Abstract
Aim
This retrospective study aimed to characterize the baseline status of patients following periodontal maintenance, analysing the association between the long‐term outcome of these patients, smoking, bruxism, and the main clinical and radiographic variables.
Material and methods
A sample of 174 patients with moderate to severe periodontitis was refined into homogeneous subsamples according to smoking and bruxism and the rate of tooth loss due to periodontal disease (TLPD): 0, 1–2, and >2 teeth. The association and the distribution (χ² test) of the variables within the subsamples were analysed.
Results
Smoking and bruxism were significantly associated with higher TLPD rates. Vertical and circumferential bone defects (p < .0001), and abfractions (p < .0001) were associated with bruxism and particularly with bruxism and TLPD >2.
Furcation defects (p = .0002), fewer radio‐opaque subgingival calculus (χ² p < .0001), a lower mean Gingival index (χ² p = .027), and increased mean recessions >1.5 mm (χ² p = .0026) were associated with smoking and higher TLPD rates. The mean baseline mobility, abfractions, and recessions characterized two basic types of TLPD.
Conclusions
Smoking, bruxism, and routine clinical and radiological parameters can be used to characterize the baseline status of patients with worse outcomes.
Keywords: abfractions, bruxism, periodontal disease, periodontal prognosis, tooth loss
1. INTRODUCTION
The identification of patients at risk of presenting a worse outcome and experiencing higher rates of tooth loss due to periodontal disease (TLPD) is a major research goal in periodontal prognosis. There are two distinct approaches to addressing the issue: characterizing the baseline status of patients with higher rates of TLPD during periodontal maintenance (PM), and assessing risk of disease progression and the resulting TLPD.
The study by Martinez‐Canut (2015) analysed a subsample of 85 patients with higher TLPD rates and reported that these patients were characterized by severe periodontitis (OR 3.8–7.1), smoking combined with bruxism (OR 3.8), fewer baseline teeth, and a younger age.
Several risk assessment tools in the second approach have been developed based on well‐documented risk factors and have been assessed in longitudinal studies (Lang & Tonetti, 2003; Lindskog et al., 2010; Page et al., 2002; Page, Krall, Martin, Mancl, & Garcia, 2002) and their ability to identify patients with different levels of risk has been established (Lang, Suvan, & Tonetti, 2015).
However, due to incomplete knowledge on the subject, several risk factors have been analysed to predict the outcomes of patients following PM without considering possible differences between risk and prognostic factors (Martinez‐Canut, 2015) and utilizing both terms interchangeably (Fardal, Grytten, Martin, Houlihan, & Heasman, 2016).
Baseline subgingival calculus and gingival inflammation are well‐known risk factors whose role as prognostic factors has yet to be elucidated. The pioneer study characterizing patients under PM found that better outcomes were correlated with increased gingival inflammation while subgingival calculus did not correlate with a worse outcome (Wasserman & Hirschfeld, 1988).
The intra‐bony component of a vertical defect has been associated with a reduced probability of tooth loss (Muzzi et al., 2006), whereas certain tools consider vertical defects to be a risk factor (Lindskog et al., 2010; Page et al., 2002) . Data supporting the latter are limited to an animal study (Lindhe & Svamberg, 1974) and a study in a non‐treated population in which the vertical defects were associated with further bone loss (Papapanou & Wennström, 1991).
Only two studies have addressed the association between bruxism and TLPD in patients following PM (Martinez‐Canut, 2015; McGuire & Nunn, 1996). The results of these studies were consistent and showed that bruxism doubled the risk of TLPD, which is similar to smoking. However, bruxism is poorly understood and represents one of the most controversial issues in dentistry. Consequently, it is a matter that deserves serious scientific discussion (Manfredini, Ahlberg, Mura, & Lobbezoo, 2015; Manfredini, Mura, & Lobbezoo, 2016; Perlitsh, 2016).
A recent review (Manfredini, Winocur, Guarda‐Nardini, Paesani, & Lobbezoo, 2013) found that the prevalence of bruxism in the general population is approximately 25%, whereas a twofold rate of bruxism (264 patients out of 500, 53%) has been reported in periodontal patients (Martinez‐Canut, 2015). Therefore, the role of bruxism merits further research.
This retrospective study aimed to characterize the baseline status of patients following PM analysing the association between the long‐term outcome of these patients, smoking, bruxism, and the main clinical and radiographic variables. These variables were gingival inflammation based on the GI, gingival recession, presence of radio‐opaque subgingival calculus (C+), tooth wear (incisal and occlusal attrition and abfractions), VCDs, FDs, and increased tooth mobility.
2. MATERIAL AND METHODS
2.1. Study population
The sample of this study consisted of 174 PM patients who were followed for a mean of 20.2 year (±2.4). These patients were selected from the baseline sample of our previous study (Martinez‐Canut, 2015) with the following criteria:
The inclusion criteria were the diagnosis of moderate and severe chronic periodontitis (Armitage, 1999), the absence of previous periodontal treatment and complete records on periapical radiographs at baseline, periapical radiographs of TLPD during the follow‐up and intra‐oral photographs at baseline and at the end of follow‐up. The exclusion criteria were mild periodontitis, aggressive periodontitis (Armitage, 1999), less than 36 and more than 70 years of age, the presence of serious disease with an influence on the periodontium, more than 6 non‐replaced missing teeth and extensive restorations with natural teeth and implants.
2.2. Treatment rendered and PM regimen
The active periodontal treatment and PM, which were previously described (Martinez‐Canut, 2015) were similar for all of the patients and included oral hygiene instructions, scaling and root planing under local anaesthesia, and surgical treatment (modified Widman flap, osseous resective surgery, root resection, and periodontal regeneration) in 82% of the patients. Systemic antibiotic therapy with amoxicillin plus clavulanic acid, metronidazole or azitromycin was prescribed in 25% of the cases, corresponding to the most severe cases of periodontitis.
PM was scheduled every 4 months and soon after the intervals were shortened or lengthened by 1 or 2 months according to changes in probing pocket depth and or bleeding upon probing.
2.3. Data collection
2.3.1. Medical history
The patients completed a medical history questionnaire upon the baseline examination, and the health status was updated during the follow‐up period.
2.3.2. Clinical findings
Two clinical parameters were obtained from the patients′ charts by one of the authors (M‐C):
Baseline number of teeth, excluding teeth extracted during active periodontal treatment and third molars.
Tooth mobility (Lindhe & Nyman, 1977), for which a mean value was calculated for the whole dentition to identify patients with generalized increased mobility.
The following clinical parameters were recorded (M‐C) at baseline and at the end of the follow‐up period and they were on the basis of baseline records from the patients′ charts and the intra‐oral photographs taken at baseline and at the end of follow‐up. Only the vestibular surface, from the second premolars to the central incisors, was evaluated.
Gingival recession: The distance from the cemento–enamel junction to the gingival margin. The maximum width of the clinical crown of an upper central incisor was the reference value for applying a rule of three to measure gingival recession on the photographs, which were scanned and magnified, using a computer.
Gingival inflammation: GI by Löe and Silness (1963) was routinely recorded at baseline in all patients on the vestibular surface of the teeth and confirmed with magnified photographs as described for gingival recession.
2.3.3. Radiographic findings
A complete set of baseline periapical radiographs for each patient was examined by the authors (Ll & M‐C) in a darkened room, using a radiographic screen (67‐0442, Dentsply Rinn, Elgin, IL, USA) and 2.5× magnification, to identify the presence and number of VCDs, the presence and degree of FDs (the most affected furcation entrance for each molar), and the presence of interproximal C+.
2.3.4. Bruxism
The presence of bruxism was identified according to criteria previously described (Martinez‐Canut, 2015), based on the self reported habits, confirmed during the follow‐up, together with signs of tooth wear. A complementary reevaluation was performed under a multidisciplinary approach that included an expert in bruxism and orofacial pain (R), a prosthodontist (Ll), and a periodontist (M‐C). Consensus was required to identify probable bruxism (Lobbezoo et al., 2012). Baseline and final photographs of the whole sample were examined under magnification to register incisal, occlusal, and cervical tooth wear (Tooth Wear Index by Smith & Knight, 1984). Particular attention was paid to differentiating clenching from grinding.
