Abstract
Fe‐N‐C catalysts with high O2 reduction performance are crucial for displacing Pt in low‐temperature fuel cells. However, insufficient understanding of which reaction steps are catalyzed by what sites limits their progress. The nature of sites were investigated that are active toward H2O2 reduction, a key intermediate during indirect O2 reduction and a source of deactivation in fuel cells. Catalysts comprising different relative contents of FeNxCy moieties and Fe particles encapsulated in N‐doped carbon layers (0–100 %) show that both types of sites are active, although moderately, toward H2O2 reduction. In contrast, N‐doped carbons free of Fe and Fe particles exposed to the electrolyte are inactive. When catalyzing the ORR, FeNxCy moieties are more selective than Fe particles encapsulated in N‐doped carbon. These novel insights offer rational approaches for more selective and therefore more durable Fe‐N‐C catalysts.
Keywords: fuel cells, heterogeneous catalysis, hydrogen peroxide, iron, oxygen reduction reaction
The oxygen reduction reaction (ORR), a key reaction for acidic fuel cells, is today most efficiently catalyzed by Pt materials.1 However, the large‐scale deployment of acidic fuel cells will be confronted with the grand challenge of developing inexpensive catalysts with high activity and stability.2 While metal–nitrogen–carbon catalysts (M‐N‐C, especially M=Fe or Co) are promising,3 further advances and fundamental insights into the factors governing their catalysis and durability are needed to meet the stringent industrial requirements.
The nature of active sites in pyrolyzed Fe‐N‐C catalysts is a highly complex and debated topic.4 The simultaneous presence of multiple Fe species in most catalysts investigated hitherto has obscured the interpretation of their ORR behaviors, especially since multiple sites may lead to unexpected synergies. In particular, several types of active sites may be necessary to catalyze the multielectronic ORR, which may proceed either through a direct 4 e− pathway on a single site (O2→H2O), or through indirect pathways involving a desorbed H2O2 intermediate (O2→H2O2→H2O) on two sites. These may be identical in nature (consequential 2 e−×2 e− mechanism), or different (bifunctional 2 e−+2 e− mechanism). If the ORR proceeds via the bifunctional mechanism, one type of site (S1) reduces O2 to H2O2 and another (S2) catalyzes the peroxide reduction reaction (PRR). Under this hypothesis, only Fe‐N‐C catalysts comprising a high density of sites S1 and S2 may catalyze the ORR with an apparent low % H2O2. PRR catalysis is also highly desirable for improved durability of Fe‐N‐C catalysts because even low H2O2 production can lead to significant degradation during fuel cell operation.5
From advanced spectroscopy (Mössbauer6 and X‐ray absorption spectroscopy (XAS),7 and mass spectrometry8) or molecular‐probe approaches,9 there is a growing consensus that FeNxCy moieties or N‐C species encapsulating Fe particles (Fe@N‐C) catalyze the transfer of the first 2 e− during ORR (S1).10 Then, if the ORR mostly follows the 2 e−+2 e− pathway, highly selective Fe‐N‐C catalysts should be characterized by a high number of sites S2.11 It has been recently suggested that Fe particles or pyridinic‐N groups might be the site S2.12 However, improved understanding on the nature of the sites active toward PRR is still pivotal for improved durability. To determine whether Fe‐N‐C materials catalyze the ORR via a direct, consequential, or bifunctional mechanism, we have investigated the PRR activity on a set of catalysts, including model catalysts only comprising either FeNxCy moieties or Fe particles.
In the continuation of our recent studies,10b, 13 the catalysts were synthesized by pyrolysis of FeII acetate, 1,10‐phenanthroline (Phen), and a ZnII zeolitic imidazolate framework (ZIF‐8). Three catalysts were first prepared, named FeNC‐wet‐1, FeNC‐dry‐1, and FeNC‐dry‐0.5, the labeling referring to homogenization conditions and Fe content before pyrolysis (see Methods in the Supporting Information). A catalyst pyrolyzed without FeII acetate was also prepared (“NC”). FeNC‐wet‐1, FeNC‐dry‐1, and FeNC‐dry‐0.5 showed Fe content of ca. 3.4, 3.0, and 1.5 wt % after pyrolysis, respectively, while about 100 ppm Fe was detected with inductively coupled plasma mass spectrometry (ICP‐MS) for NC.14 X‐ray diffraction (XRD) and Raman spectroscopy revealed carbonization of ZIF‐8 and Phen after pyrolysis (Supporting Information, Figure S1). X‐ray photoelectron spectroscopy (XPS) shows similar N‐doping level and N‐components for all catalysts (Supporting Information, Figure S2, Table S1).
