Abstract
Introduction and Hypothesis
Knowledge of the innervation of pelvic floor and sphincter muscles is of great importance to understanding the pathophysiology of female pelvic floor dysfunctions. This study aims to present our high-density intravaginal and intrarectal electromyography (EMG) probes and a comprehensive innervation zone (IZ) imaging technique based on high-density EMG readings to characterize the IZ distribution.
Methods
Both intravaginal and intrarectal probes are covered with a high-density surface electromyography electrode grid (8 X 8). Surface EMG signals were acquired in 10 healthy female subjects during maximum voluntary contractions of their pelvic floor. EMG decomposition was performed to separate the motor unit action potentials (MUAPs) and then localize their IZs.
Results
High-density surface EMG signals were successfully acquired over the vaginal and rectal surfaces. The propagation patterns of muscle activity were clearly visualized for multiple muscle groups of the pelvic floor and anal sphincter. During each contraction, up to 218 and 456 repetitions of motor units were detected by the vaginal probe and the rectal probe, respectively. MUAPs were separated with their IZs identified at various orientations and depths.
Conclusions
The proposed probes are capable of providing a comprehensive mapping of the innervation zones of the pelvic floor and sphincter muscles. They can be employed as diagnostic and preventative tools in clinical practices.
Keywords: Pelvic Floor Muscles, Electromyography, Motor Unit, Innervation Zone, Intravaginal EMG Probe, Intrarectal EMG Probe
INTRODUCTION
Pelvic floor muscles (PFMs) are intimately involved in normal pelvic floor functions. Neuromuscular injury to PFMs may cause pelvic floor dysfunctions such as incontinence or prolapse. Electromyography (EMG) has been widely used to assess the neuromuscular function of PFMs in clinical and research environments [1]. Needle EMG is known for its high selectivity and has been used to investigate the neural control of the external anal sphincter (EAS) in patients with incontinence [2]. However, this technique is not only invasive but also lacks the ability to provide global information of the muscle activation. Perineal EMG, in which the electrode is attached to the perineal skin surface, is not selective because of high risk of crosstalk [3]. Consequently, efforts have been made to develop intravaginal probes and intrarectal probes with mounted electrodes as a minimally-invasive alternative [1,3].
In order to provide a comprehensive functional map of PFMs through EMG, an ideal EMG probe should have two characteristics: 1) sufficient electrodes longitudinally to detect and differentiate superficial and deep muscles [4], and 2) sufficient electrodes circumferentially aligned parallel to the muscle fiber to perform motor unit action potential (MUAP) detection and to study the muscle innervation [5,6]. These two characteristics require that an ideal probe has high-density electrodes in both longitudinal and circumferential dimensions. A recent state-of-art review showed that current commercially available intravaginal probes are limited by their probe geometry, large detection surfaces and inappropriate electrode configurations [3]. The lack of a sufficient number of channels also prevented them from being used for MUAP detection. Recently, a high-density anal EMG probe (three circumferential arrays with 48 electrodes) has shed new light on the investigation of the innervation of EAS muscles [5–7]. The distribution of the innervation zones (IZs) of the EAS and the effect of childbirth on the EAS innervation were systematically investigated. However, its application is limited in assessing deep PFMs, such as the pubococcygeus muscle because of insufficient longitudinal electrodes. In another recently reported study, a multiple-electrode probe (with a measurement range of 50mm longitudinally) demonstrated the capability of differentiating deep and superficial muscles [4], but the low spatial circumferential resolution made it inappropriate for MUAP detections. A recent study showed the possibility of mounting EMG electrodes on a urethral catheter surface to measure the activity of urethral sphincter muscles [8]. However, compared with the urethra, the vaginal and rectal spaces have greater volumes and are more distensible. These characteristics challenge the intravaginal or intrarectal catheter probe designs because high-density electrodes require accommodation on highly-stretchable probe surfaces. Although a few attempts have been made to design compressible electrodes [9], published results are scarce in the existing literature.
In this study, we present our newly developed high-density, two-dimensional intravaginal and intrarectal surface EMG probes to feature the desired characteristics [10]. The probes are equipped with an 8X8 high-density electrode grid which allows the MUAP detection and innervation zone mapping from the entire vaginal and anorectal canal surface. It is expected that our new probes will provide a comprehensive functional mapping of the female PFM/EAS and meet the imperative need for neurogenic PFM/EAS disorder characterization [11].