2.3.5. Smoking habits
Our previous research (Martinez‐Canut, 2015) did not find significant differences between non‐smokers and light smokers (less than 10 cigarettes per day); therefore, only heavy smokers of 10 or more cigarettes per day were considered smokers. The actual habit was confirmed during the follow‐up period. Smokers who had quit for more than 5 years were considered non‐smokers.
2.3.6. Assessment of tooth loss and TLPD
For the extracted teeth, a clinical and/or a radiological evaluation was performed immediately prior to the extraction, to identify the reasons for tooth loss, which was classified as either TLPD or tooth loss because of other reasons.
The criteria to define TLPD were spontaneous exfoliation and bone loss >75% with grade III mobility, which caused pain under function or spontaneously. For molars, bone loss >50% associated with furcation lesion grade III and repeated abscesses. Teeth extracted for restorative purposes with bone loss >75 and grade III mobility, as well as endodontic complications with bone >75% without caries or root fracture were considered TLPD.
2.4. Inter‐ and intra‐examiner agreement
Intra‐ examiner agreement (clinical parameters) and inter‐examiner agreement (radiological parameters) was verified (Kappa statistic).
2.5. Statistical analysis
Data entry and descriptive and analytical statistical evaluations were performed by independent statisticians (ERATEMA, I.A & L.D.) utilizing the SSPS software program (IBM, SPSS Statistics, V.19, Armonk, NY, USA). The statistical analysis identified significant associations and differences in the distribution of the occurrence of the variables under study. Each subsample with an outcome of TLPD 0, 1–2 and >2 teeth was refined according to the smoking and bruxism status. Mann–Whitney U and Kruskal–Wallis tests were utilized for continuous variables with a non‐normal distribution (abfractions, VCDs, FDs, and gingival recession) and ANOVAs and t‐Student′s tests were utilized for continuous variables with a normal distribution (GI).
The χ² test was used to analyse the distribution of the occurrence of the following categorical variables: presence or absence of C+; VCDs 0, 1–2 and more than 2; FDs grade 0–I, II and III, abfractions 0, 1–4 and more than 4; GI <1.7 and ≥1.7 and gingival recession <1.5 and ≥1.5 mm. The homogeneity of the subsamples was also evaluated. The significance level was set at α = .005.
3. RESULTS
3.1. Patient′s sample
The mean age of the patient′s sample was 43.1 years (SD 6.95), and the age ranged from 36 to 70 year old. In addition, 102 patients were females (58.6%) and 72 males (41.4%). The subjects were mostly Caucasian and of European origin (98%) and had a high to middle socio‐economic level. None of the patients had previously undergone periodontal treatment.
3.2. Homogeneity of the samples
The TLPD groups 0, 1–2, and >2 teeth, as well as the groups characterized according to smoking and bruxism were homogeneous for age and gender, but not for severe periodontitis, which was more prevalent as TLPD increased (χ² = .002) and with smoking (χ² = .012).
3.3. Inter‐ and intra‐examiner agreement
Intra‐ and inter‐examiner agreement was well above the level of chance at 0.88–0.95 (kappa statistic p < .001 for individual variables).
3.4. Distribution of the variables in the sample
Table 1 shows the main characteristics of the sample and differentiates the TLPD subsamples 0, 1–2, and more than 2 teeth, which were distributed according to smoking (in 63 patients, 36.2%) and bruxism (in 117 patients, 67.2%), either isolated or combined. The figures for the remaining variables under study are detailed, per patient as follows: mean GI, mean gingival recession, mean VCDs, mean FDs, presence of C+, and mean abfractions. These variables are distributed within the subsamples smoking positive/negative and bruxism positive/negative, depending on their statistical association with smoking and bruxism.
Table 1.
Distribution of the baseline sample according to the the number of teeth lost (TLPD 0, 1–2 and more than 2 teeth). Each one of these three samples is distributed according to the presence of heavy smoking and bruxism, either isolated or combined (subsamples S & B). It is detailed, per patient, the figures of the remaining variables under study: mean Gingival Index, mean gingival recession, mean furaction defects grade II and III, mean vertical defects, presence of radio‐opaque calculus and mean abfractions. Changes in gingival recession and in abfractions during the follow‐up are also presented
| n. pts (%) | Subsamples | n. pts. (%) | Mean Ging | n. pts (%) Ging | n. pts (%) Ging | Mean FDs II and III/pt | Subsamples | Mean (ED) | n. (%) pts. | Mean (ED) | Mean (ED) | Mean Ging. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S & B | Subg. calculus | Index | Index <1.7 | Index ≥1.7 | S & B | VCDs/pt | >2 VDs | abfractions 1 | abfractions 2 | rec | |||
| TLPD 0 | |||||||||||||
| Total | 74 (100%) | 2.08 (0.4) | 0.45 (1.1) | 0.54 (1.1) | 2.64 (2.44) | 5 (4.10) | 1.22 (0.98) | ||||||
| S− B− | 28 (37.8%) | S− (n.65 pts) | 48 (73.8%) | 2.10 (0.4) | 16 (24.6%) | 49 (75.4%) | 0.42 (1) | B− (n.34 pts) | 0.56 (1.3) | 3 (8.8%) | 0.88 (1.49) | 1.07 (1.73) | |
| S− B+ | 37 (50%) | ||||||||||||
| S+ B− | 6 (8.1%) | S+ (n. 9 pts) | 4 (44.4%) | 1.91 (0.32) | 3 (33.3%) | 6 (66.7%) | 0.53 (1.3) | B+ (n. 40 pts) | 0.53 (0.93) | 2 (5%) | 3.80 (2.30) | 7.43 (3.12) | |
| S+ B+ | 3 (4%) | ||||||||||||
| TLPD 1–2 | |||||||||||||
| Total | 45 (100%) | 1.94 (0.47) | 1.32 (1.4) | 1.24 (1.55) | 2.24 (2.22) | 6.28 (3.37) | 1.22 (0.77) | ||||||
| S− B− | 10 (22.2%) | S− (n. 33 pts) | 25 (75.7%) | 2.02 (0.41) | 8 (24.2%) | 25 (75.8%) | 0.7 (0.82) | B− (n. 15 pts) | 0.4 (0.74) | 0 (0%) | 1.47 (1.77) | 4.63 (2.26) | |
| S− B+ | 23 (51.1%) | ||||||||||||
| S+ B− | 5 (11.1%) | S+ (n. 12 pts) | 6 (50%) | 1.74 (0.57) | 6 (50%) | 6 (50%) | 1.42 (1.32) | B+ (n. 30 pts) | 1.67 (1.69) | 10 (33.3%) | 2.63 (2.34) | 6.90 (3.55) | |
| S+ B+ | 7 (15.5%) | ||||||||||||
| TLPD >2 | |||||||||||||
| Total | 55 (100%) | 1.87 (0.47) | 2.48 (2.32) | 2.51 (2.13) | 4 (3.75) | 7.62 (3.80) | 1.61 (1.06) | ||||||
| S− B− | 3 (5.4%) | S− (n. 13 pts) | 7 (53.8%) | 2.02 (0.52) | 6 (50%) | 6 (46.2%) | 1.1 (1.26) | B− (n. 8 pts) | 0.62 (1.19) | 1 (12.5%) | 1 (1.51) | 4 (4.25) | |
| S− B+ | 10 (18.1%) | ||||||||||||
| S+ B− | 5 (9.1%) | S+ (n. 42 pts) | 5 (11.9%) | 1.83 (0.45) | 19 (45%) | 23 (54.8%) | 2.63 (2.2) | B+ (n. 47 pts) | 2.83 (2.1) | 26 (55.3%) | 4.51 (3.79) | 8.41 (3.54) | |
| S+ B+ | 37 (67.2%) | ||||||||||||
n. pts, number of patients; TLPD, Tooth loss due to periodontal disease; S, heavy smoking; B, bruxism; Sub. calculus, presence of radiopaque subgingival calculus; FDs II and III, furaction defects grade II and III; VDs, vertical and circumferential bone defects; abfractions at baseline (1) and at the end of the follow‐up (2); mean Ging. rec, mean gingival recession. The variables under analysis are distributed within the subsamples smoking and bruxism depending on their statistical association with these factors: FDs are asociated with smoking while VCDs are associated with bruxism.