To identify the Fe structures in the catalysts, we examined their morphology with transmission electron microscopy (TEM), which revealed a larger number of Fe particles in FeNC‐wet‐1 than in FeNC‐dry‐1, with most Fe particles embedded in N‐doped carbon layers (Supporting Information, Figure S3). 57Fe Mössbauer spectroscopy showed that the crystalline Fe particles seen in TEM images correspond to α‐Fe, Fe carbide, or γ‐Fe (Figure 1 a–c; Supporting Information, Table S2). The relative absorption area owing to Fe particles was 47 % for FeNC‐wet‐1 and 8 % for FeNC‐dry‐1. No signal assigned to Fe particles was detected for FeNC‐dry‐0.5, the Mössbauer spectrum of which shows only the doublets D1 and D2 (FeNxCy moieties). These observations were confirmed by extended X‐ray absorption fine structure (EXAFS), with a lower intensity of the Fe‐Fe backscattering signal for FeNC‐dry‐1 vs. FeNC‐wet‐1 (Figure 1 d). As previously reported,10b a strong Fe‐N(O) interaction without Fe‐Fe interaction for FeNC‐dry‐0.5 confirms the sole presence of FeNxCy moieties. Quantification of the absolute content of each Fe component was derived from the Mössbauer spectra fittings (Figure 1 e).6c Thus, Fe‐N‐C catalysts with high (FeNC‐wet‐1) and low content (FeNC‐dry‐1) of Fe particles are identified, along with those devoid of Fe particles (FeNC‐dry‐0.5) and nearly devoid of Fe altogether (NC). Furthermore, a catalyst labeled FePhen@MOF‐ArNH3 exclusively comprising Fe particles was investigated.10a Its Fe content was 3.1 wt % (Figure 1 e), with Fe being present as metallic, carbide, and nitride particles.
Figure 1.
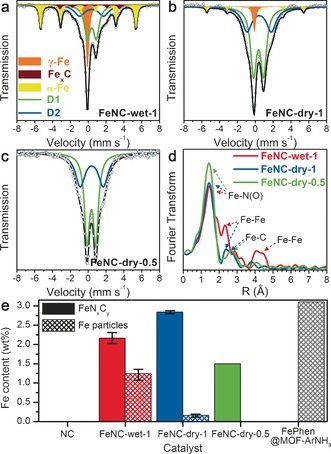
57Fe Mössbauer absorption spectra and their fittings with five spectral components for a) FeNC‐wet‐1, b) FeNC‐dry‐1, and c) FeNC‐dry‐0.5. d) Fourier transforms of the EXAFS spectra. e) Absolute Fe contents of the two main sub‐groups of Fe species.
The electrochemical properties were then measured using a rotating disk electrode (RDE) in 0.1 m HClO4 electrolyte. All four of the Fe‐N‐C catalysts showed high ORR activity (Figure 2 a), comparable to that of other high‐performing Fe‐N‐C catalysts.3c The NC catalyst showed poor ORR kinetics, as expected in acidic medium.4, 10a,10b Rotating ring disk electrode (RRDE) measurements at 800 μg cm−2 loading showed low H2O2 production (<6 %) during ORR for the four Fe‐N‐C catalysts (Figure 2 b and S4). However, at 100 μg cm−2, up to 32 % H2O2 production was measured. This indicates that H2O2 produced at a given catalytic site may be consecutively reduced to H2O. This suggests that, on some Fe sites at least, H2O2 may desorb and then re‐adsorb on the same type or on another type of Fe sites having a higher affinity for H2O2. Furthermore, the positive correlation between % H2O2 and absolute content of Fe particles at low loading (Supporting Information, Figure S4, right) suggests FeNxCy moieties catalyze the ORR to H2O more selectively than Fe@NC sites. Owing to 15–30 % H2O2 produced during ORR at low loading, investigating the PRR activity of different sites in Fe‐N‐C catalysts is important to rationally improve their selectivity.
Figure 2.
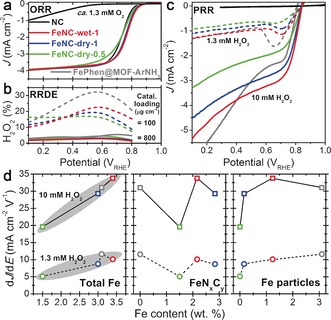
Electrochemical characterization. a) ORR polarization curves, b) H2O2 formation measured with RRDE, and c) PRR polarization curves: 900 rpm and 800 μg cm−2 (additionally, 100 μg cm−2 for RRDE studies). d) Correlations between PRR activity and absolute content of either total Fe or Fe sub‐components. PRR activity is reported as the slope dJ/dE seen in (c) at high potential (see the Supporting Information).