MATERIALS AND METHODS
Subjects
Ten healthy young female subjects (age [mean ± S.D.]: 29.1 ± 7.1 years) participated in the study. Enrollment was limited to normal, healthy female volunteers with no history of pelvic injuries or neuromuscular diseases. The EMG studies were carried out at the Houston Methodist Hospital with the protocol approved by both the University of Houston and Houston Methodist Hospital Institutional Review Boards. All participants gave informed consents.
Probe Design
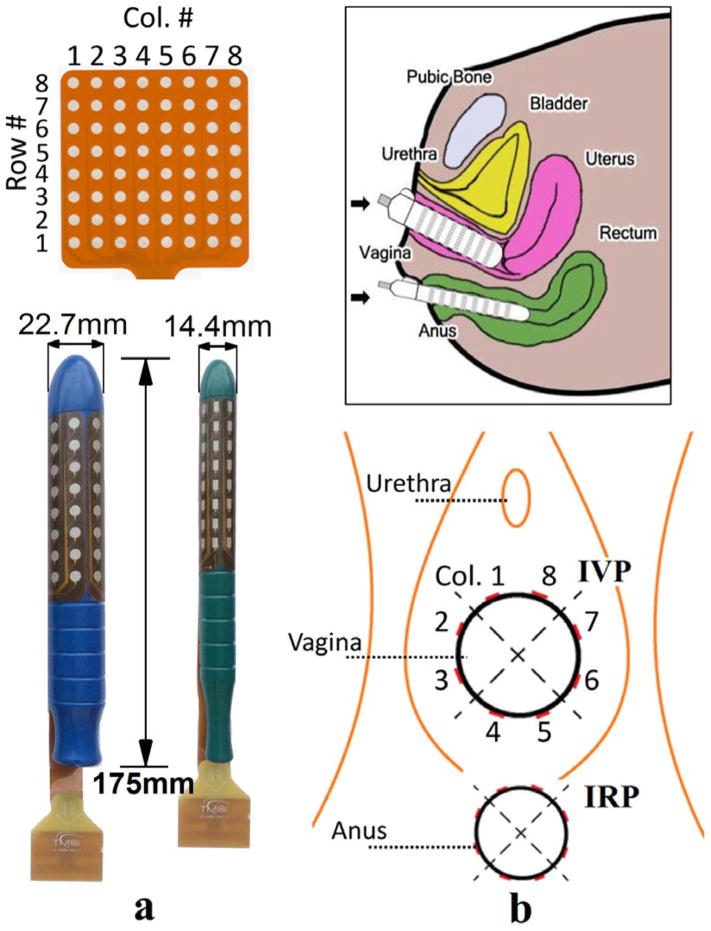
The intravaginal and intrarectal probes were designed at the University of Houston and manufactured by the Twente Medical Systems International (Enschede, The Netherlands). Both probes are cylindrical in shape with a total length of 175 mm. A high-density electrode grid (8 X 8) is coated on the surface of each probe (see Figure 1a). The intravaginal probe is 22.7 mm in diameter. The electrode surface is circular with a diameter of 4.0 mm. The inter-electrode spacing (center to center) is 8.8 mm both longitudinally and circumferentially. The intrarectal probe is 14.4 mm in diameter. The electrode surface is rectangular with a length of 4.0 mm and a width of 2.4 mm. The inter-electrode spacing (center to center) is 8.0 mm longitudinally and 5.7 mm circumferentially. The top of both probes is spherical. The application of a proper amount of gel is advised for ease of insertion.
Figure 1.
shows (a) the proposed intravaginal and intrarectal probes, together with the description of the channel numbering convention. (b) Orientations and positions of the probes on insertion. (IVP – Intravaginal Probe, IRP – Intrarectal Probe)
For a convenient reference to the electrode locations on the probe surface, we adopted a row and column numbering convention (see Figure 1a). Hereinafter, the circumferential rows are numbered from 1 (superficial) to 8 (deep). The longitudinal columns are numbered in a counter-clockwise way in the caudal view and divided into four quadrants: ventral (8, 1), right (2, 3), dorsal (4, 5) and left (6, 7).
Experimental Protocol
All tests were performed at the Houston Methodist Hospital guided by an experienced urologist (R.K). All subjects were instructed by the urologist to contract their pelvic floor muscles prior to the insertion of the probes. The individuals were re-examined for appropriate pelvic floor contractions following the insertion of the probes. The subjects were tested in the lithotomy position leaning their backs on a tilted exam table (about 30° from horizontal). The probes were inserted with the help of the urologist to maintain the correct orientation and depth, as shown in Figure 1b. A correct insertion was described as having the trademarks faced anteriorly and the edge of the electrode grid aligned with the urogenital orifices. After probe placement, a few trial PFM contractions were attempted while the signals were visually inspected in real time for quality check.