Seventy‐four patients (51.3%) did not lose teeth, 45 (25.7%) lost 1–2 teeth and 55 (31.6%) lost more than 2 teeth. As the TLPD increased, the prevalence of patients with heavy smoking and bruxism increased, especially when combined. For TLPD >2, 3 patients (5.4%) did not present smoking and bruxism while 37 patients (67.2%) presented both factors.
3.5. Analysis of the distribution of the occurrence of the variables under study
The following variables were significantly associated and could be used to determine the distribution of the occurrence of the variables.
Smoking (χ² = .001) and bruxism (χ² = .0001) were associated with increasing TLPD rate. The impact was much higher for smoking combined with bruxism (χ² < .0001) resulting in a prevalence of TLPD >2 which was much higher than for each factor in isolation (Table 2).
Table 2.
Distribution of TLPD, VCDs and FDs (mean values and categories of each variable) in the four subsamaples according to bruxism and smoking
| Total | n. pts | Total | S+/B+ | B+ | S+ | S−/B− | |
|---|---|---|---|---|---|---|---|
| 174 | 100% | 47 (100%) | 70 (100%) | 16 (100%) | 41 (100%) | ||
| TLPD | TLPD 0 | 74 | (42.5%) | 3 (6.4%) | 37 (52.9%) | 6 (37.5%) | 28 (68.3%) |
| TLPD 1–2 | 45 | (25.9%) | 7 (14.9%) | 23 (32.9%) | 5 (31.2%) | 10 (24.4%) | |
| TLPD >2 | 55 | (31.6%) | 37 (78.7%) | 10 (14.3%) | 5 (31.2%) | 3 (7.3%) | |
| Mean TLPD | 1.77 (2.14) | 3.94 (2.32) | 1.07 (1.51) | 1.63 (1.63) | 0.54 (0.92) | ||
| VDs | VDs 0 | 92 | (52.9%) | 11 (23.4%) | 38 (54.3%) | 10 (62.5%) | 33 (80.5%) |
| VDs 1–2 | 40 | (23.0%) | 12 (25.5%) | 18 (25.7%) | 4 (25%) | 6 (14.6%) | |
| VDs >2 | 42 | (24.1%) | 24 (51.1%) | 14 (20%) | 2 (12.5%) | 2 (4.9%) | |
| Mean VDs | 1.34 (1.81) | 2.68 (2.15) | 1.11 (1.50) | 0.88 (1.45) | 0.39 (1) | ||
| FDs | FDs 0–I | 61 | 35% | 3 (6.3%) | 36 (51.4%) | 1 (6.25%) | 21 (51.2%) |
| FDs 0–I and II | 34 | 19.5% | 8 (17%) | 13 (18.5%) | 4 (20%) | 9 (21.9%) | |
| FDs II and III | 79 | 45.4% | 36 (76.5) | 21 (30%) | 11 (68.7%) | 11 (26.8%) | |
| Mean FDs II | 1.17 (1.4) | 2 (1.7) | 0.5 (1.2) | 1.3 (1.5) | 0.4 (1.1) | ||
| Mean FDs III | 1.32 (1.69) | 2.5 (2.1) | 0.42 (1) | 1.1 (1.3) | 0.36 (1) |
B, bruxism; FDs, furcation defects; S, smoking; TLPD, Tooth loss due to periodontal disease; VCDs, vertical and circumferential bone defects.
The VCDs mean was three‐ to four‐times higher with bruxism and higher TLPD rates, increasing in accordance with the TLPD rate (p < .0001, χ² = .028 for TLPD 1–2 and = 0.004 for TLPD >2) and with bruxism (p = .0001; Table 1).
The mean FDs was three to four times higher with smoking and higher TLPD rates, increasing along with the TLPD rate (p < .0001, χ² = .032 for TLPD 1–2 and =0.005 for TLPD >2) and with smoking (p = .0002).
Abfractions mean increased with bruxism in the entire sample (p < .0001) and with bruxism for TLPD 0 (p < .0001, χ² < .0001), and >2 (p = .002, χ² = .006).
The prevalence of C+ decreased as TLPD increased and decreased with smoking. It was 75% for TLPD 0 and 1–2 teeth in non‐smokers and 11.9% for TLPD >2 teeth in smokers. Here C+ increased in non‐smokers in the entire sample (p = .0001) and in non‐smokers with TLPD >2 (χ² = .003, four times more prevalent).
Mean gingival recession increased as the TLPD rate increased (p = .026) and a mean recession >1.5 mm was significant for TLPD >2 (χ² = .031).
The mean GI slightly decreased with smoking and as TLPD increased. Conversely, for TLPD 0 and 1–2 teeth, the prevalence of a mean GI ≥1.7 was four times higher in non‐smokers. Mean GI <1.7 was more prevalent in smokers (χ² = .027) and for TLPD 1–2 (χ² = .007).
Table 2 depicts the associations between TLPD, FDs, and VCDs in the four subsamples depending on smoking and bruxism.
Figure 1A–L presents the 20–28 year follow‐up of patients according to the TLPD rate, smoking, and bruxism.
Figure 1.
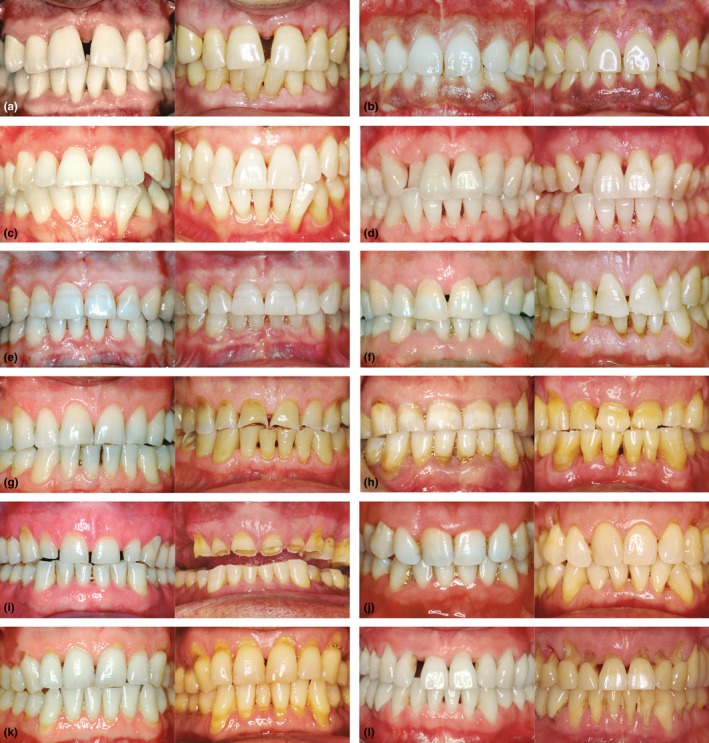
(a–d) are cases of TLPD 0 in non‐bruxists, (a and b) present a wider periodontium in a 26 year follow‐up in a non‐smoker (a) and a 20 year follow‐up in a smoker (b). (c‐d) present a narrower periodontium with developing gingival recessions in non‐smoking patients after 20 (c) and 23 years (d). (e and f) are TLPD 0 cases (after 20 and 22 years) of mild eccentric bruxism progressing to moderate bruxism (protruvise grinding) in a non‐smoking patient (e) and in a smoking patient with abfractions grade (f). (g, h, and i) (after 20, 22 and 25 years, respectively) depict moderate eccentric bruxism progressing to severe bruxism in a non‐smoking patients with TLPD 0 (g), TLPD 1–2 teeth (h) and TLPD >2 teeth (i). These three patients as well as the remaining cases of eccentric bruxism developed recession exclusively in the area of abfractions. Besides, these lesions developed to a lesser extent.(j, k, and l) (after 28, 20 and 26 years, respectively) are cases of mild to moderate centric bruxism with attrition mostly grade 0 and 1 progressing to severe centric bruxism with attrition grades 0–2 and abfractions grades 2–4. TLPD was (j), 1–2 teeth (k) and >2 teeth (l)
3.6. Characterization of patients losing more teeth
Among the 51 patients presenting the highest TLPD rate, the mean baseline tooth mobility (p < .0001 and χ² < .0001 for mobility < and ≥1) enabled two distinct profiles of patients to be differentiated (both groups were homogeneous for age, gender and severe periodontitis), which were complementarily characterized by differences in abfractions (p = .009 and χ² = .003) and in gingival recession (p = .001 and χ² = .039 for mean recession < and ≥1). Therefore, as shown in Table 3, 26 patients (termed type 1), presented a baseline mean tooth mobility of 0.30, mean gingival recession of 1.21 and mean abfractions of 5.38, whereas the second group of 25 patients (termed type 2), presented a fivefold higher mean mobility of 1.31, close to a twofold higher gingival recession and an almost twofold lower mean abfractions. The mean TLPD rate of the type 2 patients was one tooth higher.
