Abstract
Rationale
Continuously refining and advancing the strategies and methods employed in sports drug testing is critical for efficient doping controls. Besides improving and expanding the spectrum of target analytes, alternative test matrices have warranted in‐depth evaluation as they commonly allow for minimal‐/non‐invasive and non‐intrusive sample collection. In this study, the potential of exhaled breath (EB) as doping control specimen was assessed.
Methods
EB collection devices employing a non‐woven electret‐based air filter unit were used to generate test specimens, simulating a potential future application in doping controls. A multi‐analyte sports drug testing approach configured for a subset of 12 model compounds that represent specific classes of substances prohibited in sports (anabolic agents, hormone and metabolic modulators, stimulants, and beta‐blockers) was established using unispray liquid chromatography/tandem mass spectrometry (LC/MS/MS) and applied to spiked and elimination study EB samples. The test method was characterized concerning specificity, assay imprecision, and limits of detection.
Results
The EB collection device allowed for retaining and extracting all selected model compounds from the EB aerosol. Following elution and concentration, LC/MS/MS analysis enabled detection limits between 5 and 100 pg/filter and imprecisions ranging from 3% to 20% for the 12 selected model compounds. By means of EB samples from patients and participants of administration studies, the elimination of relevant compounds and, thus, their traceability in EB for doping control purposes, was investigated. Besides stimulants such as methylhexaneamine and pseudoephedrine, also the anabolic‐androgenic steroid dehydrochloromethyltestosterone, the metabolic modulator meldonium, and the beta‐blocker bisoprolol was detected in exhaled breath.
Conclusions
The EB aerosol has provided a promising proof‐of‐concept suggesting the expansion of this testing strategy as a complement to currently utilized sports drug testing programs.
1. INTRODUCTION
Routine sports drug testing has become a challenging mission in the face of the ever‐growing exigencies resulting from the breadth of relevant target analytes, drug detection windows, financial implications, etc.,1, 2 but also from the desire to reduce the intrusiveness and invasiveness of the sample collection procedure for the athlete.3 Consequently, assessing the utility of test matrices representing alternatives to commonly sampled blood and urine specimens such as dried blood spots (DBS),4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 hair,17, 18, 19 and oral fluid (OF)20, 21, 22 has been the subject of a number of studies related to doping controls. In addition, exhaled breath (EB) has received increasing attention as a potential test matrix particularly for clinical and forensic drug testing applications.23, 24, 25, 26, 27, 28, 29, 30, 31 Initially, EB was considered exclusively for the analysis of volatile compounds; however, a considerable number of non‐volatile solutes have been reported as being present in and being eliminated into EB,32 nourishing the concept of EB as a viable complement to routine doping controls. Based on the identification of these non‐volatile analytes (predominantly contained in the alveolar lining fluid and surfactant) in EB,33, 34 the formation of the breath aerosol was suggested to include bronchiolar and alveolar components.35, 36 Lung surfactant and surface lining fluid are separated from the blood stream by several barriers including the alveolar epithelium, the basement membranes of the alveolar epithelium and the capillary endothelium, and the capillary endothelium; nevertheless, plasma proteins are found in lavage fluid (transported by yet not fully clarified mechanisms exhibiting an inverse relationship between alveolar permeability and molecular mass), and pulmonary drug delivery has become routine for numerous therapeutics.37, 38 In consideration of these aspects and the proven traceability of drugs of abuse and selected therapeutics in EB,23, 26, 27, 28, 30, 39, 40 it was of interest whether EB can also be used as an alternative matrix for doping controls. As standardized protocols and easy‐to‐use sample collection devices with comprehensive analyte adsorption properties are desirable for routine sports drug testing applications, a commercially available EB sampling model equipped with an electret membrane was chosen for the present study. Following ventilation of the device and extraction of the contained membrane, conventional liquid chromatographic/tandem mass spectrometric detection (LC/MS/MS) was conducted and assay characteristics (limits of detection, analyte recovery, assay imprecision) were determined using a subset of model substances relevant for doping controls.