Subjects were involved in two test sessions. Ten short contractions were performed in session one to give as hard as possible contraction forces, and ten sustained long contractions were performed in session two. The subjects were allowed sufficient resting time between two continuous contractions and rested fully to relax their PFMs between two sessions. The ground electrode was placed at the wrist of the subject connecting to a fully-soaked wristband in accordance to the manufacturer’s recommendation. The wrist area was slightly abraded and cleaned using alcohol patches to lower the surface impedance.
Data Collection and Processing
Myoelectric signals were acquired with a 136 channel Refa amplifier (Twente Medical Systems International, The Netherlands) at a sample rate of 2048 Hz and stored in a personal computer. During offline process, the signals were digitally filtered in MATLAB R2015 (Mathworks Inc., Natick, MA) with a 4th-order band-pass Butterworth filter (15–400 Hz band) without phase distortions. The root mean squares (RMS) of the EMG recordings from a window of 0.1s (205 samples) were calculated for three muscles (the external anal sphincter, the puborectalis muscle and the pubococcygeus muscle) from the rectal recordings for each contraction of each subject [4]. To test the reliability of the probes, the intraclass correlations between two sessions (ICCs) for these three muscles were calculated to determine different sources of EMG measurement variation (between subjects and within subject) and the coefficients of variance (CV) were reported following the method previously used in assessing an intravaginal probe [12].
EMG decomposition based on the convolution kernel compensation (CKC) has been shown to be an effective tool for identifying the single MUAP from interferential surface EMG signals [13]. This technique has been validated extensively with both simulated and experimental signals [13,14]. Our newly developed K-means clustering and convolution kernel compensation (KmCKC) approach was utilized to decompose the high-density rectal and vaginal EMG signals into their constituent MUAP trains [15,16]. Briefly, the K-mean clustering method was first adopted to cluster firing times of the same motor unit (MU). The initial innervation pulse train can be estimated during this process by choosing an appropriate number of clustered groups and time instants so that the time instants fired by a single MU can be gathered into one group as completely as possible. Then an improved multi-step iterative CKC method was employed to update the estimated innervation pulse trains to improve the decomposition accuracy in a noisy environment.
The IZ is the region of a MU including the neuromuscular junction where the MUAPs are generated and propagate in two opposing directions along the muscle fibers [5]. The EMG signals propagating in opposite directions appear with opposite phases in the bipolar MUAP maps [15]. Therefore, the position of the IZ of a particular MU can be localized from the bipolar map of the decomposed high-density MUAPs by checking the phases of the propagating signals. The number of identified IZs and their MUAP repetitions per contraction will be presented as mean ± standard deviation (SD).
RESULTS
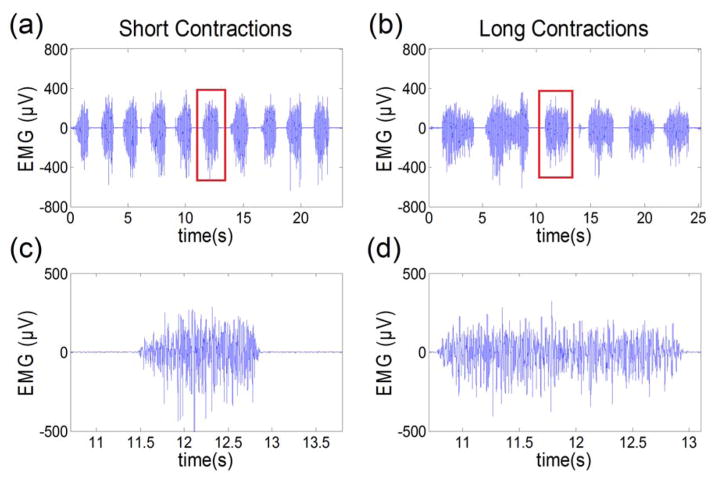
Ten healthy female subjects were enrolled in this study. EMG recordings from two of them (1009 and 1010) were excluded because of large movement artifacts during contractions and bad electrode-mucosa contacts. Figure 2 shows an example of acquired EMG signals. The ICCs between two sessions were calculated (EAS 0.95, puborectalis 0.88 and pubococcygeus 0.90), demonstrating a good (0.80<ICC<0.89) to high (ICC>0.90) reliability [12]. The EMG measurements of the external anal sphincter muscle from the rectal probe for the remaining eight subjects are shown in Table 1. Mean RMS and CV values are 25.0 ± 14.6 μV and 18.0% ± 6.1% in session I and 22.1 ± 13.5 μV and 21.3% ± 6.5% in session II.
