Abstract
WNT5A is a secreted signaling protein that promotes migration and invasion of oral squamous cell carcinoma (OSCC) cells through activation of non‐canonical WNT signaling. Here, we examined expression of WNT5A, β‐catenin, and E‐cadherin by immunohistochemistry in 21 human diagnostic incision biopsies that each had regions of oral mucosa with a normal appearance adjacent to the affected tissue, dysplasia, and OSCC. We also investigated the effect of recombinant WNT5A (rWNT5A) on expression of the cell‐adhesion proteins E‐cadherin and β‐catenin by western blot analysis. No expression of WNT5A protein was present in oral mucosa with a normal appearance or in mild grade dysplasia. However, expression of WNT5A increased along with increasing grade of dysplasia, and the highest expression was detected in OSCCs. Expression of membranous β‐catenin and of E‐cadherin was lower, whereas expression of cytoplasmic β‐catenin was higher, in OSCCs than in non‐cancerous regions. However, there was no correlation between expression of WNT5A and expression of either β‐catenin or E‐cadherin. Furthermore, treatment of OSCC cells with rWNT5A had no effect on the expression of β‐catenin or E‐cadherin. Taken together with previous results, we conclude that WNT5A influences the progression of OSCC without affecting the canonical WNT/β‐catenin pathway and without down‐regulating E‐cadherin. WNT5A may have potential as a biological marker for malignant transformation of dysplasia to OSCC.
Keywords: E‐cadherin, immunohistochemistry, oral cancer, WNT5A, β‐catenin
Oral squamous cell carcinoma (OSCC) is a heterogeneous cancer and the most prevalent type of cancer in the oral cavity 1, 2. It can spread via vascular, lymphatic 3, or perineural invasion 4 to regional cervical nodes of the neck, and to the lungs, liver, or bones 5, 6. Oral carcinogenesis begins with an accumulation of genetic and epigenetic alterations in keratinocytes of the basal layer of the oral epithelium. These alterations affect normal growth, differentiation, and apoptosis of keratinocytes and can, in time, lead to transformation of normal keratinocytes into precancerous cells and to the development of precancerous lesions. These lesions clinically appear as leukoplakia or erythroplakia but histologically appear as benign hyperkeratosis, dysplasia, or carcinoma in situ. Further genetic alterations lead to transformation of the precancerous cells into cancer cells, which degrade the basement membrane and invade the underlying connective tissue, forming OSCC 7, 8, 9.
WNT5A is a secreted signaling protein that belongs to the WNT family of cysteine‐rich proteins. It appears to be important in the development of organs and to regulate cellular functions, including proliferation, differentiation, apoptosis, survival, polarity, migration, and invasion 10. WNT5A mainly activates the non‐canonical WNT/Ca2+ and WNT/planner cell polarity signaling pathways. It can also either inhibit or activate the canonical WNT/β‐catenin pathway, and the nature of this effect depends on the receptor and cellular context 11, 12, 13. WNT5A has been reported to increase the migration and invasion of pancreatic cancer cells by activating the canonical WNT/β‐catenin pathway and reducing expression of E‐cadherin 14. In malignant melanoma cells, WNT5A increases cell migration by down‐regulating E‐cadherin without affecting the canonical WNT/β‐catenin signaling 15. In breast cancer, on the other hand, WNT5A increases membranous expression of E‐cadherin and β‐catenin, increases cell adhesion, and consequently decreases cell motility 16.
β‐catenin is involved in cell adhesion but also in cell signaling as a part of the canonical WNT signaling pathway. When associated with E‐cadherin, β‐catenin localizes to the cell membrane and the complex contributes to the establishment of the epithelial structure and to cell–cell adhesion. Loss of E‐cadherin or the presence of canonical WNT signaling promotes accumulation of β‐catenin in the cytoplasm and translocation into the nucleus, where it can induce transcription of genes affecting cell proliferation, migration, and invasion 17, 18. Decreased expression of membranous β‐catenin and increased expression of cytoplasmic β‐catenin has been associated with progression of OSCC 19, 20.
E‐cadherin is a Ca2+‐dependent transmembrane glycoprotein that is involved in cell–cell adhesion and rearrangement of epithelial cells. The expression of E‐cadherin is regulated by different signaling pathways 21. Down‐regulation of E‐cadherin expression has a key role in the epithelial–mesenchymal transition (EMT) and leads to decreased cell adhesion, increased cell migration, and invasion 22. Decreased expression of E‐cadherin has been reported to correlate with increased invasiveness and poor prognosis in OSCC 23, 24.
So far, the expression of WNT5A protein has not been thoroughly examined in human OSCC samples by immunohistochemistry (IHC). To our knowledge, there are three published studies on the expression of WNT5A in OSCC. Two of these were in fresh‐frozen human OSCC tissues and employed quantitative PCR (qPCR) and immunoblotting to determine the levels of WNT5A mRNA 25 and WNT5A protein 26, respectively. The single study that analyzed WNT5A protein by IHC was performed on tissue samples of rat tongue squamous cell carcinoma (SCC) 27. In the present study, we aimed to examine the expression of WNT5A, by IHC, in human formalin‐fixed paraffin‐embedded tissues exhibiting regions of oral mucosa with a normal appearance adjacent to the affected tissue, dysplasia, and OSCC. Because OSCC cells usually migrate collectively, thus keeping the cell‐cell junctions intact 28, and WNT5A increases the collective migration of OSCC cells 29, we also aimed to investigate if WNT5A affects expression of the cell‐adhesion proteins E‐cadherin and β‐catenin.
Material and methods
Tissue samples
The experimental design of this study was approved by The Ethics Committee of the Swedish Southern Health Care Region. We reviewed anonymous diagnostic incision biopsies, from all biopsies histologically diagnosed as OSCC, at the Department of Oral Pathology, Faculty of Odontology, Malmö University, Malmö, Sweden, from 2003 to 2010. Of the 207 diagnostic incision biopsies reviewed, only 21 were included in this study according to the following criteria: the presence, in the same tissue sample, of oral mucosa of normal appearance adjacent to the affected tissue, dysplasia, and OSCC; no previous history of cancer; technically sufficient tissue material; and availability of TNM data for the primary tumor from the Swedish Cancer Register. The biopsies were extracted from different locations of the mouth: tongue (n = 10); buccal mucosa (n = 4); trigonum retromolare (n = 1); alveolar ridge (n = 5); and the floor of the mouth (n = 1) (Table 1).