2. EXPERIMENTAL
2.1. Chemicals and consumables
Breath sampling units were obtained from SensAbues (Huddinge, Sweden). Methanol, acetonitrile, formic acid, and ammonium acetate were of analytical grade and purchased from Merck (Darmstadt, Germany). The model substances were provided by different suppliers: bisoprolol dehydrochloromethyltestosterone (DHCMT), meldonium, metoprolol, methylhexaneamine, and pseudoephedrine were from LGC Standards GmbH (Wesel, Germany). Acebutolol, (S)‐2‐aminooctane, anastrozole, letrozole, methylphenidate, stanozolol, D3‐testosterone, and D7‐propranolol were from Sigma (Schnelldorf, Germany). D3‐Meldonium was from Toronto Research Chemicals (Toronto, Canada), and YK‐11 was synthesized in‐house as described elsewhere.
2.2. Liquid chromatography/tandem mass spectrometry (LC/MS/MS)
All analyses were conducted using a Aquity I‐Class ultra‐performance liquid chromatography (UPLC) system (Waters, Eschborn, Germany) interfaced via unispray (US) ionization to a Xevo TQ‐XS tandem mass spectrometer (Waters). The LC system was equipped with a Poroshell C‐8 analytical column (50 × 3.0 mm, 2.7 μm particle size; Agilent, Waldbronn, Germany). The LC method employed 10 mM aqueous ammonium acetate (solvent A) and acetonitrile (solvent B) and gradient elution starting with 95% A, decreasing to 0% A in 10 min, maintaining 0% A for 2 min before re‐equilibration at 95% A for 2.5 min. The US source was operated in positive mode using an impactor voltage of 1 kV and the mass spectrometer recorded two diagnostic precursor/product ion pairs per analyte in multiple reaction monitoring (MRM) mode. The collision gas was nitrogen (provided by a nitrogen generator, CMC, Eschborn, Germany), and collision energies were optimized for each ion transition as summarized in Table 1.
Table 1.
Target analyte and test method characteristics
| Assay imprecision [%] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Analyte | Substance class | Precursor/product ion pairs | CE [eV] | LOD* [pg/filter] | ID capability** [pg/filter] | Recovery [%] | n = 18 100 pg/500 pg/1000 pg | Confirmed in authentic EB sample | |
| 1 | Dehydrochloromethyltestosterone | S1 | 335 → 155/149 | 34/16 | 30 | 300 | 104 | 19/8/8 | ✓ |
| 2 | YK‐11 | S1 | 357 → 325/307 | 12/14 | 50 | 100 | 84 | 19/11/10 | not tested |
| 3 | Stanozolol | S1 | 329 → 95/81 | 42/42 | 60 | 90 | 70 | 17/12/16 | not tested |
| 4 | Anastrozole | S4 | 294 → 225/142 | 20/ 40 | 5 | 5 | 59 | 5/7/5 | not tested |
| 5 | Letrozole | S4 | 286 → 217/190 | 15/32 | 100 | 200 | 76 | 16/18/7 | not tested |
| 6 | Meldonium | S4 | 147 → 59/58 | 12/12 | 5 | 5 | 93 | 5/3/4 | ✓ |
| 7 | Methylhexaneamine | S6 | 116 → 57/43 | 8/16 | 70 | 1000 | 64 | 13/8/13 | ✓ |
| 8 | Pseudoephedrine | S6 | 166 → 133/117 | 20/20 | 60 | 100 | 86 | 16/20/13 | ✓ |
| 9 | Methylphenidate | S6 | 234 → 174/84 | 10/18 | 5 | 10 | 90 | 8/10/12 | ✓a |
| 10 | Acebutolol | P2 | 337 → 116/98 | 25/19 | 5 | 5 | 66 | 11/6/6 | not tested |
| 11 | Bisoprolol | P2 | 326 → 116/74 | 16/22 | 5 | 10 | 54 | 10/5/4 | (✓)b |
| 12 | Metoprolol | P2 | 268 → 116/74 | 18/20 | 70 | 120 | 71 | 16/10/6 | not tested |
| ISTD 1 | (S)‐2‐Aminooctane | 130 → 71 | 12 | ||||||
| ISTD 2 | D3‐Meldonium | 150 → 61 | 12 | ||||||
| ISTD 3 | D7‐Propranolol | 267 → 72 | 20 | ||||||
| ISTD 4 | D3‐Testosterone | 292 → 109 | 21 | ||||||
See Beck et al.26
Only one precursor/product ion pair found at a S/N > 3.