Figure 2.
shows an example of (a) consecutive short contractions, (b) consecutive long contractions, (c) the zoomed-in view of one short contraction and (d) the zoomed-in view of one long contraction.
Table 1.
EMG measurements and number of identified innervation zones for all subjects
| EMG measurement of the EAS muscle | EMG Decomposition Results | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Session I Short contractions |
Session II Long contractions |
Vaginal Probe | Rectal Probe | ||||||
|
|
|||||||||
| Subject ID | RMS (μV) | C.V. (%) | RMS (μV) | C.V. (%) | # of MUs | # of Total MUAP Repetitions | # of MUs | # of Total MUAP Repetitions | |
| 01 | 4.1 ± 0.8 | 19.5 | 7.5 ± 1.5 | 20.0 | 2 | 35 | 7 | 258 | |
| 02 | 23.6 ± 3.0 | 12.7 | 19.3 ± 3.1 | 16.1 | 8 | 218 | 15 | 456 | |
| 03 | 26.0 ± 3.4 | 13.1 | 20.0 ± 4.2 | 21.0 | 10 | 183 | 10 | 240 | |
| 04 | 32.4 ± 6.0 | 18.5 | 31.7 ± 5.7 | 18.0 | 6 | 99 | 8 | 136 | |
| 05 | 22.5 ± 2.5 | 11.1 | 17.9 ± 2.1 | 11.7 | N.A. | N.A. | 8 | 335 | |
| 06 | 16.2 ± 2.3 | 14.2 | 17.2 ± 3.8 | 22.1 | 1 | 28 | 8 | 229 | |
| 07 | 57.6 ± 16.6 | 28.8 | 53.0 ± 14.3 | 27.0 | 6 | 158 | 11 | 331 | |
| 08 | 17.7 ± 4.6 | 26.0 | 10.4 ± 3.6 | 34.6 | 3 | 30 | 9 | 115 | |
|
| |||||||||
| Mean | 25.0 | 18.0 | 22.1 | 21.3 | 5.1 | 107 | 9.5 | 262 | |
| S.D. | 14.6 | 6.1 | 13.5 | 6.5 | 3.0 | 74 | 2.4 | 104 | |
Data are presented as mean ± SD; Decomposition was performed using one contraction dataSignals from subject 09 and 10 and vaginal EMG signals from subject 05 were discarded due to contaminated data
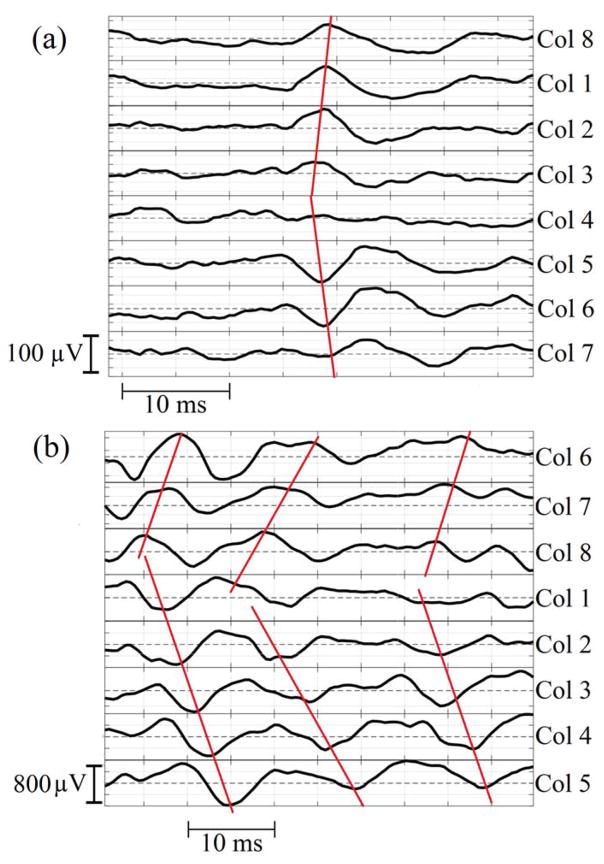
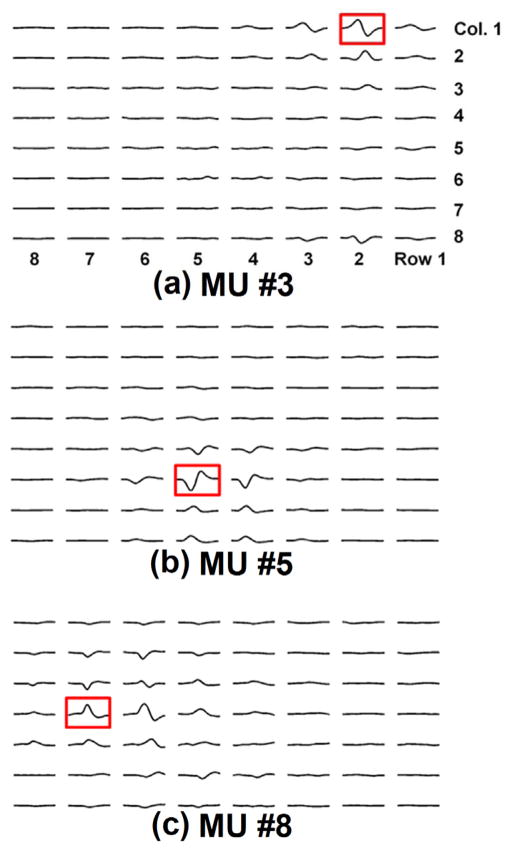