Table 1.
Clinicopathological features of 21 patients
| Patient no. | Gender | Age (yr) | Anatomical site | Grade of dysplasia | TNM stage | OSCC differentiation |
|---|---|---|---|---|---|---|
| 1 | M | 73 | Tongue | Severe | T2N0Mx | Well |
| 2 | F | 76 | Tongue | Severe | T1N0M0 | Well |
| 3 | F | 64 | Tongue | Moderate | T1N0M0 | Well |
| 4 | F | 26 | Tongue | Severe | T2N0M0 | Well |
| 5 | F | 77 | Alveolar ridge | Moderate | T4N0M0 | Well |
| 6 | M | 59 | Buccal mucosa | Moderate | TxNxMx | Well |
| 7 | F | 71 | Alveolar ridge | Severe | T1N0M0 | Well |
| 8 | F | 34 | Tongue | Severe | T1N0M0 | Well |
| 9 | F | 66 | Tongue | Severe | TisN0M0 | Well |
| 10 | F | 65 | Trigonum retromolare | Mild | T1N0M0 | Well |
| 11 | M | 70 | Tongue | Mild | T1N0M0 | Well |
| 12 | M | 71 | Alveolar ridge | Moderate | T2N0M0 | Well |
| 13 | M | 55 | Tongue | Severe | T1N0M0 | Well |
| 14 | F | 54 | Buccal mucosa | Moderate | T1N0M0 | Well |
| 15 | M | 65 | Buccal mucosa | Moderate | T2N0M0 | Moderate |
| 16 | M | 69 | Tongue | Mild | T2N0M0 | Poor |
| 17 | F | 72 | Buccal mucosa | Mild | T4aN0M0 | Poor |
| 18 | M | 77 | Alveolar ridge | Severe | T1N0M0 | Well |
| 19 | M | 69 | Floor of the mouth | Mild | T1N0M0 | Moderate |
| 20 | F | 79 | Alveolar ridge | Moderate | T2N0M0 | Well |
| 21 | F | 68 | Tongue | Moderate | T1N0M0 | Well |
F, female; M, male; OSCC, oral squamous cell carcinoma.
Antibodies
Antibodies used for IHC were: monoclonal mouse WNT5A clone 3A4 (H00007474‐M04) from Abnova (Taipei, Taiwan); monoclonal mouse β‐catenin (610154) from BD Biosciences (San Jose, CA, USA); and monoclonal rabbit E‐cadherin clone 24E10 (#3195) from Cell Signaling Technology (Danvers, MA, USA). Antibodies used for western blot analysis were: monoclonal mouse E‐cadherin (610181) and monoclonal mouse β‐catenin (610154) from BD Biosciences; monoclonal rabbit non‐phospho (active) β‐catenin (Ser33/37/Thr41) clone D13A1 (#8814) from Cell Signaling Technology; and monoclonal mouse α‐tubulin clone DM1A (sc‐32293) from Santa Cruz (Dallas, TX, USA). Goat anti‐rabbit/horseradish peroxidase (HRP) (P0448) and goat anti‐mouse/HRP (P0447) were purchased from DAKO (Glostrup, Denmark).
Immunohistochemistry
Immunohistochemistry was performed on 3‐μm‐thick serial sections of formalin‐fixed paraffin‐embedded tissues. Tissue sections were deparaffinized and rehydrated prior to heat‐induced antigen retrieval in 10 mM citrate buffer, pH 6.0, at 95°C for 20 min (for β‐catenin and E‐cadherin) or 40 min (for WNT5A) in a Decloaking Chamber (NxGen; Biocare Medical, Concord, CA, USA). After cooling and washing the sections with TRIS‐buffered saline containing 0.1% Tween 20 (TBS‐T, pH 7.6), non‐specific background staining was blocked with background sniper (BS966L10; Biocare Medical) for 10 min at room temperature. The sections were then incubated overnight at 4°C with the primary antibodies diluted in antibody diluent containing background reducing components (S3022; DAKO). The optimal antibody dilutions were obtained by serial dilution experiments and were as follows: WNT5A, 1:75 dilution; β‐catenin, 1:1,000 dilution; and E‐cadherin, 1:400 dilution. Next, endogenous peroxidase was blocked for 10 min with peroxidase blocking reagent (HPBK; S2023; DAKO) before addition of secondary antibody (goat anti‐mouse/rabbit/HRP; ENVISION System, K5007; DAKO) for 20 min at room temperature. The immunoreaction was then visualized with diaminobenzidine (DAB; K5007; DAKO). The tissue sections were counterstained with hematoxylin, then dehydrated and mounted. After each step, the slides were washed with TBS‐T. N‐Universal negative‐control mouse (N1698; DAKO), N‐Universal negative‐control rabbit (N1699; DAKO), and omission of primary antibody were the negative controls. Placenta, normal breast tissue, and breast cancer tissue were the positive controls.
Evaluation of IHC
Expression of WNT5A, β‐catenin, and E‐cadherin protein was evaluated semiquantitatively by two of the authors (Z.P. and P.L.). Each author assessed expression of these proteins in serial tissue sections of oral mucosa with a normal appearance, dysplasia, and OSCC using a Nikon Eclipse 80i light microscope (Nikon, Tokyo, Japan), with 40 × objective, in at least two visual fields in two to four cell layers of normal appearing oral mucosa and of dysplasia, and in the periphery of OSCC islands located at the invasive front. The periphery was considered as the first or second cancer‐cell layer closest to the connective tissue. The tissues were graded as positive if ≥50% of cells showed immunoreactivity for a protein, and negative if <50% of cells showed immunoreactivity for this protein 24, 30. Furthermore, the intensity of the positive immunoreaction was graded as weak or strong and was assessed in cytoplasm for WNT5A; in membrane, cytoplasm for β‐catenin; and in membrane for E‐cadherin.