one precursor/product ion pair per analyte
two precursor/product ion pairs per analyte meeting World Anti‐Doping Agency (WADA) criteria for the identification of non‐threshold substances
2.3. Stock and working solutions
Stock solutions of all model compounds were prepared at 1 mg/mL in methanol and stored at –20°C. Working solutions (10 μg/mL and 0.1 μg/mL) were prepared by dilution of the stock solution on the day of use and discarded after 24 h. A mixture of internal standards (ISTDs) containing (S)‐2‐aminooctane, D3‐meldonium, D3‐testosterone, and D7‐propranolol was prepared in methanol at a concentration of 10 μg/mL, which was diluted to a working solution of 0.1 μg/mL for daily batch processing purposes.
2.4. Sample preparation
The SensAbues EB collection devices were extracted by adding a total of 4 mL of methanol (plus 1 ng each of aminooctane, D3‐meldonium, D3‐testosterone, and D7‐propranolol as ISTDs) to the filter membrane containing cartridge, gentle agitation for 5 min, and subsequent elution of the solvent into a glass test tube. Therefore, the outlet of the cartridge was placed into the ground orifice of the test tube and centrifuged at 660 g for 2 min. The obtained extract was evaporated to dryness in a stream of nitrogen (obtained from a N2 generator), the residue was dissolved in 100 μL of a mixture of methanol and water (1:1, v/v), and 10 μL were injected into the LC/MS/MS system.
2.5. Blank and post‐administration EB samples
EB specimens were collected according to the manufacturer's guidelines, i.e. volunteers were asked to breathe through a mouthpiece into the cartridge until an approximate volume of 20 L had passed the electrostatic filter as indicated by an attached plastic bag. The mouthpiece and bag were subsequently removed from the collection device, which was then closed by stoppers and stored at room temperature until analysis.
Blank EB samples were collected from 20 employees and students of the German Sport University Cologne (10 males and 10 females, age 24–61 years). The volunteers provided written consent and declared no use of drugs for at least 7 days prior to providing the blank specimen. Post‐administration EB samples were obtained either from patients using the drugs in question in regular therapeutic settings or from individuals participating in elimination studies investigating the same substance classified as doping agent. Also here, written consent was obtained from all participants, and ethical approval for the collection and analysis of EB samples was obtained from the local ethics committee of the German Sport University Cologne (#170/2016).
2.6. Method characterization
The characteristics of the test method, including limits of detection (LODs), analyte recovery, assay imprecision, and specificity, were determined by different experiments. Specificity was assessed by analyzing 20 blank samples from different individuals (10 male and 10 female volunteers) and probing for interfering signals at diagnostic precursor/product ion pairs at expected analyte retention times. LODs were deduced from the same set of blank specimens calculating the average noise for each target analyte at the expected retention time using one diagnostic precursor/product ion pair. The signal obtained from spiked EB samples (prepared at 10, 100, 500, and 1000 pg/filter) had to exceed the average noise plus a threefold standard deviation by at least a factor of 3 (signal‐to‐noise ratio (S/N) >3). Accordingly, the identification capability was computed, necessitating two diagnostic precursor/product ion pairs meeting the S/N > 3 criterion. Analyte recoveries were measured by spiking 500 pg of each model substance either onto the blank breath ventilated filter unit prior to extraction or by adding 500 pg of the analytes into the generated methanolic solution obtained from extracting the filter. The comparison of peak areas obtained from six replicates provided the information required to calculate the analyte recovery. The method's imprecision was computed from repeated preparation and analysis (n = 18) of spiked blank EB samples fortified at three different concentration levels (low: 100 pg/filter, medium: 500 pg/filter, high: 1000 pg/filter).