The PRR activity was measured in Ar‐saturated 0.1 m HClO4 electrolyte containing 1.3 or 10 mm H2O2 (Figure 2 c). The NC catalyst showed no PRR activity, indicating that surface N‐groups unpromoted by Fe cannot be a site S2. In contrast, high PRR current on FeNC‐dry‐0.5 demonstrates that FeNxCy moieties are PRR‐active. This is a first major finding of the present study, enabled by the synthesis of Fe‐N‐C catalysts free of Fe particles.10b, 15
Previous work hypothesized that Fe‐N‐C catalysts catalyze the ORR via a bifunctional 2 e−+2 e− mechanism in acidic medium; the site S1 for 2 e− ORR being FeNxCy moieties and site S2 for PRR being Fe particles.12a However, the turnover frequency of FeNxCy moieties for PRR is much lower than that for ORR (FeNC‐dry‐0.5: lower PRR than ORR current at 0.8 VRHE for the same reactant concentration of 1.3 mm, Figure 2 a,c). This suggests that ORR on FeNxCy moieties predominantly occurs via the direct 4 e− pathway. This is also supported by the low H2O2 production measured for FeNC‐dry‐0.5 at low loading (Figure 2 b).
At this stage, we however do not know yet whether Fe particles present in other Fe‐N‐C catalysts are PRR active. If such particles are PRR inactive, the PRR activity should be linearly correlated with the absolute FeNxCy content. However, no linear correlation between FeNxCy content and PRR activity is observed (Figure 2 d, center). This suggests that FeNxCy sites are not the sole active site for PRR but that Fe particles should also be considered. Some correlation is observed between PRR activity and content of Fe particles (Figure 2 d, right), but a better correlation is observed between PRR activity and the total Fe content (Figure 2 d, left). Significant PRR activity of FePhen@MOF‐ArNH3 also demonstrates that Fe particles are PRR active, with an activity commensurate to those of FeNxCy moieties (Figure 2 c,d).
While Fe particles are PRR‐active, it is still unclear whether they need to be directly exposed to the electrolyte for catalyzing the PRR. Fe particles in direct contact with electrolyte have previously been suggested to be a site S2.12a, 16 Recently, we showed that electrolyte‐exposed Fe particles can survive in acidic environments at >0.7 VRHE, which is due to stabilization of ferric hydroxide species.13 The presence of Fe particles exposed to acidic electrolyte during short RDE studies is thus possible.
To elucidate whether Fe particles must be directly exposed to the electrolyte to catalyze the PRR, The PRR activity of FeNC‐wet‐1 and FeNC‐dry‐1 was measured before and after dissolution (electrochemical dissolution13b) of electrolyte‐exposed particles (Figure 3). Operando analysis using a scanning flow cell (SFC) connected with ICP‐MS (Supporting Information, Figure S5) showed that a significant fraction of electrolyte‐exposed Fe particles in FeNC‐wet‐1 were removed during 100 cyclic voltammograms (CV) between 0 and 1 VRHE (Figure 3 a). The dissolved Fe reaches 9 % of the total content of Fe particles present in pristine FeNC‐wet‐1. FeNxCy moieties are highly stable during this treatment, as previously demonstrated by negligible Fe leaching from FeNC‐dry‐0.5.13b
Figure 3.
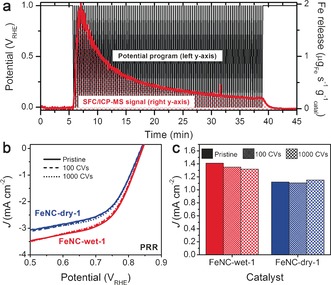
PRR activity before and after electrochemical dissolution of electrolyte‐exposed Fe particles a) Fe dissolution rates from FeNC‐wet‐1 during 100 cycles. b) PRR polarization curves before and after 100 or 1000 cycles and c) PRR current density at 0.8 VRHE before and after the cycling.