Cell culture and treatment
Two human tongue SCC cell lines – SCC9 (CRL‐1629, lot 4372272) and SCC25 (CRL‐1628, lot 58075871) – were acquired from ATCC (Manassas, VA, USA) and maintained as previously described 29. All incubations were at 37°C and 5% CO2. The cells were cultured in six‐well plates until 80% confluence was reached, rinsed with PBS, incubated with serum‐free medium overnight, rinsed again with PBS, and treated either with 0.1% BSA in PBS as the control or with 0.4 μg/ml of recombinant WNT5A (rWNT5A; 645‐WN; R&D Systems, Minneapolis, MN, USA) in serum‐free medium. After 48 h of treatment, expression of protein in the cells was analyzed using western blotting.
Western blot analysis
Western blot analysis was carried out as previously described 29. In brief, cells were lysed, and 30 μg of protein was loaded onto an 8% sodium dodecyl sulfate polyacrylamide electrophoresis gel (SDS‐PAGE). Following semi‐dry blotting, the membranes were either incubated with 3% (w/v) non‐fat skimmed milk powder (06‐019; Scharlab, Barcelona, Spain) in TBS‐T for 30 min before addition of primary antibodies against E‐cadherin (1:1,000 dilution) and α‐tubulin (1:10,000 dilution), or incubated with 3% (w/v) BSA in TBS‐T for 30 min before addition of the primary antibodies against non‐phosphorylated (active) β‐catenin (1:1,000 dilution) and total β‐catenin (1:2,000 dilution). After incubation with secondary antibodies, the immunoreaction was developed with a Chemiluminescence HRP substrate (Millipore, Temecula, CA, USA).
Statistical analysis
The immunohistochemical data were statistically analyzed using SPSS Statistics 23.0 software (IBM, Armonk, NY, USA). The differences, within a single biopsy, in expression of WNT5A, β‐catenin, and E‐cadherin between oral mucosa with normal appearance, dysplasia, and OSCC were estimated using the two‐tailed Wilcoxon signed‐rank test. The correlation between expression of WNT5A and β‐catenin or E‐cadherin, as well as the correlation between expression of WNT5A protein and tumor size (T), were determined using two‐tailed Spearman rank correlations. Statistical analysis of the western blot analysis data was performed using the GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA). The experiments were performed at least three times and data are presented as mean ± standard error of the mean. Differences in the expression of protein between two groups were determined using the paired, two‐tailed Student's t‐test.
Results
Histopathologic findings
The clinicopathologic characteristics of the 21 patients in this study are presented in Table 1. The patient cohort, with a median age of 69 yr, consisted predominantly of female patients (57.1%) and the most common location of OSCC was in the tongue (47.6%). The grade of dysplasia differed within the group – five (23.8%) patients had mild grade, eight (38.1%) patients had moderate grade, and eight (38.1%) patients had severe grade. According to the TNM staging that had been determined at the primary surgery, most of the OSCCs were small: T1 (11; 52.4%) and T2 (six; 28.6%). Seventeen (81%) of the 21 patients had been evaluated as having well‐differentiated OSCCs, while two (9.5%) each had moderately or poorly differentiated OSCCs.
Expression of WNT5A protein in normal appearing oral mucosa, dysplasia, and OSCC
The expression of WNT5A protein in normal appearing oral mucosa, dysplasia, and OSCC is presented in Fig. 1. Cytoplasmic expression of WNT5A was absent in all samples of normal appearing oral mucosa, while it was detected in eight (38.1%) of the dysplastic tissues. Of these eight samples, three were diagnosed with moderate dysplasia while five were diagnosed with severe dysplasia. There was a statistically significantly higher expression of WNT5A in dysplasia compared with normal appearing oral mucosa (P = 0.005; Table 2). In 17 (81%) OSCCs, there was cytoplasmic WNT5A expression in the periphery of cancer islands located at the invasive front of OSCC. Among the well‐differentiated OSCCs, WNT5A was expressed in 76.5% (13/17) of samples, and all moderately and poorly differentiated OSCCs were positive for WNT5A. There was a statistically significant higher expression of WNT5A in OSCCs compared with both normal appearing oral mucosa (P < 0.001) and dysplasia (P = 0.001; Table 2). Furthermore, no correlation was found between cytoplasmic WNT5A expression and tumor size (T) (ρ = −0.013; P = 0.955). Nuclear WNT5A immunostaining was detected in 61.9% of normal appearing oral mucosa samples, 81% of dysplastic tissues, and 61.9% of OSCCs (data not shown).
Figure 1.
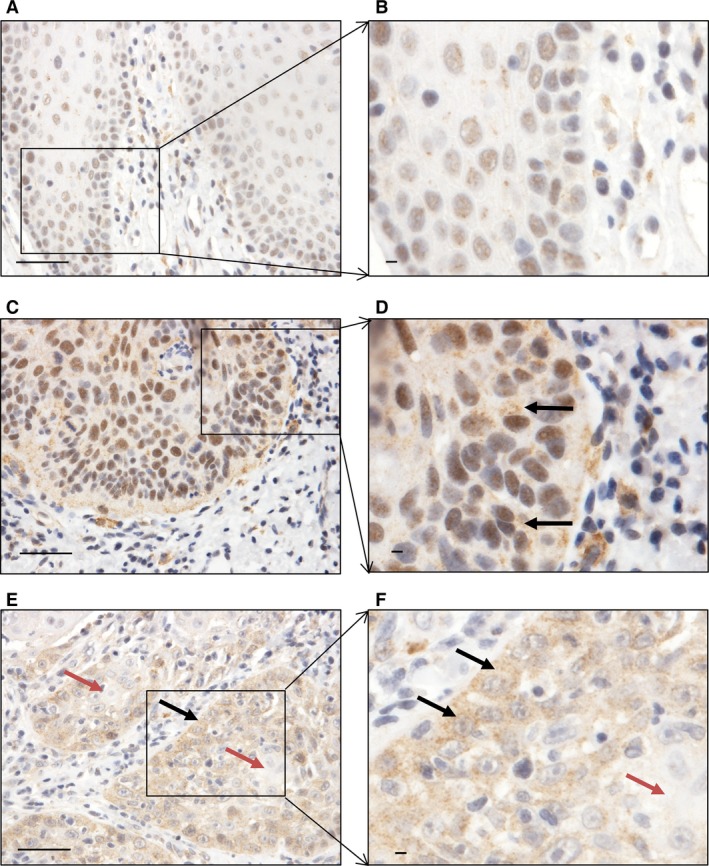
Expression of WNT5A in different tissue regions. (A and B) Nuclear expression of WNT5A in oral mucosa with normal appearance. (C and D) Cytoplasmic and nuclear expression of WNT5A in severe‐grade dysplasia (black arrows). (E and F) Cancer islands; black arrows indicate expression of WNT5A in the cytoplasm in the periphery of cancer islands and red arrows indicate the absence of expression of WNT5A in the central part of the cancer islands. Scale bar = 50 μm.