3. RESULTS AND DISCUSSION
Collecting analytes from EB has been accomplished by various different strategies in the past including cooling traps (yielding exhaled breath condensate, EBC),32, 36 breathing through tubing with silica‐coated glass fiber discs,23 and capturing aerosol particles by means of electrostatic filtration media.28, 29, 30 In consideration of the modus operandi of electrostatic filtration media, i.e. the interaction and retention of charged aerosol particles on the electret fiber material supported by Coulombic and dielectrophoretic forces, and the breadth of target analytes relevant for doping controls, commercially available standardized EB collection devices equipped with electrostatic filter units were employed in the present study. This pilot study aimed at assessing whether EB sampling can contribute to modern sports drug testing programs and included the development of a test method focusing on four classes of substances prohibited in sports,41 i.e. anabolic agents (substance class S1), hormone and metabolic modulators (S4), stimulants (S6), and beta‐blockers (P2), and the analysis of proof‐of‐concept samples indicating the practicality or impracticality of EB as alternative matrix in doping controls.
3.1. Method characterization
Method development and subsequent characterization was conducted by means of breath‐ventilated filter media of EB collection devices, which were spiked with 12 model substances (Table 1) at levels commonly targeted and found in EB analysis.29, 31 For all analytes, two diagnostic precursor/product ion pairs were selected and corresponding collision energies were optimized for maximum signal abundance (Table 1). The ion transitions conventionally consisted of the protonated molecules and respective characteristic product ions in all cases except for the steroidal selective androgen receptor modulator (SARM) drug candidate YK‐11 (analyte 2, Table 1). For YK‐11, the ion at m/z 357 (i.e. [M + H]+ – acetic acid methyl ester) was selected as the precursor ion owing to the fragile nature of the protonated molecule.42, 43 All chosen ion transitions proved specific as no interfering signals were observed in extracted ion chromatograms of 20 blank EB samples, one of which is illustrated in Figure 1A. In contrast, specimens spiked with 500 pg of each model substance resulted in unequivocal signals using the established LC/MS/MS method (Figure 1B). As comprehensiveness of the analytical approach is particularly desirable, only one sample preparation protocol was chosen and parameters potentially influencing analyte recovery were not optimized for the different classes of compounds. However, the observed recoveries (54–104%) and corresponding LODs of the tested analytes (5–100 pg/filter) as well as assay imprecision data (3–20%) were found appropriate for the intended purpose of allowing for an initial testing procedure indicating the presence or absence of a doping agent in EB as summarized in Table 1.
Figure 1.
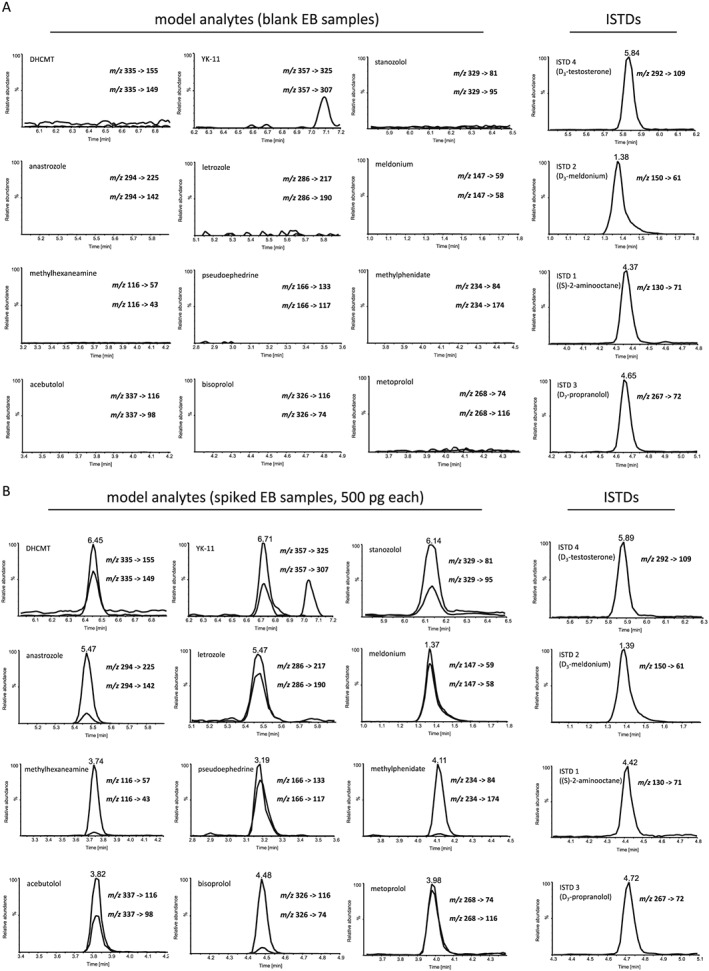
Exhaled breath (EB) samples tested by reversed‐phase liquid chromatography/tandem mass spectrometry for 12 model compounds including anabolic agents, hormone and metabolic modulators, stimulants, and beta‐blockers: (A) blank EB specimen containing only the internal standards D3‐testosterone, D3‐meldonium, (S)‐2‐aminooctane, and D7‐propranolol. Y‐axes are normalized to the abundance of the corresponding spiked specimen shown under (B), which illustrates the results of an EB sample fortified with 500 pg of each target analyte plus ISTDs