The PRR activity of FeNC‐dry‐1 and FeNC‐wet‐1 were however unmodified, even after extended dissolution up to 1000 CVs (Figure 3 b and c). An extrapolation predicts that the vast majority of electrolyte‐exposed Fe particles are removed after 1000 CVs (Supporting Information, Figure S6). As previously reported,13 the dissolution of exposed Fe particles did not decrease the ORR activity (Supporting Information, Figure S7). Therefore, it is concluded that electrolyte‐exposed Fe particles in pristine FeNC‐wet‐1 are inactive toward PRR and ORR. It demonstrates that Fe@N‐C sites are therefore PRR active and can be a site S2, as previously hypothesized.10a
In conclusion, we elucidated the PRR and ORR reactivity of four different structures existing in Fe‐N‐C catalysts, that is, metal‐free N‐C groups, electrolyte‐exposed Fe particles, FeNxCy moieties and Fe@N‐C. The ORR pathways on Fe‐N‐C catalysts could consequently be established (Figure 4). Contrary to previous hypotheses, N‐C groups and electrolyte‐exposed Fe particles were shown to be inactive toward PRR in acidic medium. We also demonstrated that FeNxCy moieties and Fe@N‐C species are moderately active toward PRR, proving their possible roles in both direct (major path) and indirect 4 e− ORR pathways. Since the synthesis of Fe‐N‐C catalysts with only FeNxCy moieties,10b, 15 only Fe@N‐C particles,10a or their combination3b,3d, 17 is now controllable, the understanding of the nature of active sites for PRR provided herein offers new insights for the rational design of advanced Fe‐N‐C catalysts with high selectivity and expectedly improved durability in polymer electrolyte fuel cells.
Figure 4.
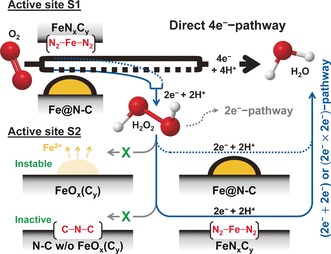
ORR mechanisms on Fe‐N‐C catalysts in acid medium. The site S1 is FeNxCy moieties or Fe@N‐C. FeNxCy mostly catalyzes direct 4 e− ORR, but also releases a minor fraction of H2O2. Fe@N‐C produces higher fraction of H2O2 in comparison to FeNxCy. The released H2O2 is then reduced to H2O on site S2 (either FeNxCy or Fe@N‐C). Surface‐exposed Fe particles and N‐groups without subsurface Fe are PRR‐inactive.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
This research was supported by MAXNET Energy, by ANR under contract 2011 CHEX 004 01, and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF‐2017R1C1B2002918). The authors deeply appreciate financial assistance from the U.S. Department of Energy, EERE (DE‐EE‐0000459), and Alexander von Humboldt Foundation.
C. H. Choi, W. S. Choi, O. Kasian, A. K. Mechler, M. T. Sougrati, S. Brüller, K. Strickland, Q. Jia, S. Mukerjee, K. J. J. Mayrhofer, F. Jaouen, Angew. Chem. Int. Ed. 2017, 56, 8809.
Contributor Information
Prof. Chang Hyuck Choi, Email: chchoi@gist.ac.kr.
Dr. Frédéric Jaouen, Email: frederic.jaouen@umontpellier.fr.
References
- 1. Cui C. H., Gan L., Heggen M., Rudi S., Strasser P., Nat. Mater. 2013, 12, 765. [DOI] [PubMed] [Google Scholar]
- 2. Shui J., Wang M., Du F., Dai L., Sci. Adv. 2015, 1, e1400129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.
- 3a. Bashyam R., Zelenay P., Nature 2006, 443, 63; [DOI] [PubMed] [Google Scholar]
- 3b. Lefèvre M., Proietti E., Jaouen F., Dodelet J. P., Science 2009, 324, 71; [DOI] [PubMed] [Google Scholar]
- 3c. Jaouen F., et al., ACS Appl. Mater. Inter. 2009, 1, 1623; [DOI] [PubMed] [Google Scholar]
- 3d. Wu G., More K. L., Johnston C. M., Zelenay P., Science 2011, 332, 443; [DOI] [PubMed] [Google Scholar]
- 3e. Cheon J. Y., et al., Sci. Rep. 2013, 3, 2715; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3f. Guan B. Y., Yu L., Lou X. W., Energy Environ. Sci. 2016, 9, 3092; [Google Scholar]
- 3g. Xia B. Y., Yan Y., Li N., Wu H. B., Lou X. W., Wang X., Nat. Energy 2016, 1, 15006; [Google Scholar]
- 3h. Liu T., Zhao P., Hua X., Luo W., Chen S., Cheng G., J. Mater. Chem. A 2016, 4, 11357. [Google Scholar]
- 4. Masa J., Xia W., Muhler M., Schuhmann W., Angew. Chem. Int. Ed. 2015, 54, 10102; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 10240. [Google Scholar]
- 5. Goellner V., Armel V., Zitolo A., Fonda E., Jaouen F., J. Electrochem. Soc. 2015, 162, H403. [Google Scholar]
- 6.