Table 2.
Expression of WNT5A, β‐catenin, and E‐cadherin in oral mucosa with a normal appearance, dysplasia, and at the invasive front of oral squamous cell carcinoma (OSCC)
| Staining | Oral mucosa | Dysplasia | OSCC | |||||
|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Well | Moderate | Poor | |||
| Cytoplasmic | Negative | 21 | 5 | 5 | 3 | 4 | 0 | 0 |
| WNT5A | Weak | 0 | 0 | 3 | 5 | 8 | 1 | 1 |
| Strong | 0 | 0 | 0 | 0 | 5 | 1 | 1 | |
| P | <0.001a | 0.001b | ||||||
| 0.005c | ||||||||
| Membranous | Negative | 0 | 0 | 1 | 1 | 3 | 0 | 0 |
| β‐catenin | Weak | 0 | 1 | 0 | 2 | 10 | 2 | 2 |
| Strong | 21 | 4 | 7 | 5 | 4 | 0 | 0 | |
| P | <0.001a | 0.001b | ||||||
| 0.038c | ||||||||
| Cytoplasmic | Negative | 21 | 3 | 4 | 0 | 8 | 2 | 0 |
| β‐catenin | Weak | 0 | 2 | 4 | 6 | 8 | 0 | 1 |
| Strong | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| P | 0.002a | 0.763b | ||||||
| 0.001c | ||||||||
| Membranous | Negative | 0 | 0 | 0 | 0 | 5 | 0 | 2 |
| E‐cadherin | Weak | 0 | 2 | 2 | 1 | 11 | 1 | 0 |
| Strong | 21 | 3 | 6 | 7 | 1 | 1 | 0 | |
| P | <0.001a | <0.001b | ||||||
| 0.025c | ||||||||
OSCC compared with oral mucosa with a normal appearance.
OSCC compared with dysplasia.
Dysplasia compared with oral mucosa with a normal appearance.
Expression of β‐catenin protein in normal appearing oral mucosa, dysplasia, and OSCC
The expression of β‐catenin is presented in Fig. 2. Its expression in all normal appearing epithelial cells was exclusively membranous, with no expression detected in the cytoplasm. Nineteen (90.5%) of the dysplastic tissues showed expression of membranous β‐catenin, which was distributed almost equally among the three grades of dysplasia (Table 2), and 12 (57.1%) of the dysplastic samples showed expression of cytoplasmic β‐catenin. This expression increased with grade of dysplasia: cytoplasmic expression was present in two samples with mild‐grade dysplasia, in four samples with moderate‐grade dysplasia, and in six samples with severe‐grade dysplasia. There was a statistically significantly higher expression of cytoplasmic β‐catenin in dysplasia compared with normal appearing oral mucosa (P = 0.001) (Table 2). Membranous β‐catenin was expressed in 18 (85.7%) of OSCCs, and there was no difference with respect to differentiation grade. Cytoplasmic β‐catenin was expressed in 11 (52.4%) of the OSCCs, which is more than in normal appearing oral mucosa (P = 0.002; Table 2). There was less expression of membranous β‐catenin in OSCC tissues than in either normal appearing oral mucosa (P < 0.001) or dysplasia (P = 0.001; Table 2). Nuclear β‐catenin expression was detected in only one OSCC sample (data not shown).
Figure 2.
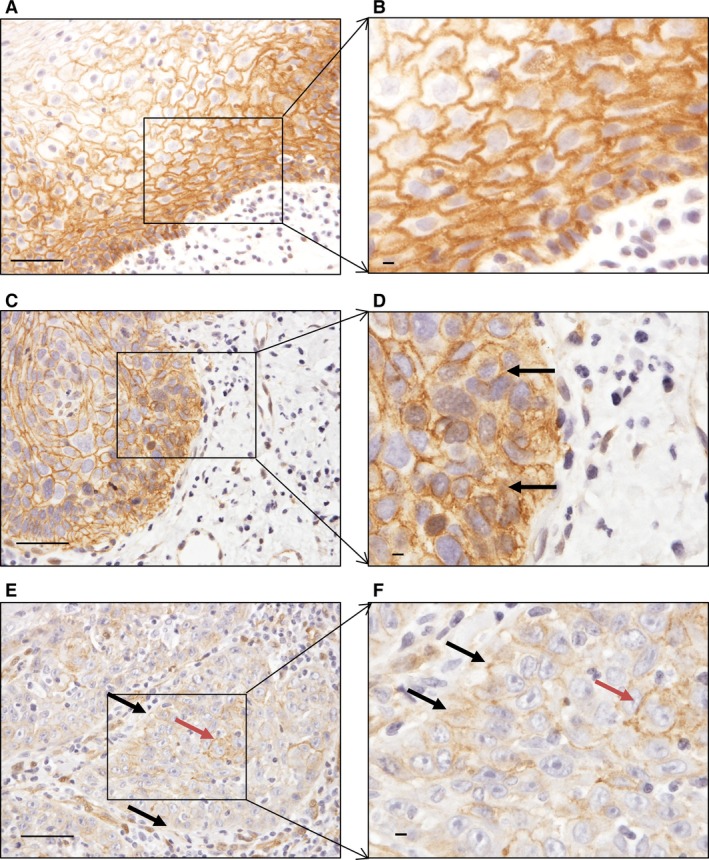
Expression of β‐catenin in different tissue regions. (A, B) Normal appearing oral mucosa showing membranous expression of β‐catenin. (C, D) Membranous and cytoplasmic expression of β‐catenin in severe‐grade dysplasia (black arrows). (E, F) Cancer islands: black arrows indicate expression of β‐catenin in the membrane in the periphery of cancer islands and red arrows indicate expression of β‐catenin in the central part of the cancer islands. Scale bar = 50 μm.
