the pivotal trial of sunitinib versus interferon-α,2 which could indicate potential hidden biases. Having said that, cabozantinib might represent the first drug to show superiority over sunitinib in a randomised trial, which represents a potential milestone in the treatment of advanced RCC and warrants further investigations.
Lenvatinib and everolimus combination
The combination of different approved drugs on account of a potential biological rationale of synergistic activity based on the fact that they have different molecular targets or different mechanisms of action has long been a classic treatment strategy in advanced RCC. Given the pre-eminence of VEGFR and mTOR pathways in the treatment of RCC, the combination of a VEGFR and mTOR inhibitors has always been considered an attractive treatment strategy. However, most trials testing this treatment combination in the first-line setting using first-generation inhibitors such as sorafenib, bevacizumab, everolimus and temsirolimus only showed modest antitumour activity and greater toxicity compared with single agents.14–17
Recently, the first study to ever show a PFS benefit combining VEGFR and mTOR inhibitors was published.18 It was a randomised phase II trial comparing the third-generation VEGFR inhibitor lenvatinib combined with everolimus versus single-agent lenvatinib or single-agent everolimus as second-line therapy in 153 patients with advanced RCC previously treated with VEGF-targeted therapy. Lenvatinib is a multi-TKI of VEGFR1-3, with inhibitory activity against fibroblast growth factor receptors (FGFR1-4), PDGFRα, RET and KIT. Lenvatinib plus everolimus significantly prolonged PFS compared with everolimus alone (14.6 vs 5.5 months, HR 0.40, 95% CI 0.24 to 0.68, p=0.0005). Single-agent lenvatinib also significantly prolonged PFS compared with everolimus alone (HR 0.61, 95% CI 0.38 to 0.98; p=0.048). At the primary data cut-off, OS did not differ significantly between treatment arms (median OS of 25.5 months with lenvatinib plus everolimus, versus 17.5 months with single-agent everolimus). However, in the post-hoc updated analysis, the combination resulted in extended OS compared with everolimus alone (25.5 vs 15.4 months, HR 0.51, 95% CI 0.30 to 0.88; p=0.024).18 An objective response was achieved in 43% of patients treated with lenvatinib plus everolimus compared with 6% with everolimus alone (p<0.0001). However, this significant antitumour activity with the treatment combination was at the expense of a greater toxicity: grade 3–4 AEs occurred more frequently in the combination arm (71%) than with single-agent everolimus (50%). Almost a quarter of the patients on the combination arm (24%) discontinued treatment due to AEs as compared with only 12% in the single-agent everolimus arm. The most common AEs of in the lenvatinib plus everolimus arm were diarrhoea, hyporexia, fatigue and vomiting.18 The main results of this clinical trial are summarised in table 1. Nevertheless, despite the small sample size of the study and the tolerability issues, the FDA approved the combination of lenvatinib plus everolimus in May 2016 for the treatment of advanced RCC following one prior antiangiogenic therapy. EMA subsequently granted approval in Europe. Of note, a phase III is currently comparing the efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab versus sunitinib as first-line treatment.
PD-1 and PD-L1 inhibitors
PD-1 is a major immune checkpoint molecule implicated in immune-suppression and immune-tolerance. PD-1 is physiologically expressed in macrophages, dendritic cells, activated T cells and B cells, and binds to two ligands, PD-L1 and PD-L2. The interaction between PD-1 and PD-L1 negatively regulates activated T cell effector functions and acts as physiological brakes on tumour immune surveillance. The PD-1/PD-L1 signalling pathway can be used by cancers as an adaptative evolutionary advantage to evade the immune system. PD-L1 is overexpressed in up to 30% of RCC tumours and its expression is correlated with advanced tumour stage, higher Fuhrman grade, sarcomatoid differentiation and poorer survival.19–22 Therefore, PD-1/PD-L1 pathway represents an attractive target since its inhibition can restore antitumour T cell activity and promote immune-mediated tumour destruction. Moreover, PD-L1 inhibitors leave the PD-L2/PD-1 interaction intact, maintaining immune homeostasis and potentially preventing toxicity. Consequently, several PD-1 and PD-L1 inhibitors are currently being investigated in RCC and are at different stages of clinical development.
Nivolumab
Nivolumab, a fully human immunoglobulin-G4 PD-1 immune-checkpoint inhibitor antibody that selectively blocks the interaction between PD-1 and PD-L1 and PD-L2, is the first new immunotherapy agent to get regulatory approval for the treatment of advanced clear cell RCC. Motzer et al conducted a phase III randomised trial (CheckMate 025 trial) of nivolumab versus everolimus in advanced clear cell RCC.23 In total, 821 patients previously treated with one or two regimens of antiangiogenic therapies were randomised to receive 3 mg/kg of nivolumab or a 10 mg/day of everolimus. The primary endpoint was OS. The secondary endpoints included the ORR and safety. Importantly, nivolumab prolonged OS as compared with everolimus. The median OS was 25.0 months (95% CI 21.8 to not estimable) with nivolumab and 19.6 months (95% CI 17.6 to 23.1) with everolimus (HR 0.73, 98.5% CI 0.57 to 0.93, p=0.002). The OS benefit was observed irrespective of the MSKCC group and number of prior antiangiogenic therapies. Similarly, the benefit with nivolumab over everolimus was seen regardless of PD-L1 tumour immunohistochemistry expression. Interestingly, the median OS was consistently lower in the PD-L1 positive group irrespective of the treatment arm, which indicates a negative prognostic role of PD-L1 expression but do not support its role as a predictive marker of response to PD-1 blockade.