Table 3.
Characteristics of patients losing more steeth. Two basic types, 1 and 2, are distinguished according to several clinical and radiological parameters. Mean tooth mobility was the key parameter differentiating both types, followed by abfractions
| n. pts | Mean (ED) | Mean (ED) | % Mean | S & B | Mean (ED) | Mean (ED) | Mean (ED) | Mean (ED) | n. pts Ging | Mean (ED) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TLPD/pt | mobility | Mobility | groups | VCD | Abfrs/pt 1 | Abfrs/pt 2 | Ging recess | recess >1 | Ging Index | ||
| Total | 51 | 4.48 (1.16) | 0.77 (0.63) | 2.61 (2.12) | 4.35 (3.72) | 8.25 (3.34) | 1.64 (0.98) | ||||
| Type 1 | |||||||||||
| Total | 26 | 4 (1.11) | 0.30 (0.21) | 100% <1 | 5.38 (3.43) | 8.67 (2.64) | 1.21 (0.8) | 14 (56%) | 1.85 (0.48) | ||
| S+/B+ | 21 | B+ | 2.50 (2.14) | ||||||||
| B+ | 4 | ||||||||||
| S+ | 1 | S+ | 0 | ||||||||
| Type 2 | |||||||||||
| Total | 25 | 4.96 (1.32) | 1.31 (0.50) | 100% ≥1 | 3.28 (3.78) | 7.63 (4.72) | 2.08 (0.97) | 19 (82%) | 1.97 (0.5) | ||
| S+/B+ | 14 | B+ | 2.72 (2.12) | ||||||||
| B+ | 5 | ||||||||||
| S+ | 4 | S+ | 1 | ||||||||
| S−/B− | 2 | B−/S− | 0 | ||||||||
Abfrs, abfractions; B, bruxism; Ging recess, gingival recession; n. pts, number of patients; S, heavy smoking; TLPD, tooth loss due to periodontal disease; VCD, vertical and circumferential bone defects.
The clinical features of some of these patients is presented in Figures [Link], [Link], [Link]. Figure S4 depicts the magnified image of several emerging abfractions. More detailed information on the role of bruxism and occlusal overload in these patients is presented in the supplementary material (Appendix S1).
3.7. Distribution of centric and eccentric bruxism, tooth wear, tooth loss, and complications
Only 10 (8.5%) out of 117 patients with bruxism presented eccentric bruxism (incisal and occlusal wear 2 and 3) with flattening of the incisal and occlusal planes. The remaining 107 patients (91.4%) presented centric bruxism with occlusal wear 1 (wear facets). Group function and lack of canine guidance were common findings at baseline and almost the rule by the end of the follow‐up period.
Abfractions were much more clearly associated with bruxism than occlusal wear. Table 4 details the mean abfractions and associated categories, in patients with and without bruxism, at baseline and at the end of the follow‐up period. Abfractions were four times more prevalent and increased twice as much in degree in bruxists compared to non‐bruxists. The baseline abfractions doubled in prevalence and degree at the end of the follow‐up, and some of these final lesions emerged with a localized gingival recession, in the shape of Stillman′s cleft (Stillman, 1921).
Table 4.
Mean abfractions per patient and categories at T1 and T2 according to bruxism
| Subsamples: | n. pts | Abfrs category | Mean abfrs | Abfrs category | |
|---|---|---|---|---|---|
| (SD)/pt T1 | at T1 (n. pts and %) | (SD)/pt T 2 | at T2 (n. pts and %) | ||
| S‐ B‐/S+ B‐ | 57 | 1.05 (1.56) | 0 = 26, 45.6% | 2.65 (2.71) | 0 = 10, 17.5% |
| 1 = 31, 54.3% | 1 = 44, 77.2% | ||||
| 1–2 = 3, 5.2% | |||||
| S‐ B+/S+ B+ | 117 | 3.79 (3.06) | 0 = 13, 11% | 7.52 (3.38) | 1 = 5, 4.2% |
| 1 = 85, 72.6% | 1–2 = 99, 84.6% | ||||
| 1–2 = 16% | 2–3 = 13, 11% |
Abfrs, abfractions and category (Tooth Wear Index by Smith & Knight, 1984); B, bruxism; n. pts, number of patients; pt, patient; S, heavy smoking; T1, at baseline; T2, at the end of the follow‐up.
The majority of teeth lost in bruxists were those lacking abfractions. Only 39 (12.6%) out of the 308 teeth extracted presented grade II and III abfractions. The teeth lost presented a widened periodontal space and a VCD.
Abfractions developed to a lesser extent in 8 out of 10 patients with eccentric bruxism (occlusal wear 2 and 3). Abfractions 2 and especially 3 developed on either the upper or the lower arches, but not on both. These lesions were extremely uncommon in mobile and pathologically migrated teeth.
Eighty‐three patients (80%) presented acute symptoms of diffuse pain, 15 crown and root fractures (in 19 teeth, 12.8%), and 2 horizontal implant fractures.
3.8. Development of an index to predict TLPD
Based on the analysed variables, a predictive index for TLPD that consisted of a simple addition of one score for each variable involved was developed. Thus, the final value ranged from the presence of 0–5 of the following variables: fewer C+ deposits, a GI below 1.7, VCDs and/or FDs grade II and III, mean gingival recession >1.5 mm and abfractions.
Table 5 shows the distribution of patients according to the index. For TLPD 0, 71 patients out of 74 (96%) presented an index of 0–2. For TLPD >2, 45 out of 51 (97.7%) presented an index of 3–5.
Table 5.
Distribution of patients with the corresponding value of the index and the mean index value within each subsample of TLPD according to smoking and bruxism
| n. patients | n. teeth | n. patients | n. patients with the corresponding value (0– 5) of the index | Mean index | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| lost | 0 | 1 | 2 | 3 | 4 | 5 | value/pt. | |||
| TLPD 0 | ||||||||||
| Total | 74 | 1.51 | ||||||||
| S− B− | 28 | 0 | 3 | 17 | 8 | 1.17 | ||||
| S− B+ | 37 | 0 | 12 | 22 | 2 | 1 | 1.78 | |||
| S+ B− | 6 | 0 | 5 | 1 | 1.16 | |||||
| S+ B+ | 3 | 0 | 3 | 2 | ||||||
| TLPD 1–2 | ||||||||||
| Total | 45 | 2.48 | ||||||||
| S− B− | 10 | 1 − 2 | 1 | 4 | 3 | 2 | 1.68 | |||
| S− B+ | 23 | 1 − 2 | 1 | 12 | 6 | 4 | 2.56 | |||
| S+ B− | 5 | 1 − 2 | 1 | 3 | 1 | 3 | ||||
| S+ B+ | 7 | 1 − 2 | 1 | 4 | 2 | 3 | ||||
| TLPD >2 | ||||||||||
| Total | 55 | |||||||||
| S− B− | 3 | 2 | ||||||||
| 3 | 3 | 3 | ||||||||
| S− B+ | 10 | 3.6 | ||||||||
| 3 | 3 | 2 | 1 | |||||||
| 4 | 4 | 3 | 1 | |||||||
| 5 | 2 | 1 | 1 | |||||||
| 6 | 1 | 1 | ||||||||
| S+ B− | 5 | 3 | ||||||||
| 3 | 2 | 1 | 1 | |||||||
| 4 | 1 | 1 | ||||||||
| >4 | 2 | 1 | 1 | |||||||
| S+ B+ | 37 | 4 | ||||||||
| 3 | 11 | 7 | 3 | 1 | ||||||
| 4 | 11 | 1 | 4 | 6 | ||||||
| 5 | 6 | 3 | 2 | 1 | ||||||
| >5 | 9 | 5 | 4 | |||||||
B, bruxism; n. pts, number of patients; n. teeth lost; number of teeth lost; S, heavy smoking; TLPD, tooth loss due to periodontal disease.