Expression of E‐cadherin protein in normal appearing oral mucosa, dysplasia, and OSCC
The expression of E‐cadherin protein in normal appearing oral mucosa, dysplasia, and OSCC is presented in Fig. 3. Based on positive immunostaining, expression of membranous E‐cadherin was present in all normal appearing oral mucosa and dysplastic tissues (Table 2), but in only 14 (66.7%) of OSCCs. Also, membranous E‐cadherin was not expressed in the poorly differentiated carcinomas. The difference in expression of E‐cadherin between OSCCs and the non‐cancerous regions was statistically significant: P < 0.001 for OSCCs compared with normal appearing oral mucosa, and P < 0.001 for OSCCs compared with dysplasia.
Figure 3.
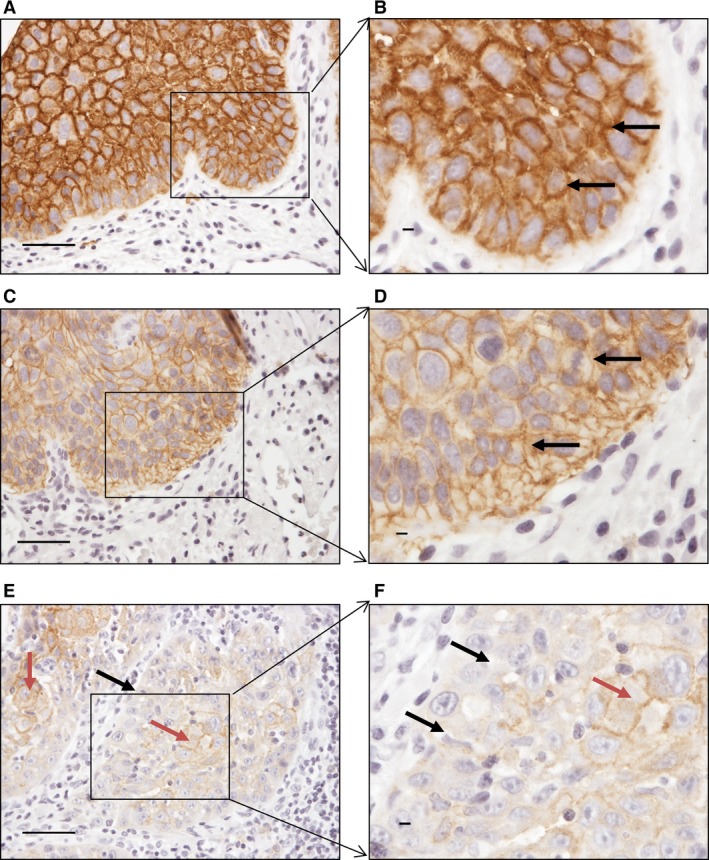
Expression of E‐cadherin in different tissue regions. (A, B) Normal appearing oral mucosa showing membranous expression of E‐cadherin. (C, D) Membranous expression of E‐cadherin in severe‐grade dysplasia. (E, F) Expression of E‐cadherin in cancer islands: black arrows indicate the absence of membranous expression of E‐cadherin in the periphery of cancer islands and red arrows indicate the presence of membranous expression of E‐cadherin in the central part of the cancer islands. Scale bar = 50 μm.
Association of WNT5A expression with β‐catenin and E‐cadherin in OSCC
Correlation was not observed between the expression of WNT5A and cytoplasmic β‐catenin (ρ = 0.026; P = 0.910), between WNT5A and membranous β‐catenin (ρ = −0.155; P = 0.504), or between WNT5A and E‐cadherin (ρ = −0.155; P = 0.502) in OSCC tissues (data not shown). Furthermore, stimulation of OSCC cells, SCC9 and SCC25, with rWNT5A for 48 h had no effect on the expression of E‐cadherin, active β‐catenin, or total β‐catenin proteins (Fig. 4).
Figure 4.
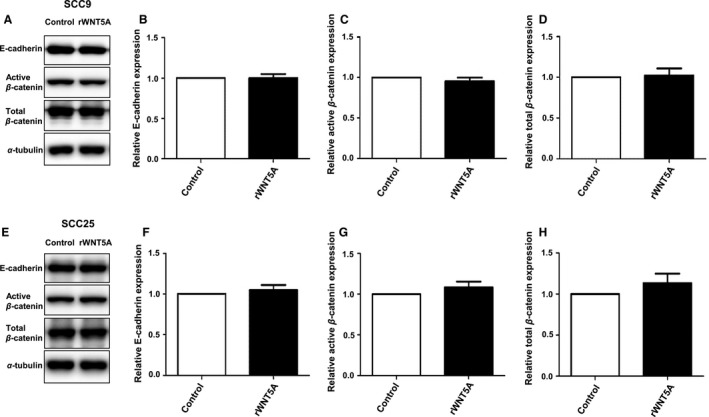
Effect of recombinant WNT5A (rWNT5A) on expression of β‐catenin and E‐cadherin in SCC9 and SCC25. (A, E) Representative western blots of expression of active β‐catenin, total β‐catenin, and E‐cadherin after stimulation with rWNT5A. (B, F) Quantification of relative E‐cadherin; (C, G) quantification of active β‐catenin; (D, H) quantification of total β‐catenin. All quantifications were performed on four separate experiments.
Discussion
The function of WNT5A has been extensively studied in different types of cancer 30, 31, 32, 33, and WNT5A was suggested to promote migration and invasion of OSCC cells 29. The expression of WNT5A protein in human OSCC tissues has, however, been less well studied 25, 26. Therefore, we aimed to analyse, by IHC, the expression of WNT5A in normal appearing oral mucosa adjacent to the affected tissue, dysplasia, and OSCC in human tissues and to examine the relationship between expression of WNT5A protein and that of β‐catenin and E‐cadherin.