The ORR was also significantly greater with nivolumab than with everolimus (25% vs 5%; OR 5.98, 95% CI 3.68 to 9.72, p<0.001). The median PFS however was similar in both arms: 4.6 months (95% CI 3.7 to 5.4) with nivolumab and 4.4 months (95% CI 3.7 to 5.5) with everolimus (HR 0.88, 95% CI 0.75 to 1.03, p=0.11). Nivolumab safety profile was acceptable. The most common AEs with nivolumab were fatigue (33%), nausea (14%) and pruritus (14%). Nivolumab was better tolerated than everolimus, grade 3 or 4 treatment-related AEs occurring in 19% of the patients receiving nivolumab as compared with 37% with everolimus. Quality of life as measured by the Functional Assessment of Cancer Therapy- Kidney Symptom Index- Disease related Symptoms (FKSI-DRS) questionnaire was also significantly improved with nivolumab as compared with everolimus (p<0.05).23 The authors conclude that this is the first study to show improvement in OS in advanced RCC since the publication of the pivotal trial of temsirolimus.5 Consequently, in November 2015 the FDA approved the use of nivolumab to treat patients with metastatic RCC who have previously progressed to one or two regimens of antiangiogenic therapy, becoming a new standard-of-care treatment option in that setting. Nivolumab was subsequently EMA-approved for RCC in February 2016. The main results of this clinical trial are summarised in table 1.
The fact that OS but not PFS was prolonged in the phase III trial of nivolumab may be related to the intrinsic immunostimulatory mechanism of action of nivolumab. Immune cell activation requires time to take place, and therefore tumour kinetics could initially surpass that time and show a transient progression before experiencing objective response. Moreover, tumour infiltration by immune cell might increase the volume of tumour lesions and mimic progression, in what has been called pseudo-progression or flare phenomenon. Together, these events could lead to the observation of transient progression when using Response Evaluation Criteria In Solid Tumors (RECIST) criteria assessment that could lead to premature discontinuation of an active treatment affecting the assessment of PFS, but not necessarily OS. Similar findings have been observed with ipilimumab and nivolumab in patients with malignant melanoma and other immunotherapy agents in other tumour types. To address this issue, a modified version of RECIST, the immune-related response criteria, has been proposed to more adequately assess the delayed and mixed responses observed with new immunotherapy agents. Moreover, in order to deal with this issue, most clinical trials with PD1/PD-L1 inhibitors allow patients to continue on study therapy beyond initial disease progression if there is clinical benefit and the side-effect profile remains acceptable.
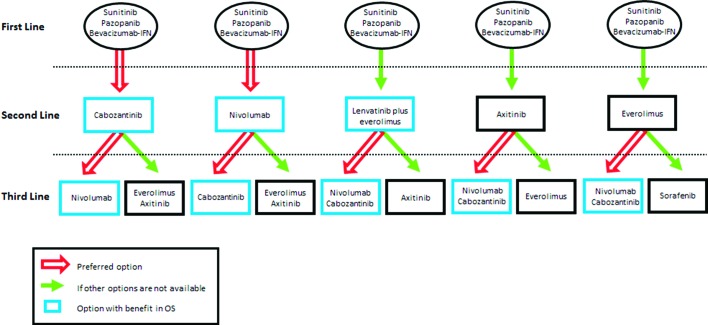
Finally, the recent approval of these three new treatment options (nivolumab, cabozantinib, lenvatinib plus everolimus) as second-line therapies has dramatically changed the landscape of advanced RCC. In view of the improvement in OS seen in large randomised phase III trials, both the National Comprehensive Cancer Network (NCCN) guidelines24 and the European Society for Medical Oncology (ESMO) guidelines25 now recommend nivolumab and cabozantinib as the new preferred second-line treatment options. Lenvatinib plus everolimus is also considered a valid option with an OS benefit. Of note, only the NCCN guidelines have included this latter treatment combination as an option, while the ESMO guidelines have not included it yet, pending the results of the corresponding phase III trial. If none of these three options are available, axitinib or everolimus should be considered. As for the third and subsequent treatment line, patients should ideally be assessed whenever possible for inclusion in clinical trials. If no clinical trial is available, and due to the absence of randomised clinical trials comparing one sequential therapy with another, several possible treatment sequences may exist depending on the drugs administered in the first and second line. A proposal of different possible sequential therapies for advanced RCC is summarised in figure 1.
Figure 1.
Proposal of different possible sequential approved therapies for advanced renal cell carcinoma. IFN, interferon; OS, overall survival.
PD-L1 inhibitors
Available data on the efficacy of PD-L1 inhibitors in advanced RCC are more limited given the earlier stage of drug development as compared with anti-PD-1 agents. BMS-936559, a fully human anti-PD-L1 IgG4 monoclonal antibody, was tested in a phase I study including 17 patients with metastatic RCC. The study drug was administered at escalating doses ranging from 0.3 to 10 mg/kg every 2 weeks in 6-week cycles for up to 16 cycles or until the patient had a complete response or confirmed disease progression. The ORR in patients with RCC was 12%, with responses lasting between 4 and 17 months, whereas disease stabilisation lasting at least 24 weeks occurred in 41% of the patients. A maximum tolerated dose (MTD) was not reached. Grade 3 or 4 related AEs occurred in 9% of patients for the whole study and tumour types.26 However, despite these promising efficacy data, the development of BMS-936559 in solid tumours has not pursued.
Atezolizumab, an engineered anti-PD-L1 monoclonal antibody, was investigated in a phase I trial, which included 70 patients with metastatic RCC.27 The study included a significant proportion of patients with unfavourable factors: 34% had a MSKCC poor-risk status, and 29% had Fuhrman grade 4 and/or sarcomatoid histology. Most patients were previously treated (87%), including VEGF pathway inhibitors (63%) and mTOR inhibitors (34%), and 57% had received two or more prior treatment lines. The median treatment duration was 8 months (range 1.0–35 months). Regarding clinical activity, the ORR was 15% (95% CI 7 to 26) in the whole group with a median duration of response of 17.4 months. ORR was also evaluated on the basis of PD-L1 immunohistochemistry expression: 18% in the PD-L1 positive group and 9% in the PD-L1 negative patients. Interestingly, ORR for sarcomatoid or Fuhrman grade 4 cases was 22% (95% CI 6 to 48). Overall, the median PFS was 5.6 months (95% CI 3.9 to 8.2), and the median OS was 28.9 months (95% CI 20.0 to not reached). Treatment was well tolerated, with only 17% of patients experiencing grade 3 related AEs, which included anaemia (4%), fatigue (4%) and hypophosphatemia (3%). No grade 4 AEs or deaths occurred. Immune-mediated AEs were reported in 30 patients (43%), most commonly grade 1 rash (20%) and grade 2 hypothyroidism (10%).27 Giving these promising results, the investigation of the role of atezolizumab in metastatic RCC has mainly pursued in combination with the VEGF inhibitor bevacizumab (see the following section).