- 6a. Tian J., Morozan A., Sougrati M. T., Lefèvre M., Chenitz R., Dodelet J. P., Jones D., Jaouen F., Angew. Chem. Int. Ed. 2013, 52, 6867; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 7005; [Google Scholar]
- 6b. Kramm U. I., Lefevre M., Larouche N., Schmeisser D., Dodelet J. P., J. Am. Chem. Soc. 2014, 136, 978; [DOI] [PubMed] [Google Scholar]
- 6c. Sougrati M. T., Goellner V., Schuppert A. K., Stievano L., Jaouen F., Catal. Today 2016, 262, 110. [Google Scholar]
- 7.
- 7a. Ramaswamy N., Tylus U., Jia Q. Y., Mukerjee S., J. Am. Chem. Soc. 2013, 135, 15443; [DOI] [PubMed] [Google Scholar]
- 7b. Jia Q., et al., ACS Nano 2015, 9, 12496; [DOI] [PubMed] [Google Scholar]
- 7c. Li J., et al., Energy Environ. Sci. 2016, 9, 2418. [Google Scholar]
- 8.
- 8a. Lefèvre M., Dodelet J. P., Bertrand P., J. Phys. Chem. B 2005, 109, 16718; [DOI] [PubMed] [Google Scholar]
- 8b. Li W. M., Wu J., Higgins D. C., Choi J. Y., Chen Z. W., ACS Catal. 2012, 2, 2761. [Google Scholar]
- 9. Sahraie N. R., Kramm U. I., Steinberg J., Zhang Y. J., Thomas A., Reier T., Paraknowitsch J. P., Strasser P., Nat. Commun. 2015, 6, 8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.
- 10a. Strickland K., Elise M. W., Jia Q. Y., Tylus U., Ramaswamy N., Liang W. T., Sougrati M. T., Jaouen F., Mukerjee S., Nat. Commun. 2015, 6, 7343; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10b. Zitolo A., Goellner V., Armel V., Sougrati M.-T., Mineva T., Stievano L., Fonda E., Jaouen F., Nat. Mater. 2015, 14, 937; [DOI] [PubMed] [Google Scholar]
- 10c. Varnell J. A., Tse E. C. M., Schulz C. E., Fister T. T., Haasch R. T., Timoshenko J., Frenkel A. I., Gewirth A. A., Nat. Commun. 2016, 7, 12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.
- 11a. Olson T. S., Pylypenko S., Fulghum J. E., Atanassov P., J. Electrochem. Soc. 2010, 157, B54; [Google Scholar]
- 11b. Robson M. H., Serov A., Artyushkova K., Atanassov P., Electrochim. Acta 2013, 90, 656; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11c. Serov A., Tylus U., Artyushkova K., Mukerjee S., Atanassov P., Appl. Catal. B 2014, 150, 179. [Google Scholar]
- 12.
- 12a. Tylus U., Jia Q., Strickland K., Ramaswamy N., Serov A., Atanassov P., Mukerjee S., J. Phys. Chem. C 2014, 118, 8999; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12b. Artyushkova K., Serov A., Rojas-Carbonell S., Atanassov P., J. Phys. Chem. C 2015, 119, 25917. [Google Scholar]
- 13.
- 13a. Choi C. H., Baldizzone C., Grote J. P., Schuppert A. K., Jaouen F., Mayrhofer K. J. J., Angew. Chem. Int. Ed. 2015, 54, 12753; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 12944; [Google Scholar]
- 13b. Choi C. H., et al., ACS Catal. 2016, 6, 3136. [Google Scholar]
- 14. Zhang G., Chenitz R., Lefèvre M., Sun S., Dodelet J.-P., Nano Energy 2016, 29, 111. [Google Scholar]
- 15. Kramm U. I., Herrmann-Geppert I., Behrends J., Lips K., Fiechter S., Bogdanoff P., J. Am. Chem. Soc. 2016, 138, 635. [DOI] [PubMed] [Google Scholar]
- 16. Wiggins-Camacho J. D., Stevenson K. J., J. Phys. Chem. C 2011, 115, 20002. [Google Scholar]
- 17. Proietti E., Jaouen F., Lefevre M., Larouche N., Tian J., Herranz J., Dodelet J. P., Nat. Commun. 2011, 2, 416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