The diagnostic incision biopsies used in this study were selected according to specific criteria: the presence of normal appearing oral mucosa adjacent to the affected tissue, dysplasia, and OSCC in the same tissue sample; no previous history of cancer; technically sufficient tissue material; and available TNM data of the primary tumor from Swedish Cancer Register. Only 21 out of the 207 diagnostic incision biopsies diagnosed as OSCC, qualified for use in this study. The OSCCs of this cohort were predominantly well differentiated and at the early stage of OSCC (T1 and T2), which provided a reliable basis for evaluation of how early in the process of the oral carcinogenesis WNT5A protein can be detected, as well as the importance of WNT5A in the progression of OSCC. The epithelium of the oral mucosa in the patient samples used in this study is actually an epithelium that might be genetically altered by the adjacent precancerous and cancerous epithelium and thus it cannot be considered as normal epithelium. We found no expression of WNT5A protein in the normal appearing mucosa, but we found a statistically significant difference between expression of WNT5A in OSCC and in normal appearing oral mucosa; between expression of WNT5A in OSCC and in dysplasia; and between expression of WNT5A in dysplasia and in normal appearing oral mucosa. It seems that expression of WNT5A gradually increased throughout the multistep process of carcinogenesis. Furthermore, we observed more WNT5A expression in the OSCC islands present in the muscle layer compared to OSCC islands in the lamina propria (data not shown). The high expression of WNT5A found in our study is supported by results of previous studies in which high levels of WNT5A mRNA and WNT5A protein have been reported in human oral tissue samples, as well as in rat tongue carcinoma 25, 26, 27. prgomet et al. showed that rWNT5A increased migration and invasion of the OSCC cell lines, SCC9 and SCC25 29. Because these cell lines are obtained from early‐stage OSCCs (SCC9 T1N1 and SCC25 T1N1M0) 34 and the present patient cohort comprised predominantly well‐differentiated and early‐stage OSCCs (T1 and T2), it seems reasonable to conclude that WNT5A has an early role in the invasiveness of OSCC. Regarding the possible mechanisms, the presence of WNT5A may up‐regulate other molecules, for example, matrix metalloproteinases 15, 33, 35 and Laminin γ2 30, which are involved in degradation of the basal lamina and metastatic spread of OSCC 36, 37. Thus, WNT5A may have potential as a biological marker for progression of dysplasia to OSCC and a cancer‐promoting role in OSCC. Further investigations of how WNT5A affects downstream molecules involved in metastatic spread of OSCC, as well as further analyses of WNT5A expression in larger patient cohorts, are required to confirm these speculations.
Our next step was based on the fact that WNT5A is expressed in OSCC and increases migration of OSCC cells. We chose to study the effect of WNT5A on expression of β‐catenin and E‐cadherin, in order to evaluate if the WNT5A‐induced migration involved alterations in cell‐adhesion proteins β‐catenin and E‐cadherin. In our study, expression of membranous β‐catenin was lower in OSCCs than in dysplasia or normal appearing oral mucosa, while expression of cytoplasmic β‐catenin increased with accumulative severity of dysplasia and was present in half of the OSCCs. Similar findings on decreased expression of membranous β‐catenin and progression of oral carcinogenesis have been reported 19, 38, 39. The slight decrease in expression of membranous β‐catenin and increase in expression of cytoplasmic β‐catenin that we observed in OSCCs was not a result of increased expression of WNT5A, because stimulation of OSCC cells with rWNT5A had no effect on β‐catenin expression. A possible explanation for this lack of effect on β‐catenin expression could be that WNT5A increases protein kinase C (PKC) in the same OSCC cell lines 29. Evidence that WNT5A increased migration of ovarian cancer cells 40 and melanoma cells 15 through PKC, without affecting expression of β‐catenin, supports our explanation. In our study, expression of nuclear β‐catenin was present in only one OSCC sample. However, ishida et al. found nuclear β‐catenin to be expressed in 67% of OSCC samples and they suggested that expression of nuclear β‐catenin is associated with cell invasion 41. The small number of samples (i.e. one) showing expression of nuclear β‐catenin in this study, along with the findings that WNT5A did not have any effect on β‐catenin expression, may indicate that β‐catenin is not involved in cell signaling but rather in cell adhesion in OSCC tissue. This has been previously reported by yu et al. 39.
E‐cadherin is involved in cell–cell adhesion and has been widely studied in OSCCs 23, 24, 42, 43, 44, although its association with WNT5A in OSCC is uncertain. We observed different levels of E‐cadherin expression in the three tissue regions we studied: less membranous E‐cadherin expression was seen in OSCC than in dysplasia or normal appearing oral mucosa. Other studies have reported even greater differences of membranous E‐cadherin expression at the invasive front of OSCCs, and the loss of membranous E‐cadherin expression was suggested to enhance the invasiveness of OSCC 43, 45. Although our work is in agreement with costa et al. 43, additional immunohistochemical experiments should be performed with a larger patient cohort to confirm this pattern. Furthermore, we evaluated the correlation of E‐cadherin and WNT5A expression based on previous studies reporting the effect of WNT5A on expression of E‐cadherin 14, 15, 16. We found no correlation between low membranous expression of E‐cadherin and the high expression of WNT5A. Moreover, expression of E‐cadherin in OSCC cell lines was not affected by stimulation of the cells with rWNT5A. These findings suggest that WNT5A might be involved in the invasiveness of OSCC without affecting the expression of E‐cadherin. A similar suggestion has been proposed by ren et al., who showed that WNT5A can enhance the migration and invasion of epidermoid carcinoma cells without down‐regulating E‐cadherin 46. Our findings, which are in accordance with jensen et al. 47, suggest that E‐cadherin is not always required for the migration and invasion of OSCC.
Based on our results, we conclude that WNT5A promotes progression of OSCC without affecting the canonical WNT/β‐catenin pathway or down‐regulation of E‐cadherin, and propose that WNT5A expression should be explored as an indicator of transformation of dysplasia to OSCC.
Conflicts of interest
T.A. is a shareholder in WntResearch and part‐time Chief Scientific Officer of WntResearch. This does not alter the author′s adherence to all policies on sharing data and materials as stated for the European Journal of Oral Sciences. Z.P. and P.L report no conflicts of interest.
Acknowledgements
This work was supported by the Swedish Cancer Foundation, the Swedish Research Council, Skåne University Hospital Research Foundations, Gunnar Nilsson′s Cancer Foundation (all to T.A.), and Malmö Allmänna Sjukhus Cancer Foundation (to Z.P. and T.A.).