The mean TLPD corresponding to an index of 4 and 5 were, respectively, 2.7 and 4.6. For TLPD >2 teeth, index values of 3, 4, and 5 matched the number of teeth lost ±1 in 43 out of 55 patients (78.1%). The higher the value of the Index, especially with bruxism and smoking, the higher the resulting TLPD rate, and the accuracy of the Index (Spearman correlation .680, p = .0001).
4. DISCUSSION
The inclusion and exclusion criteria of this study were defined to overcome the inherent limitations and potential source of bias of a retrospective study design. This approach enabled gathering a patient′s sample with reliable clinical and radiographic records. The final clinical photographs were actually useful to ensure the identification of bruxism in the long‐term and under a multidisciplinary approach.
The present research has analysed the long‐term outcome of patients following PM according to smoking, bruxism, and certain characteristic features associated with both factors. This approach provided a better understanding of bruxism, which lacked on longitudinal studies and defined criteria for identifying it, especially clenching.
Smoking has been found associated with higher TLPD rates in the present research, consistently with the results of many studies in patients undergoing PM for more than 5 years (McGuire & Nunn, 1996, 1999; König, Plagmann, Rühling, & Kocher, 2002; Fardal, Johannessen, & Linden, 2004; Chambrone & Chambrone, 2006; Eickholz, Kaltschmitt, Berbig, Reitmeir, & Pretzl, 2008; Jansson & Lagervall, 2008; Tsami, Pepelassi, Kodovazenitis, & Komboli, 2009; Leininger, Tenenbaum, & Davideau, 2010; Ravald & Starkhammar Johanson, 2012; Costa et al., 2014; Salvi et al. 2014, Martinez‐Canut, 2015; Dannewitz et al., 2016), with few exceptions (Tonetti, Muller‐Campanile, & Lang, 1998; Matthews, Smith, & Hanscom, 2001; Carnevale, Cairo, & Tonetti, 2007; Matuliene et al. 2010, Baümer et al., 2011) .
The increase on the risk of tooth loss reported in some of these studies (1.8, 2.1, 2.9, 3.3, 4, 5, and 8) was usually lower than the one reported in general population (2.5, 4, 7.2, and 14.1) (Bergstrom, 1989; Linden & Mullaly, 1994; Grossi et al., 1995; Tomar & Asma, 2000). When only heavy smoking was analysed, the increase on the risk was around 2 in patients following PM (Martinez‐Canut, 2015; McGuire & Nunn, 1999) as compared to 7 in the general population (Grossi et al., 1995).
These findings might reflect, to some extent, certain differences on the impact of a prognostic factor (in patients following PM) as compared to a risk factor in the general population (during the natural course of the disease) and these differences may be partially attributed to the efficacy of PM. A recent systematic review concluded that the risk of TLPD was much lower in patients complying regularly with PM as compared to irregular compliers (Lee et al. 2015). Most of the patients analysed in the present research complied regularly with PM.”
The impact of bruxism resulted in a threefold increase on the mean VDs and an almost sixfold increased when smoking participated. However, bruxism did not increase the mean FDs unless smoking participated, resulting in a sixfold increase on the mean FDs.
Data supporting the association of occlusal contacts and bruxism with periodontal disease is contradictory (Hanamura et al., 1987; Jin & Cao, 1992; Pihltrom, Anderson, Aeppli, & Schaffer, 1986; Shefter & McFall, 1984; Yuodelis & Mann, 1965). However, two studies in patients following PM reported a twofold increase in the risk of TLPD associated with bruxism (Martinez‐Canut, 2015; McGuire & Nunn, 1996) and an almost fourfold increase in the risk of losing more teeth when bruxism was associated with smoking (Martinez‐Canut, 2015). The present research reinforced these findings and characterized these patients according to several clinical and radiological features. Our findings seem to indicate that VCDs and FDs are characteristic features of bruxism and smoking respectively. A particular type of bone defect seen in bruxists could not be categorized as either VCD or FD, since it was a localized extreme loss of supporting bone. This lesion was characteristic of posterior teeth with short and/or fused roots.
It has been shown that smoking increases bone loss (Bergström, Eliasson, & Preber, 1991; Rosa, Lucas, & Lucas, 2008) and is associated with increased prevalence of furcation involvement (Axelsson, Paulander, & Lindhe, 1988; Kerdvongnundit, 2000; Mullally & Linden, 1996) . Our results confirm these findings and provide additional information on VCDs. Additional information on the link between bruxism and smoking is presented in supplementary material (Appendix S2).
Abfractions have been attributed to occlusal forces on the cervical area of the teeth and fall within the multi‐factorial aetiology of non‐carious cervical lesions (Grippo, Simring, & Coleman, 2012). However, these lesions have remained a theoretical process supported by engineering analysis using finite element models (Sarode & Sarode, 2013). Only one study reporting a 14‐year follow‐up of a patient with bruxism, abfractions, and occlusal wear was found in the literature at the time of writing (Pintado, DeLong, Ko, Sakaguchi, & Douglas, 2000). Consequently, the findings presented in this paper contribute to a better understanding of these lesions.
Abfractions have already been associated with bruxism (McCoy, 1982; Xhonga, 1977), wear facets (Badder, McClure, Scurria, Shugars, & Heymann, 1996; Mayhew, Jessee, & Martin, 1988; Schiller, Marquardt, & Albers, 1985; Telles, Pegoraro, & Pereira, 2000), and occlusal disturbances (Miller, Penaud, Ambrosini, Bisson‐Boutelliez, & Briancon, 2003). The study by Miller et al. (2003) found that 10% of patients with abfractions presented bruxism (eccentric bruxism with increased occlusal attrition), while the remaining 90% presented occlusal disturbances (wear facets, lack of canine guidance, and group function).
Furthermore, a similar percentage of patients with eccentric bruxism (8.5%) was found in this study. However, the 90% of occlusal disturbances reported by Miller et al. (2003) might to some extent correspond to the 92.4% of our patients with clenching, occlusal disturbances and abfractions. The lack of defined criteria to identify clenching would explain the different results and might indicate the possibility of under diagnosing a relevant and prevalent factor involved in TLPD. Only 10% of bruxists might present the conventional pattern of increased attrition.
The very low prevalence of abfractions found in mobile teeth has been previously reported (Miller et al., 2003).
Cemental tears have been attributed to occlusal trauma (Leknes, Lie, & Selvig, 1996), dental attrition (Lin et al., 2011), poor tissue repair capacity (Ishikawa, Oda, Hayashi, & Arakawa, 1996), and structural weakness of the cementum (Watanabe, Watanabe, Miyauchi, Minoru, & Watanabe, 2012). Several cemental tears progressing to abfractions or developing on existing abfractions were identified in this study.
The association of smoking, alcohol, and other substances with bruxism (Bertazzo‐Silveira et al., 2016; Lavigne, Lobbezoo, Rompré, Nielsen, & Montplaisi, 1997; Ohayon, Li, & Guilleminault, 2001) might represent a possible additional pathway implicated in periodontal disease.
This study found a decreased GI associated with smoking and higher TLPD rates, which might be partially explained by the effect of smoking, decreasing bleeding on probing (Al‐Bayaty, Baharuddin, Abdulla, Ali, & Al‐Bayaty, 2013; Dietrich, Bernimoulin, & Glynn, 2004; Ramseier et al., 2015; Shimazaki et al., 2006) and the inflammatory reactions at the histopathological level (Naderi, Semyari, & Alahinia, 2015). Bleeding on probing and even spontaneous bleeding with other signs of inflammation were not associated with TLPD in patients under PM (Baümer et al., 2011; Faggion, Petersilka, Lange, Gerss, & Fleming, 2007; Tonetti et al., 1998). Irrespective of the level of evidence indicating that bleeding on probing predicts further attachment loss and tooth loss (for review, see Renvert & Persson, 2002), baseline gingival inflammation might represent a distinct condition from that after treatment.