Combining PD-1/PD-L1 inhibitors with VEGF pathway inhibitors
Despite the excellent results seen in the pivotal phase III study of nivolumab, most patients will ultimately developed progressive disease, as only 1% of patients had a complete response, and only 31% of patients had a durable response longer that 12 months. Moreover, the median duration of response was equal to that of everolimus (12.0 months). In order to improve patients’ outcome, several combination trials are currently under investigation. Given the fact that VEGF pathway inhibitors also modulate immune responses by increasing trafficking of T cells into tumour28 29 and reducing suppressing cytokines and infiltrating T regs,30 31 it has been hypothesised that combining PD-1/PD-L1 inhibitors and drugs targeting the VEGF pathway could attenuate tumour-induced immunosuppression, allowing the tumour to become more responsive to immunotherapy.32
Nivolumab in combination with sunitinib or pazopanib for previously treated advanced RCC has been investigated in a dose-escalation phase I trial.33 Patients received sunitinib (50 mg, 4 weeks on/2 weeks off) or pazopanib (800 mg daily) combined to nivolumab (starting dose 2 mg/kg with planned escalation to 5 mg/kg, 3-weekly) until progression/unacceptable toxicity. Patients who had been previously treated with sunitinib were included in the pazopanib arm and vice versa; patients who had received prior systemic therapy, but not pazopanib or sunitinib, were assigned alternately to one of the two arms. Fourteen patients were treated on the sunitinib arms, but no dose-limiting toxicities (DLTs) were observed and the MTD was not reached; thus, the 5 mg/kg arm was expanded by 19 patients. Twenty patients were included in the pazopanib arm at the 2 mg/kg cohort. Four DLTs (three elevated transaminases, and one fatigue) were observed, leading to closure of the arm. Grade 3–4 related AEs were observed in 82% and 70% of patients in the sunitinib and pazopanib arms, respectively. Most common grade 3–4 AEs were elevated alanine aminotransferase (ALT) and hypertension (18% each), hyponatraemia and lymphopaenia (15% each) in sunitinib arm, and elevated transaminases and diarrhoea (20% each) and fatigue (15%) in pazopanib arm. ORR was 52% and 45% in the sunitinib and pazopanib arms, respectively. Responses occurred early: in the first assessment (6 weeks) in 41% (sunitinib arm) and 56% (pazopanib arm) of patients.33
Similar results were seen in a dose-escalation phase I trial combining PD-1 inhibitor pembrolizumab and pazopanib in patients with treatment-naïve RCC.34 Twenty patients were enrolled and received combination therapy with pazopanib 800 mg daily and pembrolizumab 2 mg/kg 2-weekly, with planned escalation to 10 mg/kg. One patient experienced DLT with grade 3 elevations of ALT, aspartate aminotransferase (AST) and total bilirubin, and a safety review determined not to escalate the dose of pembrolizumab and also to reduce the starting dose of pazopanib to 600 mg. Despite this modification, all 20 patients experienced AEs. Grade 3 elevations of ALT/AST occurred in 65% of patients. Regarding efficacy, ORR was 60% in the 800 mg group and 20% in the 600 mg group.34 These preliminary results highlight the fact that although the combination of nivolumab or pembrolizumab with a VEGFR inhibitor can lead to encouraging antitumour activity and durable responses, it can also exacerbate TKI-related toxicity as seen with pazopanib-related hepatotoxicity. Therefore, combining PD-1/PD-L1 and VEGFR inhibition warrants further investigation but should be approached with caution regarding tolerability.
A better toxicity profile has so far been described in combination studies using bevacizumab or axitinib. Pembrolizumab in combination with bevacizumab is currently being investigated in a dose-escalation phase I trial for patients with metastatic RCC previously treated with at least one systemic therapy.35 Sixteen patients have been enrolled so far and no DLT or serious AEs related to the study drug have been reported. There were no grade 3–4 drug-related AEs, and the most common grade 1–2 toxicities were diarrhoea, fatigue, fever and hypertension. The ORR was 71%. The 200 mg fixed dose of pembrolizumab and 15 mg/kg dose of bevacizumab, both given every 3 weeks, were determined to be safe and recommended for a phase II study, which is ongoing.