Prgomet Z, Andersson T, Lindberg P. Higher expression of WNT5A protein in oral squamous cell carcinoma compared with dysplasia and oral mucosa with a normal appearance. Eur J Oral Sci 2017; 125: 237–246. © 2017 The Authors. Eur J Oral Sci published by John Wiley & Sons Ltd
References
- 1. Vig N, Mackenzie IC, Biddle A. Phenotypic plasticity and epithelial‐to‐mesenchymal transition in the behaviour and therapeutic response of oral squamous cell carcinoma. J Oral Pathol Med 2015; 44: 649–655. [DOI] [PubMed] [Google Scholar]
- 2. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol 2009; 45: 309–316. [DOI] [PubMed] [Google Scholar]
- 3. Xie L, Zhou X, Huang W, Chen J, Yu J, Li Z. Facial lymph node involvement as a prognostic factor for patient survival in oral cavity squamous cell carcinoma. Tumour Biol 2016; 37: 3489–3496. [DOI] [PubMed] [Google Scholar]
- 4. Binmadi NO, Basile JR. Perineural invasion in oral squamous cell carcinoma: a discussion of significance and review of the literature. Oral Oncol 2011; 47: 1005–1010. [DOI] [PubMed] [Google Scholar]
- 5. Ebrahimi A, Murali R, Gao K, Elliott MS, Clark JR. The prognostic and staging implications of bone invasion in oral squamous cell carcinoma. Cancer 2011; 117: 4460–4467. [DOI] [PubMed] [Google Scholar]
- 6. Takes RP, Rinaldo A, Silver CE, Haigentz M Jr, Woolgar JA, Triantafyllou A, Mondin V, Paccagnella D, de Bree R, Shaha AR, Hartl DM, Ferlito A. Distant metastases from head and neck squamous cell carcinoma. Part I. Basic aspects. Oral Oncol 2012; 48: 775–779. [DOI] [PubMed] [Google Scholar]
- 7. Feller LL, Khammissa RR, Kramer BB, Lemmer JJ. Oral squamous cell carcinoma in relation to field precancerisation: pathobiology. Cancer Cell Int 2013; 13: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scanlon CS, Van Tubergen EA, Inglehart RC, D'Silva NJ. Biomarkers of epithelial‐mesenchymal transition in squamous cell carcinoma. J Dent Res 2013; 92: 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer 2011; 11: 9–22. [DOI] [PubMed] [Google Scholar]
- 10. Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Wnt5a: its signalling, functions and implication in diseases. Acta Physiol (Oxf) 2012; 204: 17–33. [DOI] [PubMed] [Google Scholar]
- 11. Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta‐catenin‐TCF signaling depending on receptor context. PLoS Biol 2006; 4: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev 2010; 24: 2517–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J 2010; 29: 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bo H, Zhang S, Gao L, Chen Y, Zhang J, Chang X, Zhu M. Upregulation of Wnt5a promotes epithelial‐to‐mesenchymal transition and metastasis of pancreatic cancer cells. BMC Cancer 2013; 13: 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dissanayake SK, Wade M, Johnson CE, O'Connell MP, Leotlela PD, French AD, Shah KV, Hewitt KJ, Rosenthal DT, Indig FE, Jiang Y, Nickoloff BJ, Taub DD, Trent JM, Moon RT, Bittner M, Weeraratna AT. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem 2007; 282: 17259–17271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Medrek C, Landberg G, Andersson T, Leandersson K. Wnt‐5a‐CKI{alpha} signaling promotes {beta}‐catenin/E‐cadherin complex formation and intercellular adhesion in human breast epithelial cells. J Biol Chem 2009; 284: 10968–10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valenta T, Hausmann G, Basler K. The many faces and functions of beta‐catenin. EMBO J 2012; 31: 2714–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gonzalez‐Moles MA, Ruiz‐Avila I, Gil‐Montoya JA, Plaza‐Campillo J, Scully C. Beta‐Catenin in Oral Cancer: an Update on Current Knowledge. Oral Oncol 2014; 50: 818–824. [DOI] [PubMed] [Google Scholar]
- 19. Moles MA, Montoya JA, Salvago MD, Avila IR, Campillo JJ, Bravo M. Implications of Differential Expression of beta‐Catenin in Oral Carcinoma. Anticancer Res 2016; 36: 1599–1604. [PubMed] [Google Scholar]
- 20. Reyes M, Rojas‐Alcayaga G, Maturana A, Aitken JP, Rojas C, Ortega AV. Increased nuclear beta‐catenin expression in oral potentially malignant lesions: a marker of epithelial dysplasia. Med Oral Patol Oral Cir Bucal 2015; 20: e540–e546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gumbiner BM. Regulation of cadherin‐mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol 2005; 6: 622–634. [DOI] [PubMed] [Google Scholar]
- 22. Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res 2006; 66: 8319–8326. [DOI] [PubMed] [Google Scholar]
- 23. Fan CC, Wang TY, Cheng YA, Jiang SS, Cheng CW, Lee AY, Kao TY. Expression of E‐cadherin, Twist, and p53 and their prognostic value in patients with oral squamous cell carcinoma. J Cancer Res Clin Oncol 2013; 139: 1735–1744. [DOI] [PubMed] [Google Scholar]
- 24. Kaur J, Sawhney M, DattaGupta S, Shukla NK, Srivastava A, Walfish PG, Ralhan R. Clinical significance of altered expression of beta‐catenin and E‐cadherin in oral dysplasia and cancer: potential link with ALCAM expression. PLoS ONE 2013; 8: e67361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diaz Prado SM, Medina Villaamil V, Aparicio Gallego G, Blanco Calvo M, Lopez Cedrun JL, Sironvalle Soliva S, Valladares Ayerbes M, Garcia Campelo R, Anton Aparicio LM. Expression of Wnt gene family and frizzled receptors in head and neck squamous cell carcinomas. Virchows Arch 2009; 455: 67–75. [DOI] [PubMed] [Google Scholar]