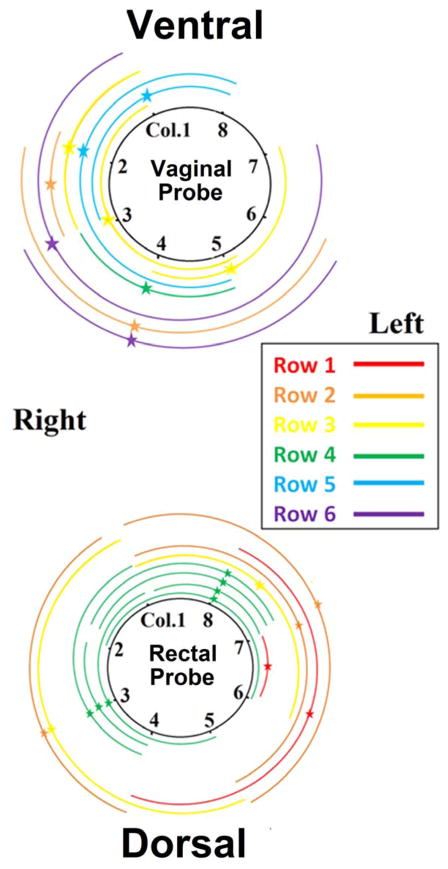
MUAPs were detected at different depths of the EMG grid. Figure 3 shows an example of MUAP propagation patterns obtained from circumferential electrodes. EMG decomposition was successfully performed. IZs were visually inspected for each MUAP of each subject. The propagations of each MUAP were estimated from the high-density surface EMG signals. Figure 4 shows an example of the identified IZs at different depths of the rectal probe. During each contraction, up to 10 (5.1 ± 3.0) motor units were detected by the vaginal probe with 107 ± 74 repetitions of the MUAPs and up to 15 (9.5 ± 2.4) motor units were detected by the rectal probe with 262 ± 104 repetitions of MUAPs. Figure 5 shows an example of the IZ distributions obtained from the vaginal and rectal EMG readings.
Figure 3.
shows an example of the MUAP propagation patterns obtained from the (a) 4th row of the vaginal probe readings and (b) 2nd row of the rectal probe readings of the same subject. Some channels were adjusted by offsetting the baseline for a better display.
Figure 4.
shows an example of the MUAP distributions among the entire grid surface of three MUs at the (a) superficial, (b) intermediate and (c) deep levels of the rectal probe. (MU – Motor Unit)
Figure 5.
shows an example of the mapped IZ distributions and MUAP propagations of one subject. Each arc represents one MU. The star represents the location of the IZ and the two arms extending from the IZ represent the propagation of the MUAP. Depth of MUAP and IZ is indicated by the arc color. The distance of each arc to the circle is only for displaying purpose and has no physical meaning.