A similar favourable toxicity profile was recently described in a phase I study of bevacizumab in combination with atezolizumab. Twelve patients with metastatic RCC were included and received bevacizumab 15 mg/kg plus atezolizumab 20 mg/kg every 3 weeks. Up to 83% of patients had no prior systemic therapy. The ORR was 40% (95% CI 15% to 73%) with a median treatment duration with atezolizumab of 9.4 months.36 The combination was well tolerated with no grade 3–4 AEs and no exacerbation of bevacizumab-associated AEs. Giving these promising results, atezolizumab was investigated in monotherapy or in combination with bevacizumab compared with sunitinib in a phase II trial including 305 patients with treatment-naïve metastatic RCC.37 Crossover to the combination arm after disease progression was allowed for patients in any of the monotherapy arms. The ORR was similar in the three treatment arms for the whole study population: 32%, 25% and 29% in the combination arm, atezolizumab monotherapy arm and sunitinib monotherapy arm, respectively. The ORR in the PD-L1 positive subgroup was higher with the treatment combination (46%), than in the monotherapy arms (atezolizumab 28%; sunitinib 27%). The median PFS was longer with atezolizumab-bevacizumab than with sunitinib alone (11.7 vs 8.4 months, respectively), but the difference was not statically significant (HR 1.00, 95% CI 0.69 to 1.45, p=0.982). In the PD-L1 positive subgroup, the magnitude of the difference in PFS was even greater (14.7 vs 7.8 months) but remained not statically significant (HR 0.64, 95% CI 0.38 to 1.08, p=0.095).37 Similar negative results in PFS were seen when atezolizumab monotherapy was compared with sunitinib monotherapy. The safety of the atezolizumab-bevacizumab was consistent with the known profile of both drugs in monotherapy. Atezolizumab-bevacizumab was better tolerated than sunitinib: treatment-related grade 3–4 AEs were seen in 40%, 16% and 57% of patients in the combination arm, atezolizumab and sunitinib monotherapy arms, respectively. AEs leading to death occurred in 3%, 2% and 2% of patients, respectively. The authors concluded that, despite the negative results in terms of PFS, atezolizumab-bevacizumab combination resulted in encouraging antitumour activity in the PD-L1 positive subgroup. A randomised phase III trial of atezolizumab alone or combined with bevacizumab versus sunitinib in patients with previously untreated metastatic RCC is currently ongoing.
With regard to axitinib, two different phase I studies are currently investigating its combination with PD-1/PD-L1 blockade agents. In the first phase I study, 52 patients with treatment-naïve metastatic RCC were treated with axitinib 5 mg twice daily continuously plus pembrolizumab 2 mg/kg intravenously every 3 weeks38. The combination showed excellent preliminary signs of antitumour activity with an ORR of 71.2% (95% CI 56.9 to 82.9) and three patients presenting with complete response (5.8%). Treatment was well tolerated, with most patients developing only grade 1 or 2 toxicities. Most common grade 3 AEs included hypertension (17.3%), diarrhoea (9.6%), fatigue (5.8%) and increased ALT (5.8%). The second phase I study is evaluating the combination of axitinib 5 mg twice daily continuously plus avelumab 10 mg/kg intravenously every 2 weeks in patients with treatment-naïve advanced RCC.39 So far, six patients have been treated and all of them have showed a partial response (ORR 100%). Treatment combination was well tolerated and enrolment is ongoing in the expansion cohort.
Thus, these five studies showing a better safety profile indicate that the viability of combining new immunotherapy agents and TKIs will depend greatly on the absence of enhanced toxicity. The fact that the randomised phase II study of atezolizumab-bevacizumab compared with sunitinib failed to show an improvement in PFS also highlights the importance of selecting the most appropriate primary endpoint of efficacy. The difficulty of assessing radiological response or progression with modern immunotherapy agents using standard RECIST criteria might hamper the interpretation of PFS as main efficacy endpoint. Importantly, several modern immunotherapy agents that demonstrated in a randomised phase III trial a significant improvement in OS in genitourinary tumours failed to show any impact on PFS, such as nivolumab in metastatic RCC,23 pembrolizumab in second-line metastatic bladder cancer40 or sipuleucel-T in metastatic castration-resistant prostate cancer.41 Consequently, most current phase III studies analysing the role of PD-1 or PD-L1 inhibitors in metastatic RCC are selecting OS as primary endpoint or as coprimary endpoint along with PFS.
CTLA-4 inhibitors
CTLA-4 is a major immune checkpoint surface receptor in T cells that acts as a down-regulator of early immune response. Its ligands are B7.1 and B7.2, which are expressed on the surface of antigen-presenting cells. Their interaction triggers down-regulation of T cells proliferation and cytokines production leading to immunosuppression and immune tolerance. Ipilimumab and tremelimumab are two fully human monoclonal antibodies against CTLA-4 that have been studied in patients with metastatic RCC. Their clinical development in RCC is however at an earlier stage compared with anti-PD-1/PD-L1 agents.
CTLA-4 inhibitors in monotherapy
Ipilimumab was tested in a phase II trial in 189 advanced solid tumours including 61 patients with metastatic RCC. All patients were either IL-2 refractory or IL-2 ineligible. Ipilimumab was given intravenously every 3 weeks in doses ranging from 1 mg/kg to 3 mg/kg. Patients were assessed for treatment efficacy and onset of autoimmune-mediated AEs. Several autoimmune-mediated AEs were described in the whole group, including enterocolitis (18%), hypophysitis (7%), dermatitis (4%), arthritis (2%) and uveitis (1%). Interestingly, the onset of enterocolitis was significantly associated with ORR in the whole group as well as in the RCC cohort. The ORR for patients with RCC with enterocolitis (n=17) was 35% compared with 2% in patients who did not develop enterocolitis (n=44) (p=0.001).42 The onset of other autoimmune AEs, such as hypophysitis, dermatitis or arthritis, also correlated significantly with objective response. ORR among patients who developed autoimmune AEs (n=20) was 30% compared with 0% in patients free of autoimmune toxicity (n=41) (p=0.0007).43 These preliminary findings indicate that autoimmune-related AEs may represent a sign of adaptative immune activity unleashed by CTLA-4 blockade and a predictive marker of treatment efficacy. This is in keeping with what has already been described with ipilimumab in malignant melanoma.44
Tremelimumab was studied in a dose-escalation phase I trial in combination with two dosing schedules of sunitinib in previously treated patients and in patients with treatment-naïve metastatic RCC. Patients received sunitinib at a dose of either 50 mg daily (4 weeks on, 2 weeks off) or 37.5 mg daily in combination with tremelimumab at 6 mg/kg, 10 mg/kg or 15 mg/kg of every 12 weeks. Tremelimumab was administered for a maximum of six cycles or until disease progression or intolerable toxicity. In total, 28 patients were enrolled, of whom only 11% had received prior systemic treatment. The ORR was 43% (95% CI 22 to 66) among evaluable patients (n=21) and disease stabilisation occurred in 33% of patients. However, treatment delivery was limited by unexpected toxicity. Two of five patients who received 50 mg of sunitinib plus tremelimumab 6 mg/kg experienced DLTs, and no further enrolment to that cohort was pursued. One of seven patients who received continuous sunitinib at 37.5 mg plus tremelimumab 10 mg/kg suffered a sudden death. Among patients who received continuous sunitinib at 37.5 mg plus tremelimumab 15 mg/kg, three of six patients experienced DLTs. Overall, rapid-onset renal failure was the most common DLT and all patients who presented with it required extensive medical management.45 Consequently, the clinical development of tremelimumab in combination with sunitinib was stopped.