3.2. Elimination study samples – proof‐of‐concept
One of the main questions to be answered in the context of doping controls has been whether relevant drugs (or diagnostic metabolites) are present in EB at concentrations detectable with commonly available chromatographic/mass spectrometric instruments. Hence, this feasibility study included the analysis of spot EB samples collected from patients using therapeutics in question or from volunteers participating in an elimination study with a compound classified as doping agent. Figure 2 displays five chromatograms of post‐administration EB specimens, demonstrating that in principle the application of the drugs can be readily monitored. Representative analytes from all four studied classes were measured from EB samples collected between 2.5 and 15 h after the drugs had been ingested. Here, abundant signals were obtained for most of the target analytes whilst one drug yielded only a moderately intense peak indicating the presence of the administered compound: The anabolic agent dehydrochloromethyltestosterone (DHCMT) was detected 4 h post‐administration following the oral administration of 20 mg of the drug, the EB sample containing meldonium was collected 15 h post‐administration of 500 mg, methylhexaneamine was detected in a specimen sampled 8 h post‐administration of 40 mg, and pseudoephedrine was determined in an EB sample collected 5 h post‐administration of 30 mg of the drug. For all of these compounds, peaks in extracted ion chromatograms of two diagnostic precursor/product ion pairs each were obtained at the analytes’ retention times. Conversely, the analysis of bisoprolol in EB (using a sample collected 3 h following the oral application of 3.75 mg of the drug) allowed only for the detection of a signal in the extracted ion chromatogram of one diagnostic precursor/product ion pair (Figure 2E).
Figure 2.
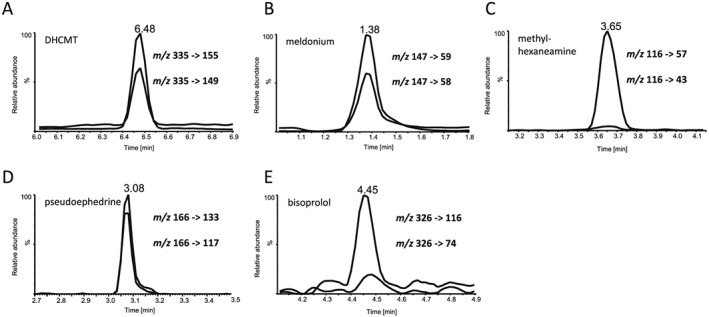
Chromatograms of exhaled breath samples collected from patients and participants of elimination studies with (A) dehydrochloromethyltestosterone (DHCMT, collected 4 h post‐administration of 20 mg), (B) meldonium (collected 15 h post‐administration of 500 mg), (C) methylhexaneamine (collected 8 h post‐administration of 40 mg), (D) pseudoephedrine (collected 5 h post‐administration of 30 mg), and (E) bisoprolol (collected 3 h post‐administration of 3.75 mg)
Various factors affecting the traceability of drugs in exhaled breath are conceivable, most of which require further studies for clarification. These factors include, amongst others, the (limited) active or passive transport of the prohibited substances into the EB matrix and potential interindividual differences; metabolic reactions (in alveolar tissue and/or fluid); the ability of the tested individual (patient/athlete) to properly use the testing device; the capability of the employed filter membrane of the sampling device to capture and immobilize the relevant analytes; the stability of drugs or its metabolites in an EB sample; etc. Whilst these parameters necessitate comprehensive follow‐up studies, the results shown in this pilot study demonstrate that diverse drugs classified as doping agents can be monitored in EB. As lowest amounts of the target analytes were expected and found, utmost analytical sensitivity and specificity are mandatory to allow for the utilization of EB in future doping control programs.