According to our findings, C+ was more prevalent in non‐smoking patients, as it has previously been reported (Martinez‐Canut, Benlloch, & Izquierdo, 1999) and this might partially explain the complementary finding that fewer C+ deposits were associated with higher TLPD rates. A higher prevalence of subgingival calculus in smokers has also been reported (Bergström, 2005) which could be explained by differences in the patient′ sample and the criteria utilized to assess calculus, without a clear distinction between supra and subgingival deposits.
Lastly, the extent to which differences in salivary composition between smokers and non‐smokers (Zuabi et al., 1999), and systemic bone mineral (Brennan, Genco, Hovey, Trevisan, & Wactawski‐Wende, 2007) influence the type of subgingival calculus might deserve further attention.
The most reliable predictors of an unfavourable outcome have been baseline VCDs and/or FDs associated with increased attrition and/or abfractions, especially in smoking patients. Therefore, the identification of emerging abfractions, VCDs, and FDs at early stages of disease might help to make a more precise diagnosis and institute the most appropriate prophylactic and therapeutic measurements for a patient at risk of losing more teeth.
The usefulness and accuracy of the long‐term outcome predictive index presented here could be validated retrospectively quite easily, using different samples of patients who followed PM over long‐term periods.
A predictive index to anticipate the long‐term outcome based on the presence of these features is proposed.
Two distinct types of patients at risk of losing more teeth were identified based on differences in the baseline mobility, abfractions and gingival recession.
5. CONCLUSIONS
This study enabled the characterization of the baseline status of patients following PM according to the final outcome.
This characterization could be useful to identify patients at risk of losing more teeth. These patients were characterized by the presence of smoking and bruxism and several clinical and radiological features which were associated with smoking (FDs, a reduced GI and fewer C+) and bruxism (VCDs and abfractions).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
6.
Clinical Relevance.
Scientific rationale for the study: Certain patients experience higher tooth loss rates during periodontal maintenance. The purpose of this study was to characterize the baseline status of these patients.
Principal findings: Higher rates of tooth loss were associated with bruxism, smoking, and certain features associated with bruxism (vertical bone defects and abfractions) and smoking (FDs, less radiographically visible subgingival calculus and decreased gingival inflammation).
Practical implications: In combination with smoking and bruxism, several clinical and radiographical parameters were useful in characterizing the baseline status of patients in relation to their final long‐term outcome, which enabled accurate predictions.
Supporting information
Martinez‐Canut P, Llobell A, Romero A . Predictors of long‐term outcomes in patients undergoing periodontal maintenance. J Clin Periodontol. 2017;44:620–631. https://doi.org/10.1111/jcpe.12730
Funding information
This study was self‐funded by the authors.
REFERENCES
- Al‐Bayaty, F.H. , Baharuddin, N. , Abdulla, M.A. , Ali, H.M. , & Al‐Bayaty, M.F. (2013). The influence of cigarette smoking on gingival bleeding and serum concentration of haptoglobin and alpha 1‐ antitrypsin. BioMed Research International, 2013, 684154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage, G. C. (1999). Development of a classification system for periodontal diseases and conditions. Annals of Periodontology, 4, 1–6. [DOI] [PubMed] [Google Scholar]
- Axelsson, P. , Paulander, J. , & Lindhe, J. (1988). Relationship between smoking and dental status in 35‐, 50‐, 65‐ and 75‐year‐old individuals. Journal of Clinical Periodontology, 25, 297–305. [DOI] [PubMed] [Google Scholar]
- Badder, J. D. , McClure, F. , Scurria, M. S. , Shugars, D. A. , & Heymann, H. O. (1996). Case‐control study of non‐carious cervical lesions. Community Dentistry and Oral Epidemiology, 24, 286–291. [DOI] [PubMed] [Google Scholar]
- Baümer, A. , El Sayed, N. , Kim, T.‐S. , Reitmeier, P. , Eickholz, P. , & Pretzl, B. (2011). Patient‐related risk factors for tooth loss in aggressive periodontitis after active periodontal therapy. Journal of Clinical Periodontology, 38, 347–354. [DOI] [PubMed] [Google Scholar]
- Bergstrom, J. (1989). Cigarette smoking as risk factor in chronic periodontal disease. Community Dentistry and Oral Epidemiology, 17, 245–247. [DOI] [PubMed] [Google Scholar]
- Bergström, J. (2005). Tobacco smoking and subgingival dental calculus. Journal of Clinical Periodontology, 32, 81–88. [DOI] [PubMed] [Google Scholar]
- Bergström, J. , Eliasson, S. , & Preber, H. (1991). Cigarette smoking and periodontal bone loss. Journal of Periodontology, 62, 242–246. [DOI] [PubMed] [Google Scholar]
- Bertazzo‐Silveira, E. , Porto De Toledo, I. , Kruger, C.M. , Porporatti, A.L. , Dick, B. , Flores‐Mir, C. , & De Luca Canto, G. (2016). The association between drug abuse and sleep bruxism: A systematic review. Journal of the American Dental Association, 11, 859–866. https://doi.org/dx.doi.org/10.1016/j.adaj.2016.06.014 [DOI] [PubMed] [Google Scholar]
- Brennan, R. M. , Genco, R. J. , Hovey, K. M. , Trevisan, M. , & Wactawski‐Wende, J. (2007). Clinical attachment loss, systemic bone density, and subgingival calculus in postmenopausal women. Journal of Periodontology, 78, 2104–2111. [DOI] [PubMed] [Google Scholar]
- Carnevale, G. , Cairo, F. , & Tonetti, M. S. (2007). Long term effects of supportive therapy in periodontal patients treated with fibre retention osseous resective surgery. II: Tooth extractions during active and supportive therapy. Journal of Clinical Periodontoilogy, 34, 342–348. [DOI] [PubMed] [Google Scholar]
- Chambrone, L. A. , & Chambrone, L. (2006). Tooth loss in well maintained patients with chronic periodontitis during long‐term supportive therapy in Brazil. Journal of Clinical Periodontology, 33, 759–764. [DOI] [PubMed] [Google Scholar]
- Costa, F. O. , Lages, E. J. , Cota, L. O. , Lorentz, T. C. , Soares, R. V. , & Cortelli, J. R. (2014). Tooth loss in individuals under periodontal maintenance therapy: 5‐year prospective study. Journal Periodontal Research, 49, 121–128. [DOI] [PubMed] [Google Scholar]
- Dannewitz, B. , Zeidler, A. , Hüsing, J. , Saure, D. , Pfefferle, T. , Eickholz, P. , & Pretzl, B. (2016). Loss of molars in periodontally treated patients: Results 10 years and more after active periodontal therapy. Journal of Clinical Periodontology, 43, 53–62. [DOI] [PubMed] [Google Scholar]
- Dietrich, T. , Bernimoulin, J. P. , & Glynn, R. J. (2004). The effect of cigarette smoking on gingival bleeding. Journal of Periodontology, 75, 16–22. [DOI] [PubMed] [Google Scholar]
- Eickholz, P. , Kaltschmitt, J. , Berbig, J. , Reitmeir, P. , & Pretzl, B. (2008). Tooth loss after active periodontal therapy. I: Patient‐related factors for risk, prognosis, and quality of outcome. Journal of Clinical Periodontology, 35, 165–174. [DOI] [PubMed] [Google Scholar]
- Faggion, C. M. Jr , Petersilka, G. , Lange, D. E. , Gerss, J. , & Fleming, T. F. (2007). Prognostic model for tooth survival in patients treated for periodontitis. Journal of Clinical Periodontology, 34, 226–231. [DOI] [PubMed] [Google Scholar]