DISCUSSION
In this study, we report the development and testing of the minimally-invasive intravaginal and intrarectal probes for simultaneous EMG acquisition of the PFM/EAS in women. Compared with existing probes [5–7], our probes have more channels that are in favor of advanced EMG analysis techniques and a better longitudinal coverage that enables the access to deep PFMs. Our intravaginal probe is the first high-density intravaginal probe. EMG decomposition was performed using the KmCKC algorithm with MUAPs from different MUs separated and their IZs identified. A global IZ distribution map was provided for each probe of each subject.
Our EMG measurements of the external anal sphincter are consistent with those reported by Voorham-van der Zalm et al. [4]. The associated coefficients of variances were achieved mostly below 20%, indicating a good reproducibility. To the best of our knowledge, this study represents the first effort made to perform MUAP detection using EMG readings recorded with an high-density intravaginal EMG probe. The number of IZs identified from the rectal probe signals was qualitatively in agreement with number of IZs in reported studies [7,17]. We found that the IZ distributions obtained from both probes were not strictly left-right symmetric (Figure 5). This may be due to the unique innervation that each side of each PFM/EAS receives and this asymmetric innervation may exist in healthy females [1,2].
We also found that the anorectal MUAPs encircled the lumen more extensively compared with vaginal MUAPs. This may be due to the fact that the vaginal canal is only bilaterally attached to the PFM, while the anorectum is more completely surrounded by the PFM/EAS musculature. This may also explain why a relatively smaller number of MUs were found from the vaginal probe signals than the rectal probe signals. These findings may provide valuable information for clinical practice. For example, the ideal incision site for the episiotomy surgery has been debated. In practice, the incision is usually performed on the right side of the vaginal wall because of the dominant hand of most operators [7]. The map of the IZ distribution and muscle fiber extensions may help surgeons objectively determine the incision location in order to minimize the obstetric trauma and the likelihood of postpartum pelvic floor disorders.
Our probes could be employed to estimate the EMG signal crosstalk, defined as undesirable EMG signals from other muscles arising from volume-propagation [18]. Crosstalk has been a persistent problem in the community and can severely misdirect the interpretation of results [19]. The complexity of the female pelvic floor musculature is a confounding factor. The MUAP propagation mapped on the high-density electrode grid surface, as shown in Figure 4, showed the possibility of quantifying the volume-propagation of one MU’s activity. The superficial muscle MU (Figure 4a, potentially from the EAS) and intermediate muscle MU (Figure 4b, likely from the puborectalis muscle) caused crosstalk only to the neighboring two rows, with about half of the original strength; however, the deep muscle MU (Figure 4c, likely from the pubococcygeus muscle) caused a more severe crosstalk, evidenced by the MUAPs seen in two rows away from the row in which the IZ was located with less attenuated magnitudes. Localizations and quantifications of these surface interference patterns would provide references for the crosstalk estimation and signal restoration.
The EMG probes can also help advance pelvic biomechanical modeling and muscle imaging research [20,21]. Many computational pelvic floor models have been developed recently to investigate the biomechanics of incontinence and prolapse [22]. Unfortunately, voluntary contraction of PFM/EAS has not been objectively considered in previously reported pelvic models because of the lack of appropriate techniques which can be used to quantitatively characterize pelvic muscle contractions. As a consequence, the performance of current pelvic modeling and biomechanical analysis approaches is limited. Incorporating the EMG measurements of different PFMs with the proposed probes in this study makes it possible to objectively quantify pelvic muscle contractions. This in turn will allow a more realistic characterization of the dynamic deformation of the female pelvic floor.
One difficulty in this study was establishing the electrode-mucosa contact, a common challenge in such studies [1,3,7]. The signal quality was affected by the subject’s cooperation with the task and by the operator’s experience and ability to use a proper amount of gel, relieve the subject’s anxiety through communication and maintain correct probe placement. From these preliminary studies, we are gaining more experience and preparing for future tests in patients with incontinence. In this pilot study, the test-retest reliability of the decomposition results using our newly developed probes has not been evaluated [16,23–25]. Although many previous studies have validated surface EMG decomposition algorithms as an accurate and reliable technique, our immediate plan as the next step is to assess the reliability of this approach for its applications in clinical practices.