4. CONCLUSIONS
The results of this pilot study and literature data from the field of therapeutic drug monitoring and forensic analyses23, 24, 25, 26, 27, 28, 29, 30, 31, 40 indicate that drugs of rather different physico‐chemical nature are traceable in EB. Consequently, a considerable number of compounds relevant for doping controls could be monitored via EB sampling in potential future sports drug testing programs that could utilize the non‐invasive sample collection approach of exhaled breath. To date, information on analyte concentrations in EB, detection times, etc., are still limited to a small number of relevant compounds. Hence, follow‐up studies are required to assess the full potential of EB as an alternative matrix in doping controls, and a considerable expansion of the spectrum of compounds that can be monitored from EB is needed. This necessitates utmost analytical sensitivity considering the minute amounts of target analytes expected to be eliminated via an athletes’ breath.
ACKNOWLEDGEMENTS
This project was supported by funding from the Partnership for Clean Competition Research Collaborative. The content of this publication does not necessarily reflect the views or policies of the Research Collaborative. Further, the authors wish to acknowledge support from the Federal Ministry of the Interior of the Federal Republic of Germany (Bonn, Germany) and the constructive collaboration with SensAbues AB (Sollentuna, Sweden).
Thevis M, Krug O, Geyer H, Schänzer W. Expanding analytical options in sports drug testing: Mass spectrometric detection of prohibited substances in exhaled breath. Rapid Commun Mass Spectrom. 2017;31:1290‐1296. https://doi.org/10.1002/rcm.7903
REFERENCES
- 1. Thevis M, Kuuranne T, Geyer H, Schänzer W. Annual banned‐substance review: analytical approaches in human sports drug testing. Drug Test Anal. 2015;7:1‐20. [DOI] [PubMed] [Google Scholar]
- 2. Thevis M, Kuuranne T, Walpurgis K, Geyer H, Schänzer W. Annual banned‐substance review: analytical approaches in human sports drug testing. Drug Test Anal. 2016;8:7‐29. [DOI] [PubMed] [Google Scholar]
- 3. Thevis M, Geyer H, Tretzel L, Schänzer W. Sports drug testing using complementary matrices: Advantages and limitations. J Pharm Biomed Anal. 2016;130:220‐230. [DOI] [PubMed] [Google Scholar]
- 4. Henion J, Oliveira RV, Chace DH. Microsample analyses via DBS: challenges and opportunities. Bioanalysis. 2013;5:2547‐2565. [DOI] [PubMed] [Google Scholar]
- 5. Thomas A, Geyer H, Guddat S, Schänzer W, Thevis M. Dried blood spots (DBS) for doping control analysis. Drug Test Anal. 2011;3:806‐813. [DOI] [PubMed] [Google Scholar]
- 6. Cox HD, Rampton J, Eichner D. Quantification of insulin‐like growth factor‐1 in dried blood spots for detection of growth hormone abuse in sport. Anal Bioanal Chem. 2013;405:1949‐1958. [DOI] [PubMed] [Google Scholar]
- 7. Höppner S, Delahaut P, Schänzer W, Thevis M. Mass spectrometric studies on the in vivo metabolism and excretion of SIRT1 activating drugs in rat urine, dried blood spots, and plasma samples for doping control purposes. J Pharm Biomed Anal. 2014;88:649‐659. [DOI] [PubMed] [Google Scholar]
- 8. Kojima A, Nishitani Y, Sato M, Kageyama S, Dohi M, Okano M. Comparison of urine analysis and dried blood spot analysis for the detection of ephedrine and methylephedrine in doping control. Drug Test Anal. 2016;8:189‐198. [DOI] [PubMed] [Google Scholar]
- 9. Möller I, Thomas A, Geyer H, Schänzer W, Thevis M. Development and validation of a mass spectrometric detection method of peginesatide in dried blood spots for sports drug testing. Anal Bioanal Chem. 2012;403:2715‐2724. [DOI] [PubMed] [Google Scholar]