- 26. Liu G, Sengupta PK, Jamal B, Yang HY, Bouchie MP, Lindner V, Varelas X, Kukuruzinska MA. N‐glycosylation induces the CTHRC1 protein and drives oral cancer cell migration. J Biol Chem 2013; 288: 20217–20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fracalossi AC, Silva Mde S, Oshima CT, Ribeiro DA. Wnt/beta‐catenin signalling pathway following rat tongue carcinogenesis induced by 4‐nitroquinoline 1‐oxide. Exp Mol Pathol 2010; 88: 176–183. [DOI] [PubMed] [Google Scholar]
- 28. Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 2009; 10: 445–457. [DOI] [PubMed] [Google Scholar]
- 29. Prgomet Z, Axelsson L, Lindberg P, Andersson T. Migration and invasion of oral squamous carcinoma cells is promoted by WNT5A, a regulator of cancer progression. J Oral Pathol Med 2014; 44: 776–784. [DOI] [PubMed] [Google Scholar]
- 30. Yamamoto H, Kitadai Y, Yamamoto H, Oue N, Ohdan H, Yasui W, Kikuchi A. Laminin gamma2 mediates Wnt5a‐induced invasion of gastric cancer cells. Gastroenterology 2009; 137: 242–252. [DOI] [PubMed] [Google Scholar]
- 31. Jenei V, Sherwood V, Howlin J, Linnskog R, Safholm A, Axelsson L, Andersson T. A t‐butyloxycarbonyl‐modified Wnt5a‐derived hexapeptide functions as a potent antagonist of Wnt5a‐dependent melanoma cell invasion. Proc Natl Acad Sci U S A 2009; 106: 19473–19478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kremenevskaja N, von Wasielewski R, Rao AS, Schofl C, Andersson T, Brabant G. Wnt‐5a has tumor suppressor activity in thyroid carcinoma. Oncogene 2005; 24: 2144–2154. [DOI] [PubMed] [Google Scholar]
- 33. Prasad CP, Chaurasiya SK, Axelsson L, Andersson T. WNT‐5A triggers Cdc42 activation leading to an ERK1/2 dependent decrease in MMP9 activity and invasive migration of breast cancer cells. Mol Oncol 2013; 7: 870–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao M, Sano D, Pickering CR, Jasser SA, Henderson YC, Clayman GL, Sturgis EM, Ow TJ, Lotan R, Carey TE, Sacks PG, Grandis JR, Sidransky D, Heldin NE, Myers JN. Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites. Clin Cancer Res 2011; 17: 7248–7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kamino M, Kishida M, Kibe T, Ikoma K, Iijima M, Hirano H, Tokudome M, Chen L, Koriyama C, Yamada K, Arita K, Kishida S. Wnt‐5a signaling is correlated with infiltrative activity in human glioma by inducing cellular migration and MMP‐2. Cancer Sci 2011; 102: 540–548. [DOI] [PubMed] [Google Scholar]
- 36. Lindberg P, Larsson A, Nielsen BS. Expression of plasminogen activator inhibitor‐1, urokinase receptor and laminin gamma‐2 chain is an early coordinated event in incipient oral squamous cell carcinoma. Int J Cancer 2006; 118: 2948–2956. [DOI] [PubMed] [Google Scholar]
- 37. Patel BP, Shah SV, Shukla SN, Shah PM, Patel PS. Clinical significance of MMP‐2 and MMP‐9 in patients with oral cancer. Head Neck 2007; 29: 564–572. [DOI] [PubMed] [Google Scholar]
- 38. Laxmidevi LB, Angadi PV, Pillai RK, Chandreshekar C. Aberrant beta‐catenin expression in the histologic differentiation of oral squamous cell carcinoma and verrucous carcinoma: an immunohistochemical study. J Oral Sci 2010; 52: 633–640. [DOI] [PubMed] [Google Scholar]
- 39. Yu Z, Weinberger PM, Provost E, Haffty BG, Sasaki C, Joe J, Camp RL, Rimm DL, Psyrri A. beta‐Catenin functions mainly as an adhesion molecule in patients with squamous cell cancer of the head and neck. Clin Cancer Res 2005; 11: 2471–2477. [DOI] [PubMed] [Google Scholar]
- 40. Qi H, Sun B, Zhao X, Du J, Gu Q, Liu Y, Cheng R, Dong X. Wnt5a promotes vasculogenic mimicry and epithelial‐mesenchymal transition via protein kinase Calpha in epithelial ovarian cancer. Oncol Rep 2014; 32: 771–779. [DOI] [PubMed] [Google Scholar]
- 41. Ishida K, Ito S, Wada N, Deguchi H, Hata T, Hosoda M, Nohno T. Nuclear localization of beta‐catenin involved in precancerous change in oral leukoplakia. Mol Cancer 2007; 6: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hashimoto T, Soeno Y, Maeda G, Taya Y, Aoba T, Nasu M, Kawashiri S, Imai K. Progression of oral squamous cell carcinoma accompanied with reduced E‐cadherin expression but not cadherin switch. PLoS ONE 2012; 7: e47899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Costa LC, Leite CF, Cardoso SV, Loyola AM, Faria PR, Souza PE, Horta MC. Expression of epithelial‐mesenchymal transition markers at the invasive front of oral squamous cell carcinoma. J Appl Oral Sci 2015; 23: 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Freitas Silva BS, Yamamoto‐Silva FP, Pontes HA, Pinto Junior Ddos S. E‐cadherin downregulation and Twist overexpression since early stages of oral carcinogenesis. J Oral Pathol Med 2014; 43: 125–131. [DOI] [PubMed] [Google Scholar]
- 45. Wang X, Zhang J, Fan M, Zhou Q, Deng H, Aisharif MJ, Chen X. The expression of E‐cadherin at the invasive tumor front of oral squamous cell carcinoma: immunohistochemical and RT‐PCR analysis with clinicopathological correlation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 107: 547–554. [DOI] [PubMed] [Google Scholar]
- 46. Ren D, Minami Y, Nishita M. Critical role of Wnt5a‐Ror2 signaling in motility and invasiveness of carcinoma cells following Snail‐mediated epithelial‐mesenchymal transition. Genes Cells 2011; 16: 304–315. [DOI] [PubMed] [Google Scholar]
- 47. Jensen DH, Reibel J, Mackenzie IC, Dabelsteen E. Single cell migration in oral squamous cell carcinoma ‐ possible evidence of epithelial‐mesenchymal transition in vivo. J Oral Pathol Med 2015; 44: 674–679. [DOI] [PubMed] [Google Scholar]