CONCLUSIONS
In this study, we describe and discuss minimally invasive intravaginal and intrarectal probes that were successfully developed and applied in MUAP detection and analysis. Preliminary results of a study of ten healthy female subjects are reported. The main findings of this study are that the muscle activity from different muscle groups can be simultaneously captured and that the distributions of the innervation zones of the pelvic floor muscle and extremal anal sphincters can be mapped with the high-density intravaginal and intrarectal surface EMG probes. The innervation zone distribution information obtained using our probes will be valuable in helping physicians better diagnose neuromuscular injuries that lead to alterations of the innervation zone distributions in PFM and EAS.
Acknowledgments
This work was supported in part by NIH DK082644 and the University of Houston.
Footnotes
Conflict of Interest
| Yun Peng | FINANCIAL DISCLAIMER/CONFLICT OF INTEREST: NONE |
| Jinbao He | FINANCIAL DISCLAIMER/CONFLICT OF INTEREST: NONE |
| Rose Khavari | FINANCIAL DISCLAIMER/CONFLICT OF INTEREST: NONE |
| Timothy B. Boone | FINANCIAL DISCLAIMER/CONFLICT OF INTEREST: NONE |
| Yingchun Zhang | FINANCIAL DISCLAIMER/CONFLICT OF INTEREST: NONE |
Authors’ contribution
| Yun Peng | Data collection; Data analysis, Manuscript writing |
| Jinbao He | Data analysis |
| Rose Khavari | Project Development; Data collection |
| Timothy B. Boone | Project Development; |
| Yingchun Zhang | Project Development; Data collection; Manuscript writing |
References
- 1.Enck P, Vodušek DB. Electromyography of pelvic floor muscles. Journal of Electromyography and Kinesiology. 2006;16(6):568–577. doi: 10.1016/j.jelekin.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Enck P, Hinninghofen H, Wietek B, Becker HD. Functional asymmetry of pelvic floor innervation and its role in the pathogenesis of fecal incontinence. Digestion. 2004;69(2):102–111. doi: 10.1159/000077876. [DOI] [PubMed] [Google Scholar]
- 3.Keshwani N, McLean L. State of the art review: intravaginal probes for recording electromyography from the pelvic floor muscles. Neurourology and urodynamics. 2015;34(2):104–112. doi: 10.1002/nau.22529. [DOI] [PubMed] [Google Scholar]
- 4.Voorham-van der Zalm PJ, Voorham JC, van den Bos TW, Ouwerkerk TJ, Putter H, Wasser MN, Webb A, DeRuiter MC, Pelger R. Reliability and differentiation of pelvic floor muscle electromyography measurements in healthy volunteers using a new device: the Multiple Array Probe Leiden (MAPLe) Neurourology and urodynamics. 2013;32(4):341–348. doi: 10.1002/nau.22311. [DOI] [PubMed] [Google Scholar]
- 5.Enck P, Franz H, Azpiroz F, Fernandez-Fraga X, Hinninghofen H, Kaske-Bretag K, Bottin A, Martina S, Merletti R. Innervation zones of the external anal sphincter in healthy male and female subjects. Digestion. 2004;69(2):123–130. doi: 10.1159/000077878. [DOI] [PubMed] [Google Scholar]
- 6.Merletti R, Bottin A, Cescon C, Farina D, Gazzoni M, Martina S, Mesin L, Pozzo M, Rainoldi A, Enck P. Multichannel surface EMG for the non-invasive assessment of the anal sphincter muscle. Digestion. 2004;69(2):112–122. doi: 10.1159/000077877. [DOI] [PubMed] [Google Scholar]
- 7.Cescon C, Riva D, Začesta V, Drusany-Starič K, Martsidis K, Protsepko O, Baessler K, Merletti R. Effect of vaginal delivery on the external anal sphincter muscle innervation pattern evaluated by multichannel surface EMG: results of the multicentre study TASI-2. International urogynecology journal. 2014;25(11):1491–1499. doi: 10.1007/s00192-014-2375-0. [DOI] [PubMed] [Google Scholar]
- 8.Stafford RE, Sapsford R, Ashton-Miller J, Hodges PW. A novel transurethral surface electrode to record male striated urethral sphincter electromyographic activity. The Journal of urology. 2010;183(1):378–385. doi: 10.1016/j.juro.2009.08.105. [DOI] [PubMed] [Google Scholar]
- 9.Sim K, Chen S, Li Y, Kammoun M, Peng Y, Xu M, Gao Y, Song J, Zhang Y, Ardebili H, Yu C. High Fidelity Tape Transfer Printing Based On Chemically Induced Adhesive Strength Modulation. Scientific Reports. 2015;5:16133. doi: 10.1038/srep16133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng Y, He J, Khavari R, Boone T, Zhang Y. PD24-03 identification of innervation zones of the pelvic floor muscle from noninvasive high-density intra-vaginal/rectal surface EMG recordings. The Journal of Urology. 2015;193(4):e487. [Google Scholar]