- Fardal, O. , Grytten, J. , Martin, J. , Houlihan, C. , & Heasman, P. (2016). Using prognostic factors from cases series and cohort studies to identify individuals with poor long‐term outcomes during periodontal maintenance. Journal of Clinical Periodontology, 43, 789–796. [DOI] [PubMed] [Google Scholar]
- Fardal, O. , Johannessen, A. C. , & Linden, G. J. (2004). Tooth loss during maintenance following periodontal practice in Norway. Journal of Clinical Periodontology, 31, 550–555. [DOI] [PubMed] [Google Scholar]
- Grippo, J. O. , Simring, M. , & Coleman, T. A. (2012). Abfraction, abrasion, biocorrosion, and the enigma of noncarious cervical lesions: A 20‐year perspective. Journal of Esthetic and Restorative Dentistry, 24, 10–25. [DOI] [PubMed] [Google Scholar]
- Grossi, S. G. , Genco, R. J. , Machtei, E. E. , Ho, A. W. , Koch, G. , Zambon, J. J. , & Hausmann, E. (1995). Assessment of risk for periodontal disease. II. Risk indicators for alveolar bone loss. Journal of Periodontology, 66, 23–29. [DOI] [PubMed] [Google Scholar]
- Hanamura, H. , Houston, F. , Rylander, H. , Carlsson, G. E. , Haraldson, T. , Nyman, S. , (1987). Periodontal status and bruxism: A comparative study of patients with periodontal disease and occlusal parafunctions. Journal of Periodontology, 58, 173–176. [DOI] [PubMed] [Google Scholar]
- Ishikawa, I. , Oda, S. , Hayashi, J. , & Arakawa, S. (1996). Cervical cemental tears in older patients with adult periodontitis. Case reports. Journal of Periodontology, 67, 15–20. [DOI] [PubMed] [Google Scholar]
- Jansson, L. , & Lagervall, M. (2008). Periodontitis progression in patients subjected to supportive maintenance care. Swedish Dental Journal, 32, 105–114. [PubMed] [Google Scholar]
- Jin, L. J. , & Cao, C. F. (1992). Clinical diagnosis of trauma from occlusion and its relation with severity of periodontitis. Journal of Clinical Periodontology, 19, 92–97. [DOI] [PubMed] [Google Scholar]
- Kerdvongnundit, V. (2000). Effect of smoking on periodontal health in molar teeth. Journal of Periodontology, 71, 433–437. [DOI] [PubMed] [Google Scholar]
- König, J. , Plagmann, H.‐C. , Rühling, A. , & Kocher, T. (2002). Tooth loss and pocket probing depths in compliant periodontally treated patiens: A retrospective analysis. Journal of Clinical Periodontology, 29, 1092–1100. [DOI] [PubMed] [Google Scholar]
- Lang, N.P. , Suvan, J.E. , & Tonetti, M.S . (2015). Risk factors assessment tools for the prevention of periodontitis progression a systematic review. Journal of Clinical Periodontology 42 (Suppl. 16): S59–S70. https://doi.org/10.1111/jcpe.12350 [DOI] [PubMed] [Google Scholar]
- Lang, N. P. , & Tonetti, M. S. (2003). Periodontal risk assessment (PRA) for patients in supportive periodontal therapy (SPT). Oral Health & Preventive Dentistry, 1, 7–16. [PubMed] [Google Scholar]
- Lavigne, G.L. , Lobbezoo, F. , Rompré, P.H. , Nielsen, T.A. , & Montplaisi, R. J. (1997). Cigarette smoking as a risk factor or an exacerbating factor for restless legs syndrome and sleep bruxism. Sleep, 20, 290–293. [PubMed] [Google Scholar]
- Lee, C. T. , Huang, H. Y. , Sun, T. C. , & Karimbux, N. (2015). Impact of patient compliance on tooth loss during supportive periodontal maintenance: A systematic review and meta‐analysis. Journal of Dental Research, 6, 777–786. [DOI] [PubMed] [Google Scholar]
- Leininger, M. , Tenenbaum, H. , & Davideau, J. L. (2010). Modified periodontal risk assessment score: Long‐term predictive value of treatment outcomes. A retrospective study. Journal of Clinical Periodontology, 37, 427–435. [DOI] [PubMed] [Google Scholar]
- Leknes, K. N. , Lie, T. , & Selvig, K. A. (1996). Cemental tear: A risk factor in periodontal attachment loss. Journal of Periodontology, 67, 583–588. [DOI] [PubMed] [Google Scholar]
- Lin, H. J. , Chan, C. P. , Yang, C. Y. , Wu, C. T. , Tsai, Y. L. , Huang, C. C. , … Jeng, J. H. (2011). Cemental tear: Clinical characteristics and its predisposing factors. Journal of Endodontics, 37, 611–618. [DOI] [PubMed] [Google Scholar]
- Linden, G. J. , & Mullaly, B. H. (1994). Cigarette smoking and periodontal destruction in young adults. Journal of Periodontology, 65, 718–723. [DOI] [PubMed] [Google Scholar]
- Lindhe, J. , & Nyman, S. (1977). The role of occlusion in periodontal diseaseand the biological rationale for splinting in treatment for periodontitis. Oral Sciences Reviews, 10, 11–43. [PubMed] [Google Scholar]
- Lindhe, J. , & Svamberg, G. (1974). Influence of trauma from acclusion on the progression of experimental periodontitis in the dog. Journal of Clinical Periodontology, 1, 3–14. [DOI] [PubMed] [Google Scholar]
- Lindskog, S. , Blomlof, J. , Persson, I. , Niklason, A. , Hedin, A. , Ericsson, L. , … Blomlof, L. (2010). Validation of an algorithm for chronic periodontitis risk assessment and prognostication: Risk predictors, explanatory values, measures of quality, and clinical use. Journal of Periodontology, 81, 584–593. [DOI] [PubMed] [Google Scholar]
- Lobbezoo, F. , Ahlberg, J. , Glaros, A. G. , Kato, T. , Koyano, K. , Lavigne, G. J. , … Winocur, E. (2012). Bruxism defined and graded: An international consensus. Journal of oral Rehabilitation, 40, 2–4. [DOI] [PubMed] [Google Scholar]
- Löe, H. , & Silness, J. (1963). Periodontal disease in pregnancy. Acta Odontologica Scandinavica, 21, 533–551. [DOI] [PubMed] [Google Scholar]
- Manfredini, D. , Ahlberg, J. , Mura, R. , & Lobbezoo, F. (2015). Bruxism is unlikely to cause damage to the periodontium: Findings from a systematic literature assessment. Journal of Periodontology, 86, 546–555. [DOI] [PubMed] [Google Scholar]
- Manfredini, D. , Mura, R. , & Lobbezoo, F. (2016). Letter to the Editor: Author′s response: Letter to the Editor: Bruxism is unlikely to cause damage to the periodontium: Findings from a systematic literature assessment. Journal of Periodontology, 87, 3–4. [DOI] [PubMed] [Google Scholar]
- Manfredini, D. , Winocur, E. , Guarda‐Nardini, L. , Paesani, D. , & Lobbezoo, F. (2013). Epidemiology of bruxism in adults: A systematic review of the literature. Journal of Orofacial Pain, 27, 99–110. [DOI] [PubMed] [Google Scholar]
- Martinez‐Canut, P. (2015). Predictors for tooth loss due to periodontal disease in patients following long‐term periodontal maintenance. Journal of Clinical Periodontology, 42, 1115–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Canut, P. , Benlloch, D. , & Izquierdo, R. (1999). Factors related to the quantity of subgingival calculus in proximal root surfaces. Journal of Clinical Periodontology, 26, 519–524. [DOI] [PubMed] [Google Scholar]
- Matthews, D. C. , Smith, C. G. , & Hanscom, S. L. (2001). Tooth loss in periodontal patients. Journal of Canadian Dental Association, 67, 207–210. [PubMed] [Google Scholar]
- Matuliene, G. , Studer, R. , Lang, N. P. , Schmidlin, K. , Pjetursson, B. E. , Salvi, G. E. , … Zwahlen, M. (2010). Significance of Periodontal Risk Assessment in the recurrence of periodontitis and tooth loss. Journal of Clinical Periodontology, 37, 191–199. [DOI] [PubMed] [Google Scholar]
- Mayhew, R. B. , Jessee, S. A. , & Martin, R. E. (1988). Association of occlusal, periodontal and dietary factors with presence of non‐carious dental lesions. American Journal of Dentistry, 11, 29–32. [PubMed] [Google Scholar]