Combining PD1/PD-L1 inhibitors and CTLA-4 inhibitors
In view of the limited clinical activity and/or significant toxicity of CTLA-4 inhibitors when given in monotherapy, their drug development in metastatic RCC has only continued in combination with PD-1 or PD-L1 inhibitors following the excellent results seen in malignant melanoma.46 CTLA-4 and PD-1/PD-L1 pathways promote tumour immune tolerance through complementary and non-redundant mechanisms. Preclinical studies and melanoma clinical trials have shown that dual inhibition of both pathways synergistically enhances antitumour responses, as compared with acting only on one pathway alone.46–48 So far, preliminary data have only been presented for the combination of ipilimumab plus nivolumab or pembrolizumab, but many other combinations are currently being investigated in clinical trials.
The phase I study CheckMate-016 is investigating the use of nivolumab combined with ipilimumab in patients with treatment-naïve and previously treated metastatic RCC.49 Patients are randomised to three possible dose cohorts: nivolumab 3 mg/kg plus ipilimumab 1 mg/kg (N3 +I1), nivolumab 1 mg/kg plus ipilimumab 3 mg/kg (N1 +I3) or nivolumab 3 mg/kg plus ipilimumab 3 mg/kg (N3 +I3) every 3 weeks for four doses, then nivolumab 3 mg/kg intravenously every 2 weeks until progression or toxicity. A total of 100 patients have been recruited so far. The N3 +I3 showed early toxicity with the first six patients included and did not proceed to expansion. N3 +I1 and N1 +I3 cohorts were expanded to 47 patients per arm. Across treatment arm, the majority of patients were aged <65 years (85%), male (76%) and had a favourable (34%) or intermediate (61%) MSKCC risk prognostic group. Approximately, half of the patients had received prior systemic therapy (51%), of whom up to 65% had only been treated with one treatment line. Treatment-related AEs were seen in 88% of patients but no grade 5 AEs were described.49 Grade 3–4 treatment-related AEs occurred in 38% of patients in the N3 +I1 group and in 62% in the N1 +I3 group. Most common AEs were increased lipase (15% and 28%), increased ALT (4% and 21%), diarrhoea (4% and 15%) and colitis (0% and 15%).50 Regarding antitumour activity, the confirmed ORR was 40% in both cohorts. Moreover, radiological responses appeared early and were prolonged, with a median duration of response of 20.4 and 19.7 months, in the N3 +I1 and N1 +I3 group, respectively. The median OS was not reached in the N3 +I1 cohort, and 32.6 months (95% CI 1.1 to 34.3) in the N1 +I3 cohort. The median PFS was 6.6 months (95% CI 1.1 to 33.7) and 9.1 months (95% CI 1.0 to 33.1), respectively.50 In view of these data showing initial safety findings and promising antitumour activity, a phase III trial of nivolumab plus ipilimumab versus sunitinib was conducted in the first-line setting using the N3 +I1 dosage. This phase III trial has completed accrual and final results are eagerly awaited.
Similarly, the phase I trial Keynote-029 is assessing the combination of low-dose ipilimumab and pembrolizumab in previously treated metastatic RCC.51 Ten patients were enrolled and received pembrolizumab 2 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four doses, followed by pembrolizumab 2 mg/kg every 3 weeks for up to 2 years. DLTs were observed in two of nine evaluable patients. Up to 54.5% of patients experienced >1 grade 3–4 related AEs. The most frequent AEs of any grade were fatigue (27.3%), diarrhoea (22.7%), increased ALT or AST (18.2% each) and colitis (18.2%). The partial response (PR) and stable disease (SD) rates were 30% (n=3) and 30% (n=3), with most patients being still on treatment at data cut-off.52 These preliminary results of combined PD-1/PD-L1 and CTLA-4 blockade are indeed promising. However, we will have to wait for the results of randomised phase III trials of these and other combinations to ascertain whether the combination holds the same efficacy as in melanoma.
Dual PI3K-mTOR inhibitors
The PI3k pathway is an important mediator of glucose metabolism and is involved in many cellular processes, including cell survival, proliferation and differentiation.53 It activates several kinases, transcription factors and proteins including mTOR, promoting cell growth, survival and metabolism. Upregulation of the pathway via activating mutations or loss of suppressor genes such as phosphatase and tensin homologue has been implicated in the initiation and progression of various cancer types including RCC. Several agents targeting the PI3K pathway, like the mTORC1 inhibitors everolimus and temsirolimus, are approved for the treatment of metastatic RCC. However, the clinical activity of these drugs and the improvement in survival in patients with metastatic RCC are modest, with most patients experiencing progressive disease due to development of drug resistance. One of the possible mechanisms of resistance to mTORC1 inhibitors is the upregulation of other PI3k signalling proteins such as mTORC2 or protein kinase B (AKT). In an effort to improve the efficacy of mTORC1 inhibitors, there are currently several molecules being investigated that target the PI3K pathway at different levels, such as PI3K, AKT, mTORC1/2, and also agents with multiple targets such as dual PI3K/mTOR inhibitors. The latter are able to suppress the mTORC1 and mTORC2 complexes downstream of PI3K, resulting in more complete pathway inhibition, and should attenuate PI3K activation triggered by inhibition of mTOR-dependent negative-feedback mechanisms.53–55 There are several PI3k pathway inhibitors that are currently being investigated in RCC, such as apitolisib or buparlisib, although the results are still preliminary.