- 11.Whitehead WE, Rao SS, Lowry A, Nagle D, Varma M, Bitar KN, Bharucha AE, Hamilton FA. Treatment of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases workshop. The American journal of gastroenterology. 2015;110(1):138–146. doi: 10.1038/ajg.2014.303. [DOI] [PubMed] [Google Scholar]
- 12.Grape HH, Dedering Å, Jonasson AF. Retest reliability of surface electromyography on the pelvic floor muscles. Neurourology and urodynamics. 2009;28(5):395–399. doi: 10.1002/nau.20648. [DOI] [PubMed] [Google Scholar]
- 13.Holobar A, Zazula D. Multichannel blind source separation using convolution kernel compensation. IEEE Transactions on Signal Processing. 2007;55(9):4487–4496. [Google Scholar]
- 14.Li X, Holobar A, Gazzoni M, Merletti R, Rymer WZ, Zhou P. Examination of Poststroke Alteration in Motor Unit Firing Behavior Using High-Density Surface EMG Decomposition. IEEE Transactions on Biomedical Engineering. 2015;62(5):1242–1252. doi: 10.1109/TBME.2014.2368514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Ning Y, Li S, Zhou P, Rymer WZ, Zhang Y. Three-dimensional innervation zone imaging from multi-channel surface EMG recordings. International journal of neural systems. 2015;25(06):1550024. doi: 10.1142/S0129065715500240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ning Y, Zhu X, Zhu S, Zhang Y. Surface EMG decomposition based on K-means clustering and convolution kernel compensation. IEEE Journal of Biomedical and Health Informatics. 2015;19(2):471–477. doi: 10.1109/JBHI.2014.2328497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cescon C, Bottin A, Fraga XLF, Azpiroz F, Merletti R. Detection of individual motor units of the puborectalis muscle by non-invasive EMG electrode arrays. Journal of Electromyography and Kinesiology. 2008;18(3):382–389. doi: 10.1016/j.jelekin.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 18.De Luca CJ, Kuznetsov M, Gilmore LD, Roy SH. Inter-electrode spacing of surface EMG sensors: reduction of crosstalk contamination during voluntary contractions. Journal of biomechanics. 2012;45(3):555–561. doi: 10.1016/j.jbiomech.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Bo K, Sherburn M. Evaluation of female pelvic-floor muscle function and strength. Physical therapy. 2005;85(3):269–282. [PubMed] [Google Scholar]
- 20.Peng Y, Khavari R, Nakib NA, Boone TB, Zhang Y. Assessment of urethral support using MRI-derived computational modeling of the female pelvis. International urogynecology journal. 2015:1–8. doi: 10.1007/s00192-015-2804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng Y, Khavari R, Nakib NA, Stewart JN, Boone TB, Zhang Y. The single-incision sling to treat female stress urinary incontinence: a dynamic computational study of outcomes and risk factors. Journal of biomechanical engineering. 2015;137(9):091007. doi: 10.1115/1.4030978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chanda A, Unnikrishnan V, Roy S, Richter HE. Computational Modeling of the Female Pelvic Support Structures and Organs to Understand the Mechanism of Pelvic Organ Prolapse: A Review. Applied Mechanics Reviews. 2015;67(4):040801. [Google Scholar]
- 23.Martinez-Valdes E, Laine C, Falla D, Mayer F, Farina D. High-density surface electromyography provides reliable estimates of motor unit behavior. Clinical Neurophysiology. 2015 doi: 10.1016/j.clinph.2015.10.065. [DOI] [PubMed] [Google Scholar]
- 24.Marateb HR, McGill KC, Holobar A, Lateva ZC, Mansourian M, Merletti R. Accuracy assessment of CKC high-density surface EMG decomposition in biceps femoris muscle. Journal of neural engineering. 2011;8(6):066002. doi: 10.1088/1741-2560/8/6/066002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holobar A, Minetto MA, Botter A, Negro F, Farina D. Experimental analysis of accuracy in the identification of motor unit spike trains from high-density surface EMG. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2010;18(3):221–229. doi: 10.1109/TNSRE.2010.2041593. [DOI] [PubMed] [Google Scholar]