- McCoy, G. (1982). The etiology of gingival erosion. Journal of Oral Implantology, 19, 361–362. [PubMed] [Google Scholar]
- McGuire, M. K. , & Nunn, M. E. (1996). Prognosis versus actual outcome. III. The effectiveness of clinical parameters in accurately predicting tooth survival. Journal of Periodontology, 67, 666–674. [DOI] [PubMed] [Google Scholar]
- McGuire, M. K. , & Nunn, M. E. (1999). Prognosis versus actual outcome. III. The effectiveness of clinical parameters in accurately predicting tooth survival. Journal of Periodontology, 67, 666–674. [DOI] [PubMed] [Google Scholar]
- Miller, N. , Penaud, J. , Ambrosini, P. , Bisson‐Boutelliez, C. , & Briancon, S. (2003). Analysis of etiologic factors and peridodontal conditions involved with 309 abfractions. Journal of Clinical Periodontology, 30, 828–832. [DOI] [PubMed] [Google Scholar]
- Mullally, B. H. , & Linden, G. J. (1996). Molar furcation involvement associated with cigarette smoking in periodontal referrals. Journal of Clinical Periodontology, 23, 658–661. [DOI] [PubMed] [Google Scholar]
- Muzzi, L. , Nieri, M. , Cattabriga, M. , Rotundo, R. , Cairo, F. , & Pini Prato, G. P. (2006). The potential prognostic value of some periodontal factors for tooth loss: a retrospective multilevel analysis on periodontal patients treated and maintained over 10 years. Journal of Periodontology, 77, 2084–2089. [DOI] [PubMed] [Google Scholar]
- Naderi, N. J. , Semyari, H. , & Alahinia, Z. (2015). The impact of smoking on gingival: A histopathological study. Iranian Journal of Pathology, 10, 214–220. [PMC free article] [PubMed] [Google Scholar]
- Page, R. C. , Krall, E. A. , Martin, J. , Mancl, L. , & Garcia, R. I. (2002). Validity and accuracy of a risk calculator in predicting periodontal disease. Journal of the American Dental Association, 133, 569–576. [DOI] [PubMed] [Google Scholar]
- Papapanou, P. N. , & Wennström, J. L. (1991). The angular bony defect as indicator of further alveolar bone loss. Journal of Clinical Periodontology, 18, 317–322. [DOI] [PubMed] [Google Scholar]
- Perlitsh, M. J. (2016). Letter to the Editor: Bruxism is unlikely to cause damage to the periodontium: Findings from a systematic literature assessment. Journal of Periodontology, 87, 1–2. [DOI] [PubMed] [Google Scholar]
- Pihltrom, B. , Anderson, K. A. , Aeppli, D. , & Schaffer, E. M. (1986). Assoociation between signs of trauma from occlusion and periodontitis. Journal of Periodontology, 57, 1–6. [DOI] [PubMed] [Google Scholar]
- Pintado, M. R. , DeLong, R. , Ko, C. , Sakaguchi, R. L. , & Douglas, W. H. (2000). Correlation of noncarious cervical lesion size and occlusal wear in a single adult over 14‐year time span. Journal of Prosthetic Dentistry, 84, 436–443. [DOI] [PubMed] [Google Scholar]
- Ramseier, C. A. , Mirra, D. , Schütz, C. , Lang, N. P. , Walter, C. , & Salvi, G. E. (2015). Bleeding on probing as it relates to smoking status in patients enrolled in supportive periodontal therapy for at least 5 years. Journal of Clinical Periodontology, 42, 150–159. [DOI] [PubMed] [Google Scholar]
- Ravald, N. , & Starkhammar Johanson, C. (2012). Tooth loss in periodontally treated patients. A long‐term study of periodontal disease and root carties. Journal of Clinical Periodontology, 39, 73–79. [DOI] [PubMed] [Google Scholar]
- Renvert, S. , & Persson, G. R. (2002). A systematic review on the use of residual probing depth, bleeding on probing and furcation status following initial periodontal therapy to predict further attachment and tooth loss. Journal of Clinical Periodontology, 29, 82–89. [DOI] [PubMed] [Google Scholar]
- Rosa, G. M. , Lucas, G. Q. , & Lucas, O. N. (2008). Cigarette smoking and alveolar bone in young adults: A study using digitized radiographs. Journal of Periodontology, 79, 232–244. [DOI] [PubMed] [Google Scholar]
- Salvi, G. E. , Mischler, D. C. , Schmidlin, G. , Pjeutursson, U. , Brägger, U. , & Lang, N. P. (2014). Risk factors associated with the longevity of multi‐rooted teeth. Long‐term outcomes after supportive periodontal therapy. Journal of Clinical Periodontology, 41, 601–707. [DOI] [PubMed] [Google Scholar]
- Sarode, G.S. , & Sarode, S.C. (2013). Abfraction: A review (2013). Journal of Oral and Maxillofacial Pathology, 10, 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller, R. , Marquardt, E. , & Albers, H. K. (1985). Connection between the polished occusal facets and pathological findings in the masticatory system of young adults. ZWR, 94, 228–232. [PubMed] [Google Scholar]
- Shefter, G. J. , & McFall, W. T. (1984). Occlusal relations and periodontal status in human adults. Journal of Periodontology, 55, 219–223. [DOI] [PubMed] [Google Scholar]
- Shimazaki, Y. , Saito, T. , Kiyohara, Y. , Kato, I. , Kubo, M. , Iida, M. , & Yamashita, Y. (2006). The influence of current and former smoking on gingival bleeding: The Hisayama Study. Journal of Periodontology, 77, 1430–1435. [DOI] [PubMed] [Google Scholar]
- Smith, B. G. , & Knight, J. K. (1984). An Index for measuring the wear of teeth. British Dental Journal, 156, 435–438. [DOI] [PubMed] [Google Scholar]
- Stillman, P. R. (1921). Early clinical evidences of disease in the gingival and pericementum. Journal of Dental Research, 3, 25–31. [Google Scholar]
- Telles, D. , Pegoraro, L. F. , & Pereira, J. C. (2000). Prevalence of non‐carious cervical lesions and their relation to occlusal aspects: A clinical study. Journal of Esthetic Dentistry, 12, 10–15. [DOI] [PubMed] [Google Scholar]
- Tomar, S. L. , & Asma, S. (2000). Smoking‐attributable periodontitis in the United States; findings from NHANES III. National Health and Nutrition Examination Survey. Journal of Periodontology, 71, 743–751. [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , Muller‐Campanile, V. , & Lang, N. P. (1998). Changes in the prevalence of residual pockets and tooth loss in treated periodontal patients during a supportive maintenance care program. Journal of Clinical Periodontology, 25, 1008–1016. [DOI] [PubMed] [Google Scholar]
- Tsami, A. , Pepelassi, E. , Kodovazenitis, G. , & Komboli, M. (2009). Parameters affecting tooth loss during periodontal maintenance in Greek population. Journal of the American Dental association, 140, 1100–1107. [DOI] [PubMed] [Google Scholar]
- Wasserman, B. , & Hirschfeld, L. (1988). The relationship of initial clinical parameters to the long‐term response in 112 cases of periodontal disease. Journal of Clinical Periodontology, 15, 38–42. [DOI] [PubMed] [Google Scholar]
- Watanabe, C. , Watanabe, Y. , Miyauchi, M. , Minoru, F. , & Watanabe, Y. (2012). Multiple cemental tears. Oral Surgery Oral Medicine Oral Pathology and Oral Radiology, 114, 365–372. [DOI] [PubMed] [Google Scholar]
- Xhonga, F. A. (1977). Bruxism and its effect on the teeth. Journal of Oral Rehabilitation, 4, 65–76. [DOI] [PubMed] [Google Scholar]
- Yuodelis, R. A. , & Mann, W. V. (1965). The prevalence and possible role of non‐working contacts in periodontal disease. Periodontics, 3, 219–223. [PubMed] [Google Scholar]
- Zuabi, O. , Machtei, E. E. , Ben‐Aryeh, H. , Ardekian, L. , Peled, M. , & Laufer, D. (1999). The effect of smoking and periodontal treatment on salivary composition in patients with stablished periodontitis. Journal of Periodontology, 70, 1240–1246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