Apitolisib (formerly known as GDC-0980) is a potent oral dual pan-PI3K and mTOR (TORC1/2) inhibitor that has been evaluated in metastatic RCC in a phase I and II study. Apitolisib was initially tested in a first-in-human dose-escalation phase I study in patients with advanced solid tumours. In total, 42 patients were recruited, including one patient with RCC. Apitolisib was well tolerated through 32 mg daily, with the MTD reached at 70 mg daily. The most common grade 3 AEs were hyperglycaemia, diarrhoea, fatigue and mucositis. A biomarker pharmacodynamic evaluation showed a significant reduction of plasma levels of pAKT inversely correlated with apitolisib concentrations. Only one patient (2.3%) experienced partial response as per RECIST criteria, whereas 69% of patients showed disease stabilisation. Of note, the patient with RCC was on treatment for around 11 months with stable disease as best response.56 With these results, a randomised phase II trial was design to directly test if a PI3K/mTOR inhibitor may improve efficacy over mTORC1 inhibitor everolimus alone in patients with metastatic RCC who progressed on or after first-line VEGF inhibition.57 Eighty-five patients were included and randomised to apitolisib 40 mg daily or everolimus 10 mg daily, stratifying by MSKCC prognostic group and time to progression on first VEGFR treatment. After 67 events, stratified analysis revealed the median PFS was significantly shorter for apitolisib than everolimus (3.7 vs 6.1 months, HR 2.12, 95% CI 1.23 to 3.63, p<0.01). The median OS was not significantly different but trended in favour of everolimus (16.5 vs 22.8 months, HR 1.77, 95% CI 0.97 to 3.24, p=0.06). Moreover, ORR was 7.1% for apitolisib and 11.6% for everolimus. Patients treated with apitolisib had greater incidence of grade 3–4 AEs and were more likely to discontinue treatment because of toxicity (31% vs 12%). Apitolisib was associated with substantially more high-grade hyperglycaemia (40% vs 9%) and rash (24% vs 2%). The study was therefore unable to demonstrate that dual inhibition of PI3K/mTOR with apitolisib provides any benefit over inhibition of MTORC1 alone with everolimus. The authors hypothesised that this may be due to the high rate of AEs associated with the potent pathway inhibition.57 Consequently, the drug development of apitolisib in metastatic RCC has been halted.
BEZ235, a potent inhibitor of both PI3K and mTOR, was tested in a dose-escalation phase Ib trial in patients with advanced RCC of any subtype previously treated with at least one systemic regimen.58 A total of 10 patients had been enrolled when the study was prematurely stopped because of the high incidence of DLTs across all dose levels tested. Around 50% of patients developed grade 3–4 AEs, and 50% of patients came off the study because of toxicities. Moreover, no objective responses were observed in the five evaluable patients. Hence, the drug development of BEZ235 in metastatic RCC was also halted.
PI3K also activates hypoxia-inducible growth factor-1α gene expression, which is a potential mechanism of resistance to VEGF-targeted therapy. There is therefore a rationale for combining VEGF-targeted therapy with a PI3K inhibitor. With that regard, the pan-PI3K inhibitor buparlisib (formerly known as BKM120) was tested in combination with bevacizumab in a phase I study for patients with metastatic RCC progressing on prior VEGFR therapies.59 A total of 32 patients were accrued and were treated with buparlisib (60–100 mg/day (d)) plus bevacizumab 10 mg/kg every 2 weeks. Most patients had clear cell histology (88%) and 50% had ≥2 prior lines of systemic therapy. Of the 30 evaluable patients, 13% had a partial response and 50% had stable disease. However, safety concerns were raised as most observed DLTs were in the form of cognitive disturbance, depression, suicidal ideation and anxiety.59 Consequently, the drug development of buparlisib has also been halted.
These first results with PI3K pathway inhibitors are indeed disappointing and are probably due to the lack of predictive biomarkers of efficacy and drugs being tested in molecularly unselected patients. Other PI3k pathway inhibitors are currently being tested in phase I trials including patients with RCC. However, their drug development is at a much earlier stage and results are still very preliminary. These include the inhibitors SF1126, XL765 or GSK2126458.
Conclusions
During the two last years, the treatment of advanced RCC has witnessed a dramatic revolution with the regulatory approval of three new treatment options for second-line therapy following progression to VEGFR-targeted therapy. To the already approved second-line options of everolimus, axitinib and sorafenib, now we can include the anti-PD-1 inhibitor nivolumab, the VEGFR/cMet inhibitor cabozantinib and the VEGFR/FGFR inhibitor lenvatinib combined with the mTOR inhibitor everolimus. Moreover, for the first time in the second-line setting, these three agents were approved based on an improvement in OS compared with an active and valid comparator drug such as everolimus. Importantly, OS is generally considered as the most relevant surrogate factor of meaningful clinical benefit with a given drug. This has led to the most influential international oncology guidelines such as the NCCN guidelines24 and the ESMO guidelines25 to recommend both nivolumab and cabozantinib as the new preferred standard-of-care, second-line options in advanced RCC.
The approval of nivolumab also represents the return of immunotherapy to the treatment of advanced RCC following the abandonment of cytokines in the early 2000s. This is extremely relevant as it confirms that RCC is an immunotherapy-sensitive disease and allows the expansion of further research of modern immune-mediated therapies for the treatment of localised and advanced RCC. However, despite the excellent results seen with nivolumab, a significant proportion of patients never benefit from treatment and most initial responders will ultimately developed progressive disease. Consequently, the advent of modern immunotherapy for the treatment of RCC has not closed the door of continued research in other drugs targeting antiangiogenesis and other relevant pathways of intracellular signal transduction. Among these, the PI3k pathway is one of the most important pathways currently being investigated as a potential target of several new drugs, although the preliminary results have been so far somewhat disappointing.