- 10. Sadones N, Capiau S, De Kesel PM, Lambert WE, Stove CP. Spot them in the spot: analysis of abused substances using dried blood spots. Bioanalysis. 2014;6:2211‐2227. [DOI] [PubMed] [Google Scholar]
- 11. Thomas A, Geyer H, Schänzer W, et al. Sensitive determination of prohibited drugs in dried blood spots (DBS) for doping controls by means of a benchtop quadrupole/Orbitrap mass spectrometer. Anal Bioanal Chem. 2012;403:1279‐1289. [DOI] [PubMed] [Google Scholar]
- 12. Tretzel L, Görgens C, Geyer H, et al. Analyses of Meldonium (Mildronate) from blood, dried blood spots (DBS), and urine suggest drug incorporation into erythrocytes. Int J Sports Med. 2016;37:500‐502. [DOI] [PubMed] [Google Scholar]
- 13. Tretzel L, Thomas A, Geyer H, Delahaut P, Schänzer W, Thevis M. Determination of Synacthen((R)) in dried blood spots for doping control analysis using liquid chromatography tandem mass spectrometry. Anal Bioanal Chem. 2015;407:4709‐4720. [DOI] [PubMed] [Google Scholar]
- 14. Tretzel L, Thomas A, Geyer H, et al. Use of dried blood spots in doping control analysis of anabolic steroid esters. J Pharm Biomed Anal. 2014;96:21‐30. [DOI] [PubMed] [Google Scholar]
- 15. Tretzel L, Thomas A, Geyer H, Pop V, Schänzer W, Thevis M. Dried blood spots (DBS) in doping controls: a complementary matrix for improved in‐ and out‐of‐competition sports drug testing strategies. Anal Methods. 2015;7:7596‐7605. [Google Scholar]
- 16. Verplaetse R, Henion J. Quantitative determination of opioids in whole blood using fully automated dried blood spot desorption coupled to on‐line SPE‐LC‐MS/MS. Drug Test Anal. 2016;8:30‐38. [DOI] [PubMed] [Google Scholar]
- 17. Segura J. Is anti‐doping analysis so far from clinical, legal or forensic targets?: The added value of close relationships between related disciplines. Drug Test Anal. 2009;1:479‐484. [DOI] [PubMed] [Google Scholar]
- 18. Thieme D. Potential and limitations of alternative specimens in doping control. Bioanalysis. 2012;4:1613‐1622. [DOI] [PubMed] [Google Scholar]
- 19. Thieme D, Grosse J, Sachs H, Mueller RK. Analytical strategy for detecting doping agents in hair. Forensic Sci Int. 2000;107:335. [DOI] [PubMed] [Google Scholar]
- 20. Anizan S, Huestis MA. The potential role of oral fluid in antidoping testing. Clin Chem. 2014;60:307‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schönfelder M, Hofmann H, Schulz T, et al. Potential detection of low‐dose transdermal testosterone administration in blood, urine and saliva. Drug Test Anal. 2016;8:1186‐1196. [DOI] [PubMed] [Google Scholar]
- 22. Thieme D, Rautenberg C, Grosse J, Schoenfelder M. Significant increase of salivary testosterone levels after single therapeutic transdermal administration of testosterone: suitability as a potential screening parameter in doping control. Drug Test Anal. 2013;5:819‐825. [DOI] [PubMed] [Google Scholar]
- 23. Beck O, Leine K, Palmskog G, Franck J. Amphetamines detected in exhaled breath from drug addicts: A new possible method for drugs‐of‐abuse testing. J Anal Toxicol. 2010;34:233‐237. [DOI] [PubMed] [Google Scholar]
- 24. Beck O, Sandqvist S, Dubbelboer I, Franck J. Detection of Δ9‐tetrahydrocannabinol in exhaled breath collected from cannabis users. J Anal Toxicol. 2011;35:541‐544. [DOI] [PubMed] [Google Scholar]
- 25. Beck O, Stephanson N, Sandqvist S, Franck J. Detection of drugs of abuse in exhaled breath using a device for rapid collection: comparison with plasma, urine and self‐reporting in 47 drug users. J Breath Res. 2013;7:026006. [DOI] [PubMed] [Google Scholar]
- 26. Beck O, Stephanson N, Sandqvist S, Franck J. Determination of amphetamine and methylphenidate in exhaled breath of patients undergoing attention‐deficit/hyperactivity disorder treatment. Ther Drug Monit. 2014;36:528‐534. [DOI] [PubMed] [Google Scholar]