Despite the fact that the treatment of advanced RCC has been dramatically modified in recent years, durable complete remissions are exceptional and advanced RCC remains a lethal disease. The success of future next-generation agents will therefore depend on our ability to select patients most likely to respond to treatment based on robust predictive biomarkers. Finally, in view of the several new treatment options that have become available for the treatment of advanced RCC from the first-line to third-line setting, further studies are urgently needed to inform on the best sequence of therapies in order to provide the best outcome to patients.
Footnotes
Contributors: All authors contributed equally to this article.
Competing interests: JB: Consultant and adboard for Eisai and Exelixis (compensated). The other authors (ARV, TEH and MHS) have no competing interests.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1. Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 2007;370:2103–11. 10.1016/S0140-6736(07)61904-7 [DOI] [PubMed] [Google Scholar]
- 2. Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–24. 10.1056/NEJMoa065044 [DOI] [PubMed] [Google Scholar]
- 3. Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061–8. 10.1200/JCO.2009.23.9764 [DOI] [PubMed] [Google Scholar]
- 4. Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931–9. 10.1016/S0140-6736(11)61613-9 [DOI] [PubMed] [Google Scholar]
- 5. Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271–81. 10.1056/NEJMoa066838 [DOI] [PubMed] [Google Scholar]
- 6. Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008;372:449–56. 10.1016/S0140-6736(08)61039-9 [DOI] [PubMed] [Google Scholar]
- 7. Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584–90. 10.1200/JCO.2008.20.1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125–34. 10.1056/NEJMoa060655 [DOI] [PubMed] [Google Scholar]
- 9. Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1814–23. 10.1056/NEJMoa1510016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou L, Liu XD, Sun M, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene 2016;35:2687–97. 10.1038/onc.2015.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016;17:917–27. 10.1016/S1470-2045(16)30107-3 [DOI] [PubMed] [Google Scholar]
- 12. Rankin EB, Fuh KC, Castellini L, et al. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc Natl Acad Sci USA 2014;111:13373–8. 10.1073/pnas.1404848111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choueiri TK, Halabi S, Sanford B, et al. CABOzantinib versus SUNitinib (CABOSUN) as initial targeted therapy for patients with metastatic renal cell carcinoma (mRCC) of poor and intermediate risk groups: results from ALLIANCE A031203 trial. Ann Oncol 2016;27:1–36. 10.1093/annonc/mdw435.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Négrier S, Gravis G, Pérol D, et al. Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncol 2011;12:673–80. 10.1016/S1470-2045(11)70124-3 [DOI] [PubMed] [Google Scholar]
- 15. Rini BI, Bellmunt J, Clancy J, et al. Randomized phase III trial of temsirolimus and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. J Clin Oncol 2014;32:752–9. 10.1200/JCO.2013.50.5305 [DOI] [PubMed] [Google Scholar]
- 16. Ravaud A, Barrios CH, Alekseev B, et al. RECORD-2: phase II randomized study of everolimus and bevacizumab versus interferon α-2a and bevacizumab as first-line therapy in patients with metastatic renal cell carcinoma. Ann Oncol 2015;26:1378–84. 10.1093/annonc/mdv170 [DOI] [PubMed] [Google Scholar]
- 17. Flaherty KT, Manola JB, Pins M, et al. BEST: a randomized phase II study of vascular endothelial growth factor, RAF kinase, and mammalian target of rapamycin combination targeted therapy with bevacizumab, sorafenib, and temsirolimus in advanced renal cell carcinoma–a trial of the ECOG-ACRIN cancer research group (E2804). J Clin Oncol 2015;33:2384–91. 10.1200/JCO.2015.60.9727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 2015;16:1473–82. 10.1016/S1470-2045(15)00290-9 [DOI] [PubMed] [Google Scholar]
- 19. Jilaveanu LB, Shuch B, Zito CR, et al. PD-L1 expression in clear cell renal cell carcinoma: an analysis of nephrectomy and sites of metastases. J Cancer 2014;5:166–72. 10.7150/jca.8167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006;66:3381–5. 10.1158/0008-5472.CAN-05-4303 [DOI] [PubMed] [Google Scholar]
- 21. Kang MJ, Kim KM, Bae JS, et al. Tumor-infiltrating PD1-positive lymphocytes and FoxP3-positive regulatory T cells predict distant metastatic relapse and survival of clear cell renal cell carcinoma. Transl Oncol 2013;6:282–9. 10.1593/tlo.13256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choueiri TK, Figueroa DJ, Fay AP, et al. Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: results from COMPARZ, a randomized controlled trial. Clin Cancer Res 2015;21:1071–7. 10.1158/1078-0432.CCR-14-1993 [DOI] [PubMed] [Google Scholar]
- 23. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf (accessed Jan 2017).
- 25. Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v58–68. 10.1093/annonc/mdw328 [DOI] [PubMed] [Google Scholar]
- 26. Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65. 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McDermott DF, Sosman JA, Sznol M, et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term Safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol 2016;34:833–42. 10.1200/JCO.2015.63.7421 [DOI] [PubMed] [Google Scholar]
- 28. Manning EA, Ullman JG, Leatherman JM, et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res 2007;13:3951–9. 10.1158/1078-0432.CCR-07-0374 [DOI] [PubMed] [Google Scholar]
- 29. Shrimali RK, Yu Z, Theoret MR, et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of Cancer. Cancer Res 2010;70:6171–80. 10.1158/0008-5472.CAN-10-0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kusmartsev S, Eruslanov E, Kübler H, et al. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol 2008;181:346–53. 10.4049/jimmunol.181.1.346 [DOI] [PubMed] [Google Scholar]
- 31. Roland CL, Lynn KD, Toombs JE, et al. Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PLoS One 2009;4:e7669 10.1371/journal.pone.0007669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 2012;12:237–51. 10.1038/nrc3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amin A, Plimack ER, Infante JR, et al. Nivolumab (N) (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib (S) or pazopanib (P) in patients (pts) with metastatic renal cell carcinoma (mRCC). Ann Oncol 2014;25:iv362–3. 10.1093/annonc/mdu342.5 [DOI] [Google Scholar]
- 34. McDermott DF, Infante JR, Chowdhury S, et al. ; 18th European Cancer Conference (ECCO 18). A phase I/II study to assess the safety and efficacy of pazopanib (paz) and pembrolizumab (pembro) in patients (pts) with advanced renal cell carcinoma (aRCC). Eur J Cancer 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dudek AZ, Sica RA, Sidani A, et al. Phase Ib study of pembrolizumab in combination with bevacizumab for the treatment of metastatic renal cell carcinoma: big ten cancer research consortium BTCRC-GU14-003. J Clin Oncol 2016;34:559. 10.1200/jco.2016.34.2_suppl.559 [DOI] [Google Scholar]
- 36. Sznol M, McDermott DF, Jones SF, et al. Phase Ib evaluation of MPDL3280A (anti-PDL1) in combination with bevacizumab (bev) in patients (pts) with metastatic renal cell carcinoma (mRCC). J Clin Oncol 2015;33:410. 10.1200/jco.2015.33.7_suppl.410 [DOI] [Google Scholar]
- 37. McDermott DF, Atkins MB, Motzer RJ, et al. A phase II study of atezolizumab (atezo) with or without bevacizumab (bev) versus sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC) patients (pts). J Clin Oncol 2017;35:431. 10.1200/JCO.2017.35.6_suppl.431 [DOI] [Google Scholar]
- 38. Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients (pts) with advanced renal cell carcinoma (aRCC): preliminary safety and efficacy results. Ann Oncol 2016;27:266–95. 10.1093/annonc/mdw373.01 [DOI] [Google Scholar]
- 39. Larkin J, Rini BI, Nathan P, et al. Phase 1b dose-finding study of avelumab (anti-PD-L1) + axitinib in treatment-naïve patients with advanced renal cell carcinoma. Ann Oncol 2016;27:266–95. 10.1093/annonc/mdw373.03 [DOI] [Google Scholar]
- 40. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26. 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411–22. 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 42. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol 2006;24:2283–9. 10.1200/JCO.2005.04.5716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother 2007;30:825–30. 10.1097/CJI.0b013e318156e47e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol 2005;23:6043–53. 10.1200/JCO.2005.06.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rini BI, Stein M, Shannon P, et al. Phase 1 dose-escalation trial of tremelimumab plus sunitinib in patients with metastatic renal cell carcinoma. Cancer 2011;117:758–67. 10.1002/cncr.25639 [DOI] [PubMed] [Google Scholar]
- 46. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Selby M, Englehardt J, L-s L, et al. Antitumor activity of concurrent blockadeof immune checkpoint molecules CTLA-4 and PD-1 in preclinical models. J Clin Oncol 2013;31:3061.23569323 [Google Scholar]
- 48. Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA 2010;107:4275–80. 10.1073/pnas.0915174107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hammers HJ, Plimack ER, Infante JR, et al. Expanded cohort results from CheckMate 016: a phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC). J Clin Oncol 2015;33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hammers H, Plimack ER, Infante JR, et al. Updated results from a phase I study of nivolumab (Nivo) in combination with ipilimumab (Ipi) in metastatic renal cell carcinoma (mRCC): the checkMate 016 study. Ann Oncol 2016;27 10.1093/annonc/mdw378.16 [DOI] [Google Scholar]
- 51. Atkins MB, Choueiri TK, Hodi FS, et al. Pembrolizumab (MK-3475) plus low-dose ipilimumab (IPI) in patients (pts) with advanced melanoma (MEL) or renal cell carcinoma (RCC): data from the KEYNOTE-029 phase 1 study. J Clin Oncol 2015;33. [Google Scholar]
- 52. Atkins MB, Hodi FS, Thompson JA, et al. Pembrolizumab (pembro) plus ipilimumab (ipi) or pegylated interferon alfa-2b (PEG-IFN) for advanced melanoma or renal cell carcinoma (RCC). J Clin Oncol 2016;34. [Google Scholar]
- 53. Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol 2010;28:1075–83. 10.1200/JCO.2009.25.3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 2006;66:1500–8. 10.1158/0008-5472.CAN-05-2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Papadopoulos KP, Tabernero J, Markman B, et al. Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245409 (XL765), a novel, orally administered PI3K/mTOR inhibitor in patients with advanced solid tumors. Clin Cancer Res 2014;20:2445–56. 10.1158/1078-0432.CCR-13-2403 [DOI] [PubMed] [Google Scholar]
- 56. Wagner AJ, Bendell JC, Dolly S, et al. A first-in-human phase I study to evaluate GDC-0980, an oral PI3K/mTOR inhibitor, administered QD in patients with advanced solid tumors. J Clin Oncol 2011;29:3020. 10.1200/jco.2011.29.15_suppl.3020 [DOI] [Google Scholar]
- 57. Powles T, Lackner MR, Oudard S, et al. Randomized open-label phase II trial of apitolisib (GDC-0980), a novel inhibitor of the PI3K/mammalian target of rapamycin pathway, versus everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol 2016;34:1660–8. 10.1200/JCO.2015.64.8808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Carlo MI, Molina AM, Lakhman Y, et al. A phase ib study of BEZ235, a dual inhibitor of phosphatidylinositol 3-kinase (PI3K) and mammalian target of rapamycin (mTOR), in patients with advanced renal cell carcinoma. Oncologist 2016;21:787–8. 10.1634/theoncologist.2016-0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McKay RR, Werner L, Harshman LC, et al. A phase I study of buparlisib (BKM120) with bevacizumab (BEV) in patients (pts) with metastatic renal cell carcinoma (mRCC) progressing on prior vascular endothelial growth factor (VEGF) therapies. J Clin Oncol 2015;33. [Google Scholar]