- 27. Ellefsen KN, Concheiro M, Beck O, Gorelick DA, Pirard S, Huestis MA. Quantification of cocaine and metabolites in exhaled breath by liquid chromatography‐high‐resolution mass spectrometry following controlled administration of intravenous cocaine. Anal Bioanal Chem. 2014;406:6213‐6223. [DOI] [PubMed] [Google Scholar]
- 28. Himes SK, Scheidweiler KB, Beck O, Gorelick DA, Desrosiers NA, Huestis MA. Cannabinoids in exhaled breath following controlled administration of smoked cannabis. Clin Chem. 2013;59:1780‐1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stephanson N, Sandqvist S, Lambert MS, Beck O. Method validation and application of a liquid chromatography‐tandem mass spectrometry method for drugs of abuse testing in exhaled breath. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;985:189‐196. [DOI] [PubMed] [Google Scholar]
- 30. Meyer MR, Rosenborg S, Stenberg M, Beck O. First report on the pharmacokinetics of tramadol and O‐desmethyltramadol in exhaled breath compared to plasma and oral fluid after a single oral dose. Biochem Pharmacol. 2015;98:502‐510. [DOI] [PubMed] [Google Scholar]
- 31. Beck O, Olin AC, Mirgorodskaya E. Potential of mass spectrometry in developing clinical laboratory biomarkers of nonvolatiles in exhaled breath. Clin Chem. 2016;62:84. [DOI] [PubMed] [Google Scholar]
- 32. Popov TA. Human exhaled breath analysis. Ann Allergy Asthma Immunol. 2011;106:451‐456. [DOI] [PubMed] [Google Scholar]
- 33. Larsson P, Mirgorodskaya E, Samuelsson L, et al. Surfactant protein A and albumin in particles in exhaled air. Respir Med. 2012;106:197‐204. [DOI] [PubMed] [Google Scholar]
- 34. Moliva JI, Rajaram MV, Sidiki S, et al. Molecular composition of the alveolar lining fluid in the aging lung. Age (Dordr). 2014;36:1187‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson GR, Morawska L. The mechanism of breath aerosol formation. J Aerosol Med Pulm Drug Deliv. 2009;22:229‐237. [DOI] [PubMed] [Google Scholar]
- 36. Horvath I, Hunt J, Barnes PJ, et al. Condensate. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26:523‐548. [DOI] [PubMed] [Google Scholar]
- 37. Kim KJ, Malik AB. Protein transport across the lung epithelial barrier. Am J Physiol Lung Cell Mol Physiol. 2003;284:L247‐259. [DOI] [PubMed] [Google Scholar]
- 38. Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56:588‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carlsson S, Olsson R, Lindkvist I, Beck O. Application of drug testing using exhaled breath for compliance monitoring of drug addicts in treatment. Scand J Clin Lab Invest. 2015;75:156‐161. [DOI] [PubMed] [Google Scholar]
- 40. Kintz P, Mathiaux F, Villeger P, Gaulier JM. Testing for drugs in exhaled breath collected with ExaBreath in a drug dependence population: Comparison with data obtained in urine after liquid chromatographic‐tandem mass spectrometric analyses. Ther Drug Monit. 2016;38:135‐139. [DOI] [PubMed] [Google Scholar]
- 41. The 2017 Prohibited List, World Anti‐Doping Agency, 2017 Available: https://wada‐main‐prod.s3.amazonaws.com/resources/files/2016‐09‐29_‐_wada_prohibited_list_2017_eng_final.pdf. Accessed October 31, 2016
- 42. Thevis M, Piper T, Dib J, et al. Mass spectrometric characterization of the selective androgen receptor modulator (SARM) YK‐11 for doping control purposes. Rapid Commun Mass Spectrom. 2017;31:1175‐1183. [DOI] [PubMed] [Google Scholar]
- 43. Thevis M, Schänzer W. Detection of SARMs in doping control analysis. Mol Cell Endocrinol. 2017, in press. https://doi.org/10.1016/j.mce.2017.01.040. [DOI] [PubMed] [Google Scholar